Increased expression of phosphorylated forms of rna-dependent protein kinase and eukaryotic initiation factor 2α may signal skeletal muscle atrophy in weight-losing cancer patients
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Previous studies suggest that the activation (autophosphorylation) of dsRNA-dependent protein kinase (PKR) can stimulate protein degradation, and depress protein synthesis in
skeletal muscle through phosphorylation of the translation initiation factor 2 (eIF2) on the _α_-subunit. To understand whether these mediators are important in muscle wasting in cancer
patients, levels of the phospho forms of PKR and eIF2_α_ have been determined in rectus abdominus muscle of weight losing patients with oesophago-gastric cancer, in comparison with healthy
controls. Levels of both phospho PKR and phospho eIF2_α_ were significantly enhanced in muscle of cancer patients with weight loss irrespective of the amount and there was a linear
relationship between phosphorylation of PKR and phosphorylation of eIF2_α_ (correlation coefficient 0.76, _P_=0.005). This suggests that phosphorylation of PKR led to phosphorylation of
eIF2_α_. Myosin levels decreased as the weight loss increased, and there was a linear relationship between myosin expression and the extent of phosphorylation of eIF2_α_ (correlation
coefficient 0.77, _P_=0.004). These results suggest that phosphorylation of PKR may be an important initiator of muscle wasting in cancer patients. SIMILAR CONTENT BEING VIEWED BY OTHERS THE
AUTOPHAGY INHIBITOR NSC185058 SUPPRESSES MTORC1-MEDIATED PROTEIN ANABOLISM IN CULTURED SKELETAL MUSCLE Article Open access 06 April 2024 MECHANISMS OF MUSCLE ATROPHY AND HYPERTROPHY:
IMPLICATIONS IN HEALTH AND DISEASE Article Open access 12 January 2021 BLOCKING MUSCLE WASTING VIA DELETION OF THE MUSCLE-SPECIFIC E3 LIGASE MURF1 IMPEDES PANCREATIC TUMOR GROWTH Article
Open access 13 May 2023 MAIN Patients with cancer, especially those of the gastrointestinal tract, show a progressive wasting of skeletal muscle, which reduces both their quality of life and
survival time (DeWys et al, 1980). Skeletal muscle atrophy is characterised by a decreased protein content, fibre diameter, force production and fatigue resistance. Muscle wasting is due to
a combination of depressed protein synthesis (Emery et al, 1984), and elevated endogenous protein breakdown, with oxidation of the resultant amino acids (O'Keefe et al, 1990). In
cancer patients the mechanism for the depression in protein synthesis is not known, while the increased protein degradation has been attributed to an increased expression of the
ubiquitin–proteasome proteolytic pathway (Khal et al, 2005). Several potential mediators of the cachectic process, including proteolysis-inducing factor (PIF) and angiotensin II (Ang II),
inhibit protein synthesis in skeletal muscle (Lorite et al, 1997; Russell et al, 2006a), and also stimulate degradation, through increased activity and expression of the ubiquitin–proteasome
pathway (Lorite et al, 2001; Sanders et al, 2005). We have recently shown (Eley and Tisdale, 2007) a link between the ability of PIF and Ang II to inhibit protein synthesis and increase
protein degradation in murine myotubes through the dsRNA-dependent protein kinase (PKR). dsRNA-dependent protein kinase is a serine/threonine-specific protein kinase, which undergoes
