Clinical outcome and prognostic factors for patients treated within the context of a phase i study: the royal marsden hospital experience
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The main aim of phase I trials is to evaluate the tolerability and pharmacology of a new compound. However, investigating the potential for clinical benefit is also a key objective.
Our phase I trial portfolio incorporates a range of new drugs, including molecular targeted agents, sometimes given together with cytotoxic agents. We performed this analysis of response
rate, progression-free (PFS) and overall survival (OS) to assess the extent of clinical benefit rate (CBR: partial response (PR)+stable disease (SD)) derived from current trials. We analysed
212 consecutive patients who were treated in 29 phase I studies, from January 2005 to June 2006. All patients had progression of disease prior to study entry. The median age was 58 years
(range: 18–86) with a male/female ratio of 2 : 1. A total of 148 patients (70%) were treated in ‘first in human trials’ involving biological agents (132 patients) or new cytotoxic compounds
(16 patients) alone, and 64 patients (30%) received chemotherapy-based regimens with or without biological agents. After a median follow-up time of 34 weeks, the median PFS and OS were 11
and 43 weeks, respectively. The CBR was 53% (9% PR and 44% SD) after the first tumour evaluation after two cycles (between weeks 6 and 8) and has been maintained at 36 and 26% at 3 and 6
months, respectively. Treatment related deaths occurred in 0.47% of our patients and treatment had to be withdrawn in 11.8% of patients due to toxicity. A multivariate analysis (MVA) of 13
factors indicated that low albumin (<35 g l−1), lactate dehydrogenase>upper normal limit and >2 sites of metastasis were independent negative prognostic factors for OS. A risk score
based on the MVA revealed that patients with a score of 2–3 had a significantly shorter OS compared to patients with a score of 0–1 (24.9 weeks, 95% CI 19.5–30.2 _vs_ 74.1 weeks, 95% CI
53.2–96.2). This analysis shows that a significant number of patients who develop disease progression while receiving standard therapy derived benefit from participation in phase I trials.
Risk scoring based on objective clinical parameters indicated that patients with a high score had a significantly shorter OS, and this may help in the process of patient selection for phase
I trial entry. SIMILAR CONTENT BEING VIEWED BY OTHERS PROGRESSION-FREE SURVIVAL AS A SURROGATE ENDPOINT IN MYELOMA CLINICAL TRIALS: AN EVOLVING PARADIGM Article Open access 12 August 2024
EFFICACY AND SAFETY PROFILE OF DEEP RESPONDERS TO CARFILZOMIB-BASED THERAPY: A SUBGROUP ANALYSIS FROM ASPIRE AND ENDEAVOR Article Open access 16 October 2020 PAMIPARIB IN COMBINATION WITH
TISLELIZUMAB IN PATIENTS WITH ADVANCED SOLID TUMOURS: RESULTS FROM THE DOSE-EXPANSION STAGE OF A MULTICENTRE, OPEN-LABEL, PHASE I TRIAL Article Open access 20 July 2023 MAIN Patients with
advanced cancers most commonly face the dilemma of having no available standard treatment option. A minority of patients with good performance status (PS) and adequate organ function are
sometimes offered treatment within the context of a phase I trial. Phase I trials are designed primarily to evaluate the tolerability and toxicity profile of new therapies. The generally
accepted inclusion and exclusion criteria for these trials include adequate organ function and reasonable PS in order to ensure safety and avoid unnecessary toxicity. Another important entry
criterion is life expectancy predicted to be more than 3 months, and this is notoriously difficult to predict. More accurate selection criteria or even prognostic scores for patients who
will potentially benefit from a clinical phase I trial may therefore be helpful. So far, there have been few studies exploring factors associated with clinical outcome, toxicity and
prognosis in this context. Multivariate analyses (MVAs) have revealed that factors such as poor PS, high lactate dehydrogenase (LDH), low albumin, and certain chemotherapy regimens could be
negative prognostic factors for survival. However, one of the drawbacks of these studies has been that most analyses have been performed over a long period of time, some of them over 10
years. Moreover, most of these studies focused on the ‘classical cytotoxic drug development era’ and not on the newer generations of molecularly targeted or biological agents (Yamamoto et
al, 1999; Han et al, 2003; Penel et al, 2008). Biological agents target a certain molecular structure or pathway relevant for cancer growth. Broadly speaking, the mechanism of action usually
results in a cytostatic rather than cytotoxic effect, resulting in lower toxicity to normal tissue. We performed this retrospective analysis in all patients who took part in phase I trials
at the Drug Development Unit, Royal Marsden Hospital, over an 18-month period, from January 2005 to June 2006. During this period, the majority of our trials involved biological agents.
