Infrequent mutation of the tumour-suppressor gene smad4 in early-stage colorectal cancer
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Smad4 is a candidate tumour-suppressor gene identified recently on chromosome 18q21.1. Both alleles are inactivated in nearly one-half of pancreatic carcinomas, but its role in the
tumorigenesis of other tumours is still unknown. The aim of this study was to investigate the potential involvement of the Smad4 locus in early-stage colorectal cancers (stages I–III) in
tumour samples from a randomised multicentre trial. Of a large collection of DNA samples, 73 with a loss of one allele of the Smad4 gene were analysed for the presence of point mutations in
the remaining gene. Patients, from whom biopsies were isolated, were part of a previous randomised multicentre study of the Swiss Group for Clinical Cancer Research on the benefit of
adjuvant chemotherapy (SAKK study 40/81). Mutation analysis was restricted to the highly conserved C-terminal domain (exons 8, 9, 10 and 11) of Smad4, using PCR and single-strand
conformational variant analysis. Two of the 73 patients (3%) with loss of one allele of Smad4 had a point mutation in the remaining allele. These results indicate that whereas Smad4 point
mautations are prevalent in pancreatic carcinoma, they are infrequent in early stages (I–III) of colorectal cancer. SIMILAR CONTENT BEING VIEWED BY OTHERS THE ATOM-SEQ SEQUENCE CAPTURE PANEL
CAN ACCURATELY PREDICT MICROSATELLITE INSTABILITY STATUS IN FORMALIN-FIXED TUMOUR SAMPLES, ALONGSIDE ROUTINE GENE MUTATION TESTING Article Open access 19 September 2024 THE PANCREATIC
CANCER GENOME REVISITED Article 04 June 2021 INTEGRATION OF TUMOUR SEQUENCING AND CASE–CONTROL DATA TO ASSESS PATHOGENICITY OF _RAD51C_ MISSENSE VARIANTS IN FAMILIAL BREAST CANCER Article
Open access 17 January 2022 MAIN Deletion of a chromosomal region is a frequent cytogenetic alteration observed in carcinogenesis. The loss of tumour-suppressor genes has been reported in
numerous types of human tumours, in particular those of the gastrointestinal tract (Vogelstein et al, 1988). APC and p53 have been widely recognised as important tumour-suppressor genes
inactivated during colorectal carcinogenesis. Several other tumour-suppressor genes have been located on chromosomes 1p, 8p, 18q and 22q. In particular, loss of heterozygosity (LOH) at 18q21
is correlated with carcinomas of the colon, and other tumours such as pancreatic carcinoma, renal cell carcinoma, melanoma and breast carcinoma (Schutte et al, 1996; Barbera et al, 2000).
Much of the interest in this region arose because reports indicated that 18q losses are associated with high metastatic potential and reduced patient survival (Iino et al, 1994). In fact,
several potentially cancer-related genes map to the 18q21 region, including bcl-2, gastrin-releasing peptide gene and cellular homologue of yes-1. However, none of these have been observed
to be mutated in colorectal cancer (CRC). Fearon et al. (1990) identified another tumour-suppressor gene localised on 18q21, designated DCC for deleted in colorectal cancer. However, there
have been several cases in which loss of expression did not correlate with LOH (Kikuchi-Yanoshita et al, 1992), and mutation in the coding region of the DCC gene has been infrequently
detected (Cho et al, 1994; Sato et al, 2001). Owing to the controversial evidence as to the role of DCC in cancer, additional genetic analysis of the 18q21 region led to the identification
of other potential tumour-suppressor genes, including three candidate tumour-suppressor genes: Smad2, Smad4 and Smad7. These genes are involved in signal transduction of the TGF_β_
signalling pathway. Members of the transforming growth factor (TGF)-_β_ family transmit their signals from the plasma membrane to the nucleus through combinations of serine/threonine kinase
receptors and their downstream effectors, known as Smads. After the Smad4 (MADH4) gene was isolated from the same region as a tumour-suppressor gene for pancreatic cancer (Hahn et al, 1996),
mutation analysis of this gene has been carried out in various cancers. In recent studies, Smad4 was identified as a genetic target in pancreatic carcinomas, inactivated through homozygous
deletion (_n_=5), intragenic mutation (_n_=3) and lack of protein (_n_=2) in 10 out of 16 pancreatic cell lines (Barbera et al, 2000). Furthermore, it could be shown that when genetically
inactivated this tumour-suppressor in the TGF_β_ signalling pathway represents a prognostic factor in invasive pancreatic cancer influenced by Smad4 status (Tascilar et al, 2001). In
addition to observations in pancreatic carcinomas, Smad4 is also known as a gene involved in juvenile polyposis tumour predisposition syndrome (Howe et al, 1998; Huang et al, 2000).