autophosphorylation at multiple serine and threonine residues, causing activation, in the presence of double-stranded (ds)RNA, in response to viral attack (Jammi and Beal, 2001). Both PIF
and Ang II were shown to induce autophosphorylation of PKR. Activated PKR can phosphorylate several protein substrates including the _α_-subunit of the heterotrimeric translation initiation
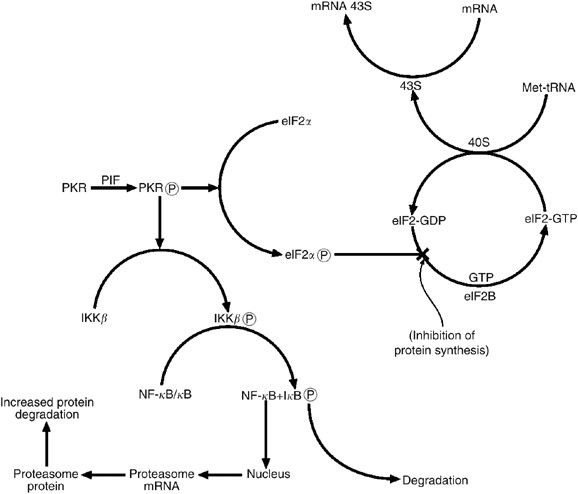
factor 2 (eIF2_α_) (deHaro et al, 1996). Phosphorylation of eIF2_α_ inhibits continued initiation of protein synthesis by the eIF2 complex, which initiates Met tRNA binding to the 40S
ribosomal subunit. During this process GTP associated with eIF2 is hydrolysed to GDP, and recycling of eIF2-GDP to eIF2-GTP requires a guanine nucleotide exchange factor eIF2B (Price and
Proud, 1994). Phosphorylation of eIF2 on the _α_-subunit causes it to act as an inhibitor of eIF2B and the reduction in eIF2-GTP levels reduces general translation (Rowlands et al, 1998)
(Figure 1). Thus activation of PKR by PIF and Ang II was responsible for the depression in protein synthesis, since transfection of myotubes with a mutant PKR incapable of
autophosphorylation and induction of phosphorylation of eIF2_α_, completely attenuated the depression in protein synthesis by both agents (Eley and Tisdale, 2007). Mutation of PKR also
completely attenuated the induction of protein degradation and upregulation of the ubiquitin–proteasome pathway. Induction of the ubiquitin–proteasome pathway by both PIF (Wyke and Tisdale,
2005) and Ang II (Russell et al, 2006b) requires activation of the transcription factor nuclear factor-_κ_B (NF-_κ_B). dsRNA-dependent protein kinase has been shown to activate the upstream
kinase I_κ_B kinase (IKK) leading to degradation of the inhibitor protein I_κ_B, leading to release of NF-_κ_B, which migrates to the nucleus and induces transcriptional activation of
specific genes (Zamanian-Daryoush et al, 2000). Myotubes containing mutant PKR showed no activation of NF-_κ_B in response to either PIF or Ang II, and no induction of the
ubiquitin–proteasome pathway (Eley and Tisdale, 2007), suggesting that NF-_κ_B activity is required for the induction of ubiquitin–proteasome pathway by PKR (Figure 1). Thus activation of
PKR leads potentially to both a depression of protein synthesis and an increase in protein degradation in skeletal muscle. These studies _in vitro_ were also reflected by changes _in vivo_
in gastrocnemius muscle of mice bearing a cachexia-inducing tumour, where levels of phosphorylated PKR and eIF2_α_ were found to increase with increasing weight loss by as much as 18-fold
for PKR at 25% weight loss (Eley and Tisdale, 2007). To determine whether changes similar to those induced by PIF and Ang II also occur in human cancer cachexia, the present study examines
the levels of phosphorylation of PKR and eIF2_α_ in skeletal muscle of weight-losing patients with upper gastrointestinal cancer, in comparison with healthy, weight-stable subjects
undergoing minor elective surgery. PATIENTS AND METHODS CANCER PATIENTS AND CONTROLS Patients provided written, informed consent, and the study was approved by the Lothian Research Ethics
Committee. Twenty-nine patients with newly-diagnosed oesophago-gastric adenocarcinoma who were undergoing elective resection of their primary cancer were recruited for the study.