Clinical parameters, blood tests (biochemistry and full blood count (FBC)), tumour type, toxicity and type of treatment were included in our univariate and MVAs. The main aim of this study
was to analyse the clinical outcome for our large patient population treated in phase I trials. Secondly, we were interested in the impact of the type of phase I trial on clinical outcome.
Our third aim was to analyse factors that could guide us in the development of an improved patient selection process for phase I trials. PATIENTS AND METHODS PATIENT CHARACTERISTICS We
analysed the outcome of 212 consecutive patients who were treated from January 2005 to June 2006 in 29 phase I trials at the Drug Development Unit, Royal Marsden Hospital, Sutton, UK. All
patients had to have objective evidence of progressive disease prior to trial entry. The median age was 58 years (range: 18–86) with a male/female ratio of 2 : 1 (142 male and 70 female).
Overall, the patients had a median of two cycles of prior systemic therapy (range 0–8). The Eastern Cooperative Oncology Group (ECOG) PS was 0, 1 and 2 in 28, 66 and 6% of patients,
respectively. A total of 33.5% of the patients had urological tumours, 15.6% had breast and gynaecological tumours, 14.2% had lung, mesothelioma and head and neck tumours, 12.3% had sarcoma,
12.3% had gastrointestinal tumours, 6.1% had melanoma, and 6.1% others. Of the patients with urological tumours, 37 of 54 had prostate cancer previously untreated with chemotherapy.
Sixty-four per cent had ⩽2 sites of metastasis and 36% had ⩾2 sites of metastasis (median 2, range: 0–8). The most common sites of metastasis were lung (41%), bone (29%) and liver (27%).
Baseline biochemistry showed decreased albumin levels in 57% of the patients (albumin <35 g l−1) and LDH levels were above the upper normal limit (UNL: >192 IU) in 49%. The FBC showed
haemoglobin levels <12 g dl−1 in 41%, a white cell count (WCC) >10 500 mm−3 in 11% and platelets >400 000 mm−3 in 24% of the patients (Table 1). TRIAL CHARACTERISTICS During the
18-month study period, all 212 consecutive patients were treated within one of 29 phase I trials. A total of 148 patients (70%) were treated in ‘first in human trials’ involving biological
agents (132 patients) or new cytotoxic compounds (16 patients) alone and 64 patients (30%) received chemotherapy-based regimens with or without biological agents. According to the various
protocols, the first tumour evaluation was performed before the third cycle, between weeks 6 and 8. Trial categories are summarised in Table 1. Of our 29 trials, 19 trials have already been
completed and phase II doses were recommended; 17 of the 19 trials reached maximum-tolerated doses (MTDs). Three trials were closed early based on the sponsor's decision. Seven trials
are still ongoing and four of them have reached MTDs. Overall, MTDs were defined for 21 of the 29 trials. STATISTICAL CONSIDERATION The SPSS Programme (Version 12.0, Chicago, IL, USA) was
used for statistical analysis. The Kaplan–Meier method was used to estimate progression-free (PFS) and overall survival (OS) and the log-rank test was used to compare the survival curves
(Kaplan and Meier, 1958). The Cox regression model has been applied for HR estimation and for MVA of prognostic factors for PFS and OS, using a backward selection approach (Cox, 1972).