Mutations of the Smad4 gene have been detected in some colorectal cancers, but its role in this specific cancer remains unclear. The frequencies of mutations (5–45%) have been found to be
low (Takagi et al, 1996; MacGrogan et al, 1997; Ohtaki et al, 2001), but data originated from relatively small studies, and the tumour populations examined were inhomogeneous explaining the
broad range of incidences found. The aim of this study was to further expand these data by Smad4 mutation analysis of a large set of early-stage (I–III) colorectal cancer patients treated in
a randomised multicentre trial of 5-fluorouracil (5-FU)/Mitomycin C adjuvant chemotherapy of the Swiss Group for Clinical Cancer Research (SAKK study 40/81). Owing to the significance of
LOH in colorectal cancer and the role of the remaining gene, this study was focused on patients with an allelic loss of one Smad4. METHODS PATIENTS Patients from whom biopsies were isolated,
were part of a previous randomised multicentre study of the SAKK on the benefit of treatment with adjuvant chemotherapy between 1981 and 1987 (Laffer, 1995). Deoxyribo nucleic acid (DNA)
samples of these patients were extracted from tumour as well as from healthy tissue derived from the same patient in order to perform genetic analyses. Paraffin-embedded material was
available from 329 of the 505 patients. To investigate genetic alterations in the 18q21 region in these tumours, a gene dosage study of the tumour-suppressor genes Smad2, Smad4 and DCC was
performed (Boulay et al, 1999). For technical reasons, high-quality DNA for analysis was available from 294 patients only. Individual dosage of the Smad4 gene showed a total deletion
frequency (one or both alleles) of 68% when compared to normal tissue. In total, 167 patients (=57%) were detected with an allelic loss of one Smad4 copy. In this study, we randomly chose 73
of these 167 patients to search for the presence of point mutations in the remaining gene. After analysis of these 73 out of 167 patients, two point mutations of Smad4 had been detected,
and for statistical reasons, further mutation analysis in the remaining 94 out of 167 patients did not seem necessary to substantiate our finding. GENE COPY STATUS SCORING Genomic samples
from 294 patients were tested for copy dosage of the Smad4 gene using TaqMan quantitative real-time PCR (Perkin-Elmer, Huenenberg, Switzerland). Copy status of the Smad4 gene was determined
by comparing tumour DNA to DNA from normal tissue derived from the same patient as described previously (Boulay et al, 1999). DUPLEX PCR Polymerase chain reaction (PCR) amplification on DNA
was performed in 15 _μ_l reaction volume, containing 1.5 _μ_l 10 × PCR buffer (Perkin-Elmer, Huenenberg, Switzerland), 10 mM 2′desoxyribonucleosoid-5′-triphosphate (dNTPs), 20 _μ_ M of each
primer, 1 U of AmpliTaq Gold (Perkin-Elmer, Huenenberg, Switzerland), 32P Oligo (2.5 _μ_l 10 × Buffer, 20 _μ_ M forward primer, 1 _μ_l PNK and 1 _μ_l _γ_32P-adenosine triphosphate (ATP)
incubated 30 min at 37°C) and 100 ng DNA. Duplex PCR for Smad4 gene was done using primers EX 8/1 and EX 8/2, EX 9/1 and EX 9/2, EX 10/1 and EX 10/2, and finally EX 11/1 and EX 11/2 (Table
1). Polymerase chain reaction conditions were as follows: 40 amplification cycles of denaturation at 94°C for 45 s, annealing at 55°C for 60 s, and extension at 72°C for 60 s, followed by
one cycle at 72°C for 10 min. Amplification products were loaded on a 0.4 mm acrylamide gel in a ‘Model S2 Sequencing Gel Electrophoresis Apparatus (Life Technologies, Switzerland)’.