Oesophago-gastric cancer patients have a high incidence of weight loss (DeWys et al, 1980) and were therefore chosen as a representative group of patients who develop cancer cachexia. Muscle
biopsies were also collected from 10 healthy, weight-stable volunteers who were undergoing elective hernia surgery and who served as controls. MUSCLE BIOPSY A sample of rectus abdominis
muscle was obtained from the edge of the patients abdominal wound within 10 min of induction of general anaesthesia. The sample was obtained without the use of diathermy and was frozen
immediately in liquid nitrogen using liquid nitrogen-resistant tubes (Corning BV, Netherlands). Samples were frozen at −70°C until analysis. NUTRITIONAL ASSESSMENT At the preoperative
assessment, preillness stable weight was self-reported by the patient. Height was measured using a wall-mounted stadiometer with the patient standing erect without shoes. Patients were
weighed on spring balance scales without shoes and wearing light clothing, and body mass index was calculated. Mid arm circumference (MAC) was measured at the midpoint between the acromion
and olecranon processes. Triceps skinfold thickness (TSF) was measured with Harpenden skin callipers (Holtain, Crymych, UK). Mid arm muscle circumference (MAMC) was calculated according to
the formula: MAC-[π × TSF]=MAMC. Karnofsky performance score was documented by the recruiting physician. MOLECULAR BIOLOGY MATERIALS Rabbit polyclonal antibody to phospho PKR (pThr 446), was
purchased from Insight Biotechnology, (Wembley, Middlesex, UK) and to total PKR (C terminus) from New England Biolabs, (Herts, UK). Rabbit polyclonal antisera to total eIF2_α_ was purchased
from Santa Cruz Biotechnology (CA, USA) and rabbit polyclonal antisera to phospho eIF2_α_ was purchased from Abcam (Cambridge, UK). Mouse monoclonal antibody to myosin heavy chain was from
Novocastra (Newcastle, UK). Rabbit polyclonal antiserum to actin was from Sigma Aldridge (Dorset, UK). Peroxidase-conjugated goat anti-rabbit antibody and peroxidase-conjugated rabbit
anti-mouse antibody were purchased from Dako Ltd (Cambridge, UK). PhosphoSafe™ extraction reagent was obtained from Merck Biosciences, (Nottingham, UK). Hybond A nitrocellulose membranes and
enhanced chemiluminescence (ECL) development kits were from Amersham Biosciences Ltd (Bucks, UK). WESTERN BLOT ANALYSIS Samples (approximately 10 mg) of muscle were homogenised in 500 _μ_l
of PhosphoSafe™ Extraction Reagent and centrifuged at 15 000 g for 15 min. Samples of cytosolic protein (10 _μ_g) were resolved on 10% sodium dodecylsulphate polyacrylamide gels (6% for
eIF2_α_) and transferred to 0.45 _μ_m nitrocellulose membranes, which had been blocked with 5% marvel in Tris-buffered saline, pH 7.5, at 4°C for 1–2 h. Membranes were then washed for 15 min
in 0.5% Tween-buffered saline or TBS Tween prior to adding the primary antibodies. The primary antibodies were used at a dilution of 1 : 1000 except for actin (1 : 250) and anti-myosin (1 :
100). Incubation was at 4°C overnight, except for total eIF2_α_ (1–2 h at room temperature). The primary antibodies were washed off the membranes for 15 min (changing the wash every 5 min),
except for actin, which was washed for 45 min (changing the wash every 15 min). TBS Tween (0.1%) was used for washing phospho antibodies and total antibodies. The secondary antibodies were
used at a dilution of 1 : 1000, and were washed off after 45 min. Development was by ECL and films were developed for 3–6 min. Blots were scanned by a densitometer to quantify differences.
STATISTICAL ANALYSIS Western blot densitometry results are presented as means±s.e.m. for at least three replicate experiments. Differences in means between groups were determined by one-way
analysis of variance, followed by Tukey–Kramer multiple comparison test. Significance level was set at _P_<0.05. RESULTS The characteristics of the patients in this study is shown in
Table 1. Muscle biopsies from healthy subjects undergoing elective surgery for hernia served as weight stable controls. The weight losing subjects had oesophago-gastric cancer and had a
weight loss at the time of operation between 2.4 and 27.5%. As a comparison some patients with oesophago-gastric cancer without weight loss were also included. Western blots for the phospho
and dephospho forms of PKR and eIF2_α_ in rectus abdominus muscle as a function of weight loss is shown in Figures 2 and 3, which display values for different patients. While there was no
major change in total PKR or eIF2_α_ with weight loss, there was a significant increase in the phosphorylated forms in all patients with weight loss, which, however, did not show a tendency
for increased expression with increasing weight loss. Cancer patients with no weight loss, or weight gain (Figure 2) showed the same low expression of phosphorylated PKR and eIF2_α_, as
nonweight-losing normal subjects. There was a linear correlation between expression of phosphorylated PKR and phosphorylated eIF2_α_ (correlation coefficient 0.76, _P_=0.005), consistent
with increased PKR activity being responsible for the increased phosphorylation of eIF2 on the _α_-subunit (Figure 4). Myosin levels decreased as the weight loss increased (Figure 5A), and
there was an inverse relationship between the expression of myosin in rectus abdominus muscle and the extent of phosphorylation of eIF2_α_ (correlation coefficient 0.77, _P_=0.004) (Figure
5B). As previously reported (Acharyya et al, 2004) in skeletal muscle of mice bearing a cachexia-inducing tumour (colon 26) myosin levels decreased, while actin levels remained constant.