Fisher's exact test and the _χ_2 test for trend were used to compare proportions of response rate (RR). All _P_-values presented are two-sided. The cutoff date for the present analysis
was the 31 May 2007. OUTCOME: RESPONSE, PFS AND OS Of the 212 patients, 202 (95%) had disease evaluable by the acknowledged standard Response Evaluation Criteria in Solid Tumours (RECIST)
and Prostate Specific Antigen Working Group criteria (PSAWG) were also used to determine progressive disease but not response in this specific setting (Bubley et al, 1999; Therasse et al,
2000). Overall, there has been a radiological proven partial response (PR) in 19 patients (9.4%), stable disease (SD) in 88 patients (44%) and progression of disease in 95 patients (47%) of
all patients, respectively. The clinical benefit rate (CBR: PR+SD) was 53% after the first tumour evaluation after two cycles of treatment (between weeks 6 and 8) and has been maintained at
36 and 26% at 3 and 6 months, respectively. Patients who received a chemotherapy-based regimen had a RR of 19.7% and patients who received a biological agent 3.6% (_P_=0.01). The median
duration of treatment was 6.9 weeks (range: 0–60.1 weeks) in patients who received a biological agent and 10.6 weeks (range: 0.1–64.4 weeks, _P_=0.027) in patients who received a
chemotherapy-based regimen with or without a biological agent. The 30- and 90-day mortality was 1.9% (4 out of 212) and 18.3% (39 out of 212), respectively. Treatment related mortality was
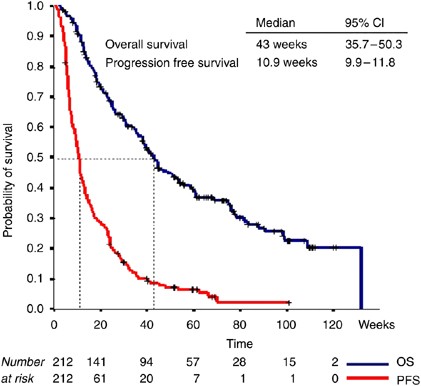
0.47% (1 out of 212) and 11.8% (25 out of 212) of the patients have been withdrawn from an ongoing study due to toxicity (Table 2). After a median follow-up of 34 weeks (range: 2.7–131.9),
the PFS was 11 weeks (95% CI: 9.9–11.8) and the median OS 43 weeks (95% CI: 3.8–50.3), respectively (Figure 1). Univariate analysis revealed that ECOG PS 2, >2 sites of metastasis,
albumin <35 g day−1, LDH>UNL, WCC >10 500 mm−3, haemoglobin <12 g dl−1, platelets >400 000 mm−3, among other factors, were highly significant negative prognosticators for OS.
In the MVA, LDH>UNL (_P_=0.003), albumin <35 g dl−1 (_P_<0.001), and >2 sites of metastasis (_P_=0.01) were significant negative prognosticators for OS (Table 3). Since our
patient population included a number of chemonaive prostate cancer patients and our trial portfolio included docetaxel regimens, we also assessed the outcome according to disease type;
urologic _vs_ non-urologic cancer. Urologic cancers were associated with a significant better outcome compared to non-urologic cancers, _P_=0.001 (Table 3). A risk score based on the results
of the outcome of the MVA (LDH normal=0 _vs_ LDH>UNL=1, albumin >35 g l−1=0 _vs_ <35 g l−1=1, site of metastasis <2=0 _vs_ >2=1) demonstrated that patients with a score <2
had a significantly longer OS (74.1 _vs_ 24.9 weeks, _P_=0.0001). Moreover, the same score remained highly predictive for urological patients, a subgroup with a significant better OS, and
could also distinguish between a good and poor prognosis cohort in this group of patients (Figure 2A, B and C). DISCUSSION The primary objectives of a phase I trial have classically been to
determine the toxicity profile of a new drug therapy and its MTD. However, the clinical outcome measured in RR, PFS and OS is usually descriptive due to the small numbers of patients
enrolled in these studies. Previous retrospective analyses have studied the outcome for patients on phase I trials and have reported RRs between 3.8 and 17.8% with higher RR in patients who
received classical cytotoxic drugs compared to patients who received biological agents (Sekine et al, 2002; Roberts et al, 2004; Horstmann et al, 2005). These studies however reviewed the
outcome over a long period of time, sometimes more than 10 years, which may not reflect the current status of drug development. Moreover, these trials analysed only the published outcome of
clinical trials rather than individual patient data. In our patient group, with a broad spectrum of different cancers, the RR was 9.4% and median PFS and OS were 11 and 43 weeks,
respectively, after a median follow-up of 34 weeks. Patients who received a chemotherapy-based regimen with or without a biological agent had significantly higher RR compared to patients who
received non-cytotoxic agents (19.7 _vs_ 3.6%). These results are in keeping with the aforementioned published analysis comprising more than 460 phase I trials over a 12-year period, which