Electrophoresis settings are: 1800 V, 35–40 mA, 60 VA and 120 min. Polymerase chain reactions without DNA templates were performed as negative controls. Bands were subsequently cut out from
the single-strand conformation polymorphism (SSCP)-gel and reamplified in a PCR. SEQUENCING ANALYSIS Reamplification of DNA was performed in a 50 _μ_l reaction volume, containing 5 _μ_l 10 ×
PCR buffer (Perkin-Elmer, Huenenberg, Switzerland), 10 mM dNTPs, 20 _μ_ M forward and backward primer, 1 U of AmpliTaq Gold (Perkin-Elmer, Huenenberg, Switzerland) and 38 _μ_l H2O. PCR
conditions were as follows: 35 amplification cycles of denaturation at 94°C for 45 s, annealing at 55°C for 60 s and extension at 72°C for 60 s, followed by one cycle at 72°C for 10 min.
Sequencing analysis was performed by Microsynth (Basel, Switzerland) on a fluorescence-based DNA sequencer that utilises capillary electrophoresis with 96 capillaries operating in parallel.
RESULTS Among the 294 tumours for which gene dosage data (Smad2, Smad4 and DCC) were available, 167 tumours (57%) showed heterozygous loss of Smad4 (Boulay et al, 1999), and 73 out of 167
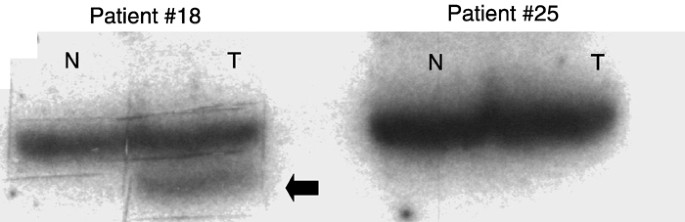
samples were randomly chosen for mutation analysis. Of these, only two (3%) carried point mutations in Smad4 in tumour but not the corresponding healthy tissue, as demonstrated by PCR–SSCP
(Figure 1). The two mutations were located in the highly conserved C-terminal Smad4 homology region. One confirmed point mutation was found in exon 9 and another point mutation in exon 11.
Both mutations were confirmed by direct sequencing analysis showing one mutation resulting in an amino-acid change from arginine to serine; the second mutation led to an exchange of alanine
to valine (Table 2). However, a caveat in the interpretation of our data needs to be mentioned: informative and reproducible data were available from a total of 174 complete exons (8, 9, 10
and 11) derived from the 73 defined patients. This shortcoming of our data was because of technical problems in the analysis, caused by the sometimes poor quality of the DNA, as is often
observed with nucleic acids isolated from paraffin-embedded tissue. Nevertheless, since the exons for which interpretable results were available were equally distributed between the eight
different amplicons we used in our study, our conclusion of a very low frequency of Smad4 point mutations in the population studied is not put into question by this technical shortcoming.
DISCUSSION Smad proteins are a novel family of proteins that function downstream of serine/threonine kinase receptors to transduce signals for members of the TGF_β_ superfamily (Massague,
1996). The three Smads (Smad2, Smad4 and Smad7) encoded in the 18q21 chromosomal region participate in the signalling mechanisms subsequent to TGF_β_-receptor complex formation. Smad4, a
co-Smad of Smad2, is known as a tumour-suppressor gene in different cancer types. Tumour-suppressor genes are often inactivated when one allele acquires a mutation and the second allele is
lost, typically through deletion (Cavenee et al, 1983). The tumour-suppressor gene p53 represents just one example for this classic concept (Miller et al, 1992), while for another
tumour-suppressor gene, DCC, these findings could not be confirmed (Sato et al, 2001). Our screen of 73 patients with early-stage colorectal cancer (I–III) carrying a loss of one Smad4
allele identified two mutations of the remaining allele (3%), a finding that is in accordance with results described in the literature (Schutte et al, 1996; Miyaki et al, 1999). Mutation
analysis was restricted to exons 8, 9, 10 and 11 of Smad4, which together span the entire conserved C-terminal Smad4 homology region. Since 90% of the Smad4 mutations reported are located in
that highly conserved region, the number of undetected mutations is expected to be low when the analysis is restricted to these mutation hot spots (Hahn et al, 1996; Takagi et al, 1996;
Kong et al, 1997). The low rate of point mutations detected by our method deserves further comments: SSCP has been shown to be a highly sensitive method to identify mutations in