This has been attributed to specific targeting of myosin by the ubiquitin–proteasome pathway. DISCUSSION This is the first report to show an increased expression of phosphorylated PKR and
eIF2_α_ in the skeletal muscle of weight-losing cancer patients compared with healthy weight-stable controls. As found in the gastrocnemius muscle of weight-losing mice bearing the MAC16
tumour (Eley and Tisdale, 2007), expression of both phospho PKR and eIF2_α_ increased in patients with weight loss, although there was no trend to increased expression with increasing weight
loss. This suggests that the same signalling mechanism is operative in the skeletal muscle of cachectic cancer patients as that in mice with experimental cancer cachexia. Similar findings
have been observed in murine myotubes in the presence of PIF or Ang II, and are thought to be responsible for the depression in protein synthesis and increase in degradation (Eley and
Tisdale, 2007). dsRNA-dependent protein kinase is normally activated in response to viral attack, and the depression of protein synthesis resulting from phosphorylation of eIF2_α_
constitutes one of the major ways in which viral replication is impaired (Clemens, 1997). However, PKR can also exert effects in uninfected cells and can be a potent growth inhibitory
protein when activated (Chong et al, 1992). dsRNA-dependent protein kinase is also linked to the induction of pro-apoptotic genes by dsRNA, and may trigger cell death in response to viral
infection and possible tumorigenesis (Balachandran et al, 1998). Activation of PKR by PIF may be responsible for its ability to induce apoptosis in murine myotubes (Smith and Tisdale, 2003).
Increased apoptosis has also been observed in the skeletal muscle of rats bearing the cachexia-inducing Yoshida AH-130 ascites hepatoma (van Royen et al, 2000) and in the early stage of
weight loss in rabbits bearing the VX2 carcinoma (Yoshida et al, 2001). In addition, muscle biopsies from weight losing patients with upper gastro-intestinal cancer showed a threefold
increase in DNA fragmentation compared with control subjects, together with an increased PARP cleavage and decrease in MyoD protein content (Busquets et al, 2007). Thus activation of PKR
might be responsible for the increased apoptosis in the skeletal muscle of weight-losing cancer patients contributing to muscle atrophy. The increased phosphorylation of eIF2_α_ is likely to
contribute to the depression of protein synthesis in the skeletal muscle of cancer patients, through the inhibition of eIF2B and subsequent translational repression (Rowlands et al, 1998),
as was previously observed in murine myotubes treated with PIF and Ang II (Eley and Tisdale, 2007). Phosphorylation of eIF2_α_ has also been shown to be responsible for the inhibition of
protein synthesis in rat liver by vasopressin (Kimball and Jefferson, 1990), and rat skeletal muscle by interleukin-1 (Cooney et al, 1999). Phosphorylation of PKR would also be expected to
lead to an increased breakdown of myofibrillar proteins in skeletal muscle by induction of the ubiquitin–proteasome pathway (Wyke and Tisdale, 2005; Russell et al, 2006b) through activation
of NF-_κ_B (Zamanian-Daryoush et al, 2000) analogous to the effect of PIF and Ang II (Eley and Tisdale, 2007). In the current study, there was a linear relationship between activation
(autophosphorylation) of PKR and phosphorylation of eIF2_α_, suggesting that PKR is responsible for this effect rather than general control of gene expression, nondepressing 2, which is
expected to be activated (Anthony et al, 2004) by the reduction in plasma levels of amino acids in cachectic subjects (Norton et al, 1985). Moreover, in myotubes expressing mutant PKR there
was no increase in phosphorylation of eIF2_α_ in response to catabolic stimuli (Eley and Tisdale, 2007) suggesting that PKR is the major eIF2_α_ kinase under such conditions. In mice bearing
the MAC16 tumour, which show a similar elevation of phospho PKR in skeletal muscle with weight loss (Eley and Tisdale, 2007), treatment with a PKR inhibitor, at a concentration which
reduced levels of phospho PKR down to that found in non tumour-bearing animals, effectively attenuated the depression of body weight, through an increase in lean body mass (Eley et al,
2007). This was achieved through attenuation in both the depression in protein synthesis and the increase in protein degradation, as observed in murine myotubes exposed to either PIF or Ang
II (Eley and Tisdale, 2007). This suggests that muscle atrophy in cachectic cancer patients may also be responsive to inhibitors of PKR. In addition to attenuation of cachexia, treatment of
mice bearing the MAC16 tumour with a PKR inhibitor also inhibited tumour growth (Eley et al, 2007). _In vitro_ studies (unpublished) also showed sensitisation of MAC16 cells to the growth
inhibitory effects of gemcitabine and 5-fluoroacil. The MAC16 tumour also shows elevated autophosphorylation of PKR and phosphorylation of eIF2_α_, which has been linked to constitutive
activation of NF-_κ_B and chemoresistance (unpublished). Other studies have also shown an increased autophosphorylation of PKR and phosphorylation of eIF2_α_ in human breast carcinoma cell
lines, compared with nontransformed epithelial cells (Kim et al, 2000), and in human melanoma cells compared with nontransformed melanocytes in culture (Kim et al, 2002). In addition
analysis of colon cancer specimens showed that transformation from normal mucosa to adenomas and carcinomas coincided with an increase in PKR expression (Kim et al, 2002). These results
suggest a positive role of PKR in cancer progression and growth control of tumour cells. This suggests that inhibitors of PKR may not only be effective in attenuating muscle wasting in
cancer patients, but may also induce antitumour effects or synergise with existing chemotherapy. The changes that are seen may be part of a common signalling mechanism found in conditions
where muscle atrophy occurs. Thus PIF has been found in the urine of weight-losing cancer patients, and when purified and administered to mice causes muscle atrophy (Cariuk et al, 1997). Ang
II has also been linked with muscle wasting in congestive heart failure (Onder et al, 2002), and tumour necrosis factor-_α_, which may be linked to muscle wasting in sepsis, AIDS and
parasitic infections, as well as cancer, has also been shown to activate PKR (Jeffrey et al, 2002). Burn injury, which also causes muscle atrophy, is also associated with an increased
phosphorylation of eIF2_α_ as a result of a 274% increase in phosphorylation of PKR (Kaneki et al, 2004). This suggests that inhibitors of PKR autophosphorylation may have a general role in
the treatment of muscle atrophy. CHANGE HISTORY * _ 16 NOVEMBER 2011 This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at
publication _ REFERENCES * Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC (2004) Cancer cachexia is regulated by selective targeting of skeletal muscle gene
products. _J Clin Invest_ 114: 370–378 Article CAS Google Scholar * Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC (2004) Preservation of liver protein
synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. _J Biol Chem_ 279: 36553–36561 Article CAS Google Scholar
* Balachandran S, Kim CN, Yeh W-C, Mak TW, Bhalla K, Barber GN (1998) Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signalling. _EMBO
J_ 17: 6888–6902 Article CAS Google Scholar * Busquets S, Deans C, Figueras M, Moore-Carrasco R, Lopez-Soriano FJ, Fearon KCH, Argiles JM (2007) Apoptosis is present in skeletal muscle of
cachectic gastro-intestinal cancer patients. _Clin Nutr_ 26: 614–618 Article CAS Google Scholar * Cariuk P, Lorite MJ, Todorov PT, Field WN, Wigmore SJ, Tisdale MJ (1997) Induction of
cachexia in mice by a product isolated from the urine of cachectic cancer patients. _Br J Cancer_ 76: 606–613 Article CAS Google Scholar * Chong KL, Feng L, Schappert K, Meurs E, Donahue
TF, Friesen JD, Hovanessian AG, Williams BR (1992) Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. _EMBO J_ 11: 1553–1562 Article CAS