PCR-generated fragments. The sensitivity of SSCP analysis is widely disputed in the literature, with reports ranging from 35% (Sarkar et al, 1992) to nearly 100% (Orita et al, 1989). Of
course, certain mutations may not be detected using this method. Furthermore, it is possible that some of our tumours had large intragenetic deletions of Smad4, which would have been missed
with the detection method used. However, the single factor having the greatest effect on SSCP sensitivity is the size of the DNA fragments. An optimal size of 200 base pairs (bp) or less was
used in our study (160–180 bp), which is described as the most sensitive for single-base substitutions (Sheffield et al, 1993). Among the mediators of TGF_β_ signalling encoded by the 18q21
chromosomal region, two were identified as involved in activating TGF_β_ signalling: Smad2 and Smad4, and one, in the inhibition of TGF_β_ signalling: Smad7. Thus, one could have expected
that inactivation of Smad4 might result in a TGF_β_ resistance that would favour tumour expansion. Interestingly, the patients with deletion of Smad4 did not show a significantly worse
prognosis than those without a deletion (Boulay et al, 2002). In contrast, in the same population, Smad4 seemed to be a predictive marker for 5FU/mitomycin adjuvant chemotherapy. However,
whether Smad4 plays a key role in tumorigenesis of colorectal cancer is still unclear. To date, a significant number of Smad4 point mutations have been found only in pancreatic carcinomas
(50–60%), biliary tract carcinomas (15%) or colorectal carcinomas (5–20%) (Hahn et al, 1996; Schutte et al, 1996; MacGrogan et al, 1997). Although the existence of additional unknown target
tumour-suppressor genes in the region of 18q21 cannot be ruled out, recently published results strongly suggest a significant contribution of Smad4 gene inactivation in advanced tumour
stages. Metastatic colorectal carcinomas including carcinomas metastasised to the liver showed a considerably higher frequency (31–35%) than invasive carcinomas without distant metastasis
(7%) (Miyaki et al, 1999; Ohtaki et al, 2001). Our findings of less than 5% point mutations are at the lower end of the spectrum and confirm the low frequency of point mutations of Smad4 in
early-stage colorectal cancer without distant metastasis. The limitation to patients with loss of one Smad4 allele–initially used to select a population with a presumably high mutation
frequency–is one possible theoretical explanation for our findings. However, in pancreatic and biliary tract carcinomas, patients with LOH represent a group with an especially high point
mutation frequency in the remaining gene, making this explanation highly unlikely (Hahn et al, 1998; Barbera et al, 2000). Other possible explanations for the absence of Smad4 point
mutations in colorectal cancer at this stage include methylation changes at the promoter and alternative splicing or changes in mRNA stability (Roth et al, 2000). The importance of genes
that undergo alterations at low prevalence, however, may as yet be underestimated. Such events may contribute significantly to the genetic variety within a tumour type and, thus, to the
complexity of human tumorigenesis. Since it is likely that many alterations of low prevalence exist in human cancers, an individual tumour might still acquire several of these different
alterations with a high probability, making low prevalence alterations a powerful driving force of the carcinogenic process. In conclusion, our findings indicate that Smad4 point mutations
are infrequent in early stages of colorectal cancer. However, it cannot be completely ruled out that inactivation of Smad4 could be a common genetic event at later stages of colorectal
cancer. Future research comparing early and advanced stages is required to investigate the tumour-suppressor pathway in colorectal cancer and to redefine the role Smad4 signalling plays in
tumorigenesis. CHANGE HISTORY * _ 16 NOVEMBER 2011 This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _
REFERENCES * Barbera VM, Martin M, Marinoso L, Munne A, Carrato A, Real FX, Fabre M (2000) The 18q21 region in colorectal and pancreatic cancer: independent loss of DCC and DPC4 expression.