Google Scholar * Clemens MJ (1997) PKR – A protein kinase regulated by double-stranded RNA. _Int J Biochem Cell Biol_ 29: 945–949 Article CAS Google Scholar * Cooney RN, Maish III GO,
Gilpin T, Shumate ML, Lang CH, Vary TC (1999) Mechanism of IL-1 induced inhibition of protein synthesis in skeletal muscle. _Shock_ II: 235–241 Article Google Scholar * deHaro C, Mendez R,
Santogo J (1996) The eIF-2_α_ kinases and the control of protein synthesis. _FASEB J_ 10: 1378–1387 Article CAS Google Scholar * DeWys WD, Begg C, Laven PT, Band PR, Bennett JM, Bertino
JR, Cohen MH, Douglass Jr HO, Engstrom PF, Edzini EZ, Horton J, Johnson GJ, Moertel CG, Oken MM, Perlia C, Rosenbaum C, Silverstein MN, Skeel RT, Sponzo RW, Tormey DC (1980) Prognostic
effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. _Am J Med_ 69: 491–497 Article CAS Google Scholar * Eley HL, Russell ST, Tisdale MJ
(2007) Attenuation of muscle atrophy in a murine model of cachexia by inhibition of the dsRNA-dependent protein kinase. _Br J Cancer_ 96: 1216–1222 Article CAS Google Scholar * Eley HL,
Tisdale MJ (2007) Skeletal muscle atrophy: a link between depression of protein synthesis and increase in degradation. _J Biol Chem_ 282: 7087–7097 Article CAS Google Scholar * Emery PW,
Edwards RHT, Rennie MJ, Souhami RL, Halliday D (1984) Protein synthesis in muscle measured _in vivo_ in cachectic patients with cancer. _Br Med J_ 289: 584–586 Article CAS Google Scholar
* Jammi NV, Beal PA (2001) Phosphorylation of the RNA-dependent protein kinase regulates its RNA-binding activity. _Nucleic Acids Res_ 29: 3020–3029 Article CAS Google Scholar * Jeffrey
IW, Bushell M, Tilleray VJ, Morley S, Clemens MJ (2002) Inhibition of protein synthesis in apoptosis: differential requirements by the tumor-necrosis factor _α_ family and a DNA-damaging
agent for caspases and the double-stranded RNA-dependent protein kinase. _Cancer Res_ 62: 2272–2280 CAS PubMed Google Scholar * Kaneki M, Kunii K, Chang K, Martyn J (2004) Inhibitory
phosphorylation of translation initiation factors, eIF2_α_ and eIF2B_ɛ_ in skeletal muscle of burned rats. _Anaesthesiology_ 101: A421 Google Scholar * Khal J, Hine AV, Fearon KCH, Dejong
CHC, Tisdale MJ (2005) Increased expression of proteasome subunits in skeletal muscle of cancer patients with weight loss. _Int J Biochem Cell Biol_ 37: 2196–2206 Article CAS Google
Scholar * Kim SH, Forman AP, Matthews MB, Gunnery S (2000) Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. _Oncogene_ 19: 3086–3094 Article CAS
Google Scholar * Kim SH, Gunnery S, Choe JK, Matthews MB (2002) Neoplastic progression in melanoma and colon cancer is associated with increased expression and activity of the
interferon-inducible protein kinase PKR. _Oncogene_ 21: 8741–8748 Article CAS Google Scholar * Kimball SR, Jefferson LS (1990) Mechanism of the inhibition of protein synthesis by
vasopressin in rat liver. _J Biol Chem_ 265: 16794–16798 CAS PubMed Google Scholar * Lorite MJ, Caruik P, Tisdale MJ (1997) Induction of muscle protein degradation by a tumour factor. _Br
J Cancer_ 76: 1035–1040 Article CAS Google Scholar * Lorite MJ, Smith HJ, Arnold JA, Morris A, Thompson MG, Tisdale MJ (2001) Activation of ATP-ubiquitin-dependent proteolysis in
skeletal muscle _in vivo_ and murine myoblasts _in vitro_ by a proteolysis-inducing factor (PIF). _Br J Cancer_ 85: 297–302 Article CAS Google Scholar * Norton JA, Gorschboth CM, Wesley
RA (1985) Fasting plasma amino acid levels in cancer patients. _Cancer_ 56: 1181–1186 Article CAS Google Scholar * O'Keefe SJD, Ogden J, Ramjee G, Rund J (1990) Contribution of
elevated protein turnover and anorexia to cachexia in patients with hepatocellular carcinoma. _Cancer Res_ 50: 1226–1230 CAS PubMed Google Scholar * Onder G, Penninx BWJH, Balkrishnan R,
Fried LP, Chaves PHM, Williamson J, Carter C, DiBari M, Guralnik JM, Pahor M (2002) Relation between use of angiotensin-converting inhibitors and muscle strength and physical function in