_Biochim Biophys Acta_ 1502: 283–296 Article CAS PubMed Google Scholar * Boulay JL, Mild G, Lowy A, Reuter J, Lagrange M, Terracciano L, Laffer U, Herrmann R, Rochlitz C (2002) SMAD4 is
a predictive marker for 5-fluorouracil-based chemotherapy in patients with colorectal cancer. _Br J Cancer_ 87: 630–634 Article CAS PubMed PubMed Central Google Scholar * Boulay JL,
Reuter J, Ritschard R, Terracciano L, Herrmann R, Rochlitz C (1999) Gene dosage by quantitative PCR. _Biotechniques_ 27: 228–230, 232 Article CAS PubMed Google Scholar * Cavenee WK,
Dryja TP, Phillips RA, Benedict WF, Godbout R, Gallie BL, Murphree AL, Strong LC, White RL (1983) Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. _Nature_ 305:
779–784 Article CAS PubMed Google Scholar * Cho KR, Oliner JD, Simons JW, Hedrick L, Fearon ER, Preisinger AC, Hedge P, Silverman GA, Vogelstein B (1994) The DCC gene: structural
analysis and mutations in colorectal carcinomas. _Genomics_ 19: 525–531 Article CAS PubMed Google Scholar * Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR,
Preisinger AC, Thomas G, Kinzler KW _et al_ (1990) Identification of a chromosome 18q gene that is altered in colorectal cancers. _Science_ 247: 49–56 Article CAS PubMed Google Scholar *
Hahn SA, Bartsch D, Schroers A, Galehdari H, Becker M, Ramaswamy A, Schwarte-Waldhoff I, Maschek H, Schmiegel W (1998) Mutations of the DPC4/Smad4 gene in biliary tract carcinoma. _Cancer
Res_ 58: 1124–1126 CAS PubMed Google Scholar * Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE (1996) DPC4, a
candidate tumor suppressor gene at human chromosome 18q21.1. _Science_ 271: 350–353 Article CAS PubMed Google Scholar * Howe JR, Roth S, Ringold JC, Summers RW, Jarvinen HJ, Sistonen P,
Tomlinson IP, Houlston RS, Bevan S, Mitros FA, Stone EM, Aaltonen LA (1998) Mutations in the SMAD4/DPC4 gene in juvenile polyposis. _Science_ 280: 1086–1088 Article CAS PubMed Google
Scholar * Huang SC, Chen CR, Lavine JE, Taylor SF, Newbury RO, Pham TT, Ricciardiello L, Carethers JM (2000) Genetic heterogeneity in familial juvenile polyposis. _Cancer Res_ 60: 6882–6885
CAS PubMed Google Scholar * Iino H, Fukayama M, Maeda Y, Koike M, Mori T, Takahashi T, Kikuchi-Yanoshita R, Miyaki M, Mizuno S, Watanabe S (1994) Molecular genetics for clinical
management of colorectal carcinoma. 17p, 18q, and 22q loss of heterozygosity and decreased DCC expression are correlated with the metastatic potential. _Cancer_ 73: 1324–1331 Article CAS
PubMed Google Scholar * Kikuchi-Yanoshita R, Konishi M, Fukunari H, Tanaka K, Miyaki M (1992) Loss of expression of the DCC gene during progression of colorectal carcinomas in familial
adenomatous polyposis and non-familial adenomatous polyposis patients. _Cancer Res_ 52: 3801–3803 CAS PubMed Google Scholar * Kong XT, Choi SH, Inoue A, Xu F, Chen T, Takita J, Yokota J,
Bessho F, Yanagisawa M, Hanada R, Yamamoto K, Hayashi Y (1997) Expression and mutational analysis of the DCC, DPC4, and MADR2/JV18-1 genes in neuroblastoma. _Cancer Res_ 57: 3772–3778 CAS
PubMed Google Scholar * Laffer U (1995) Long-term results of single course of adjuvant intraportal chemotherapy for colorectal cancer. Swiss Group for Clinical Cancer Research (SAKK).
_Lancet_ 345: 349–353 Article Google Scholar * MacGrogan D, Pegram M, Slamon D, Bookstein R (1997) Comparative mutational analysis of DPC4 (Smad4) in prostatic and colorectal carcinomas.
_Oncogene_ 15: 1111–1114 Article CAS PubMed Google Scholar * Massague J (1996) TGFbeta signaling: receptors, transducers, and Mad proteins. _Cell_ 28: 947–950 Article Google Scholar *
Miller CW, Simon K, Aslo A, Kok K, Yokota J, Buys CH, Terada M, Koeffler HP (1992) p53 mutations in human lung tumors. _Cancer Res_ 52: 1695–1698 CAS PubMed Google Scholar * Miyaki M,
Iijima T, Konishi M, Sakai K, Ishii A, Yasuno M, Hishima T, Koike M, Shitara N, Iwama T, Utsunomiya J, Kuroki T, Mori T (1999) Higher frequency of Smad4 gene mutation in human colorectal
cancer with distant metastasis. _Oncogene_ 18: 3098–3103 Article CAS PubMed Google Scholar * Ohtaki N, Yamaguchi A, Goi T, Fukaya T, Takeuchi K, Katayama K, Hirose K, Urano T (2001)
Somatic alterations of the DPC4 and Madr2 genes in colorectal cancers and relationship to metastasis. _Int J Oncol_ 18: 265–270 CAS PubMed Google Scholar * Orita M, Iwahana H, Kanazawa H,
Hayashi K, Sekiya T (1989) Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. _Proc Natl Acad Sci USA_ 86: 2766–2770 Article CAS
PubMed PubMed Central Google Scholar * Roth S, Laiho P, Salovaara R, Launonen V, Aaltonen LA (2000) No SMAD4 hypermethylation in colorectal cancer. _Br J Cancer_ 83: 1015–1019 Article
CAS PubMed PubMed Central Google Scholar * Sarkar G, Yoon HS, Sommer SS (1992) Screening for mutations by RNA single-strand conformation polymorphism (rSSCP): comparison with DNA–SSCP.