older women: an observational study. _Lancet_ 359: 926–930 Article CAS Google Scholar * Price N, Proud C (1994) The guanine nucleotide-exchange factor, eIF-2B. _Biochimie_ 76: 748–760
Article CAS Google Scholar * Rowlands AG, Panniers R, Henshaw EC (1998) The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated
eukaryotic initiation factor 2. _J Biol Chem_ 263: 5526–5533 Google Scholar * Russell ST, Sanders PM, Tisdale MJ (2006a) Angiotensin II directly inhibits protein synthesis in murine
myotubes. _Cancer Lett_ 231: 290–294 Article CAS Google Scholar * Russell ST, Wyke SM, Tisdale MJ (2006b) Mechanism of induction of muscle protein degradation by angiotensin II. _Cell
Sig_ 18: 1087–1096 Article CAS Google Scholar * Sanders PM, Russell ST, Tisdale MJ (2005) Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome
pathway and may play a role in cancer cachexia. _Br J Cancer_ 93: 425–434 Article CAS Google Scholar * Smith HJ, Tisdale MJ (2003) Induction of apoptosis by a cachectic-factor in murine
myotubes and inhibition by eicosapentaenoic acid. _Apoptosis_ 8: 161–169 Article CAS Google Scholar * Van Royen M, Carbo N, Busquets S, Alvarez B, Quin LS, Lopez-Soriano FJ, Argiles JM
(2000) DNA fragmentation occurs in skeletal muscle during tumour growth: a link with cachexia? _Biochem Biophys Res Commun_ 270: 533–537 Article CAS Google Scholar * Wyke SM, Tisdale MJ
(2005) NF-_κ_B mediates proteolysis-inducing factor induced protein degradation and expression of the ubiquitin-proteasome system in skeletal muscle. _Br J Cancer_ 92: 711–721 Article CAS
Google Scholar * Yoshida H, Ishiko O, Sumi T, Honda K, Hirai K, Ogita S (2001) Expression of apoptosis regulatory proteins in the skeletal muscle of tumour-bearing rabbits. _Jpn J Cancer
Res_ 92: 1135–1140 Google Scholar * Zamanian-Daryoush M, Mogensen TH, DiDonato JA, Williams BRG (2000) NF-_κ_B activation by double-stranded-RNA-activated protein kinase (PKR) is mediated
through NF-_κ_B-inducing kinase and I_κ_B kinase. _Mol Cell Biol_ 20: 1278–1290 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work has been supported by a grant
from Novartis Medical Nutrition. RJES is supported by the Maurice Wohl Fellowship in Surgery/Dental Surgery, and a Small Projects Grant from the Royal College of Surgeons in Edinburgh. Mr
Simon Paterson-Brown, Mr Andrew de Beaux and Mr Graeme Couper, Consultant Surgeons, Royal Infirmary of Edinburgh, were essential for the recruitment of patients and the provision of tissue
used in this study. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Nutritional Biomedicine, School of Life and Health Sciences, Aston University, Birmingham, B4 7ET, UK H L Eley & M J
Tisdale * Tissue Injury and Repair Group, Clinical and Surgical Sciences (Surgery), University of Edinburgh, 49 Little France Crescent, Edinburgh, EH16 4SB, UK R J E Skipworth, D A C Deans
& K C H Fearon Authors * H L Eley View author publications You can also search for this author inPubMed Google Scholar * R J E Skipworth View author publications You can also search for
this author inPubMed Google Scholar * D A C Deans View author publications You can also search for this author inPubMed Google Scholar * K C H Fearon View author publications You can also
search for this author inPubMed Google Scholar * M J Tisdale View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to M J
Tisdale. RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported
License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Eley, H., Skipworth, R., Deans,
D. _et al._ Increased expression of phosphorylated forms of RNA-dependent protein kinase and eukaryotic initiation factor 2_α_ may signal skeletal muscle atrophy in weight-losing cancer
patients. _Br J Cancer_ 98, 443–449 (2008). https://doi.org/10.1038/sj.bjc.6604150 Download citation * Received: 04 September 2007 * Revised: 20 November 2007 * Accepted: 20 November 2007 *
Published: 18 December 2007 * Issue Date: 29 January 2008 * DOI: https://doi.org/10.1038/sj.bjc.6604150 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
KEYWORDS * muscle atrophy * cancer patients * PKR * eIF2_α_ * p70S6k