_Nucleic Acids Res_ 20: 871–878 Article CAS PubMed PubMed Central Google Scholar * Sato K, Tamura G, Tsuchiya T, Endoh Y, Usuba O, Kimura W, Motoyama T (2001) Frequent loss of
expression without sequence mutations of the DCC gene in primary gastric cancer. _Br J Cancer_ 85: 199–203 Article CAS PubMed PubMed Central Google Scholar * Schutte M, Hruban RH,
Hedrick L, Cho KR, Nadasdy GM, Weinstein CL, Bova GS, Isaacs WB, Cairns P, Nawroz H, Sidransky D, Casero Jr RA, Meltzer PS, Hahn SA, Kern SE (1996) DPC4 gene in various tumor types. _Cancer
Res_ 56: 2527–2530 CAS PubMed Google Scholar * Sheffield VC, Beck JS, Kwitek AE, Sandstrom DW, Stone EM (1993) The sensitivity of single-strand conformation polymorphism analysis for the
detection of single base substitutions. _Genomics_ 16: 325–332 Article CAS PubMed Google Scholar * Takagi Y, Kohmura H, Futamura M, Kida H, Tanemura H, Shimokawa K, Saji S (1996) Somatic
alterations of the DPC4 gene in human colorectal cancers _in vivo_. _Gastroenterology_ 111: 1369–1372 Article CAS PubMed Google Scholar * Tascilar M, Skinner HG, Rosty C, Sohn T,
Wilentz RE, Offerhaus GJ, Adsay V, Abrams RA, Cameron JL, Kern SE, Yeo CJ, Hruban RH, Goggins M (2001) The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. _Clin Cancer Res_
7: 4115–4121 CAS PubMed Google Scholar * Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL (1988) Genetic alterations during
colorectal-tumor development. _N Engl J Med_ 319: 525–532 Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by a grant from the Swiss Cancer
League and Cancer League, Basel. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Oncology, University Hospital Basel, Basel, 4031, Switzerland C Mamot, R Herrmann & C Rochlitz
* Department of Research, University Hospital Basel, Basel, 4031, Switzerland G Mild, J Reuter, J-L Boulay, R Herrmann & C Rochlitz * The Swiss Group for Clinical Cancer Research (SAKK),
Bern, 3000, Switzerland U Laffer & U Metzger * Institute of Pathology, University Hospital Basel, Basel, 4031, Switzerland L Terracciano Authors * C Mamot View author publications You
can also search for this author inPubMed Google Scholar * G Mild View author publications You can also search for this author inPubMed Google Scholar * J Reuter View author publications You
can also search for this author inPubMed Google Scholar * U Laffer View author publications You can also search for this author inPubMed Google Scholar * U Metzger View author publications
You can also search for this author inPubMed Google Scholar * L Terracciano View author publications You can also search for this author inPubMed Google Scholar * J-L Boulay View author
publications You can also search for this author inPubMed Google Scholar * R Herrmann View author publications You can also search for this author inPubMed Google Scholar * C Rochlitz View
author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to C Rochlitz. RIGHTS AND PERMISSIONS From twelve months after its
original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mamot, C., Mild, G., Reuter, J. _et al._ Infrequent mutation of the
tumour-suppressor gene Smad4 in early-stage colorectal cancer. _Br J Cancer_ 88, 420–423 (2003). https://doi.org/10.1038/sj.bjc.6600733 Download citation * Received: 21 October 2002 *
Accepted: 07 November 2002 * Published: 10 February 2003 * Issue Date: 10 February 2003 * DOI: https://doi.org/10.1038/sj.bjc.6600733 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * DPC4 * loss of heterozygosity (LOH) * Smad4 * TGF_β_ * tumorigenesis