found an RR of 17.8% for patients who received a chemotherapy-based regimen compared to 4.4% for patients who received non-cytotoxic agents. No data were available on patients achieving SD,
nor was a survival analysis performed in that study (Horstmann et al, 2005). A patient-specific analysis was performed in a single centre retrospective analysis, which enrolled 420 patients
treated within 16 phase I trials over a 10-year period. This study showed OS rates of 38 weeks for patients who received cytotoxic-based regimens compared to 27 weeks for patients who
received non-cytotoxic treatment. These results were similar to our survival analysis, which showed that classical cytotoxic-based regimens resulted in longer OS compared to biological
agents. This trial also confirmed that RR was significantly higher in patients receiving cytotoxic agents compared to patients who received non-cytotoxic agents (14.1 _vs_ 1%, _P_<0.001)
(Han et al, 2003). Our series demonstrated a better outcome for patients who had disease control (CBR: PR+SD), which was reflected in an overall CBR of 36% at 3 months and 26% at 6 months,
respectively. It is notoriously difficult to attribute a better outcome to treatment effect in a non-randomised study analysis where patient selection clearly is a major factor. However, the
achievement of SD lasting more than 3 months in advanced cancer patients with previous disease progression is noteworthy, and in several cases has justified the further development of the
agent under investigation. The patients who died within the first 3 months of treatment reflected a group with an unfavourable prognosis. Our analysis showed that these patients had
significantly higher LDH, WCC and lower albumin and haemoglobin levels compared to the rest of the population. Similar results were demonstrated in a study, which included 70 phase I
patients. All patients who presented with the following two risk factors, albumin <38 g l−1 and lymphocyte count <0.7 × 109 l−1, died within 90 days (Penel et al, 2008). Another study,
which analysed 154 patients over an 8-year period, identified two independent risk factors, namely LDH >600 IU and PS >1, which were correlated with a shorter 90-day OS for patients
with both factors. The authors recommended that patients with these risk factors should not participate in a phase I trial (Bachelot et al, 2000). Interestingly, this study also revealed
that patients ⩾65 years had a significantly better OS than younger patients in keeping with our findings. A possible explanation includes more aggressive tumour biology in younger patients.
In our MVA, parameters such as LDH>UNL, albumin <35 g l−1, sites of metastasis >2 have been associated with a significantly poorer clinical outcome. On the basis of these results, a
prognostic score model was developed (LDH normal (0) _vs_ LDH>UNL (+1), albumin >35 g l−1 (0) _vs_ albumin <35 g l−1 (+1), site of metastasis <2 (0) _vs_ >2 (+1)). Our
prognostic score demonstrated that patient with a good risk score (0 and 1 risk factors) had significantly superior OS compared to patients with a poor risk score (>2 risk factors). This
score has been also proved to be valid in the subgroup of urological cancer patients. The use of this score might be helpful for the future as it is based solely on objective clinical
parameters. It could be a helpful tool in evaluating the eligibility of patients into phase I trials. We are currently performing a prospective analysis in our phase I patients to validate
this scoring system. This analysis demonstrated that treatment within the context of a phase I trial could be considered as a valuable therapeutic option. Interestingly, those trials
incorporating classical cytotoxics were associated with a better outcome. Clearly, this relates to patient selection, particularly when the trial may involve the use of a cytotoxic in
chemonaive cases. The treatment in our cohort was generally well tolerated and treatment-related deaths and toxicities were low. Moreover, a significant number of patients achieved disease
control for a significant duration. However, the challenge remains in appropriate patient selection and for this, the use of an objective clinical score could be a helpful tool. CHANGE
HISTORY * _ 16 NOVEMBER 2011 This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Bachelot T,
Ray-Coquard I, Catimel G, Ardiet C, Guastalla JP, Dumortier A, Chauvin F, Droz JP, Philip T, Clavel M (2000) Multivariable analysis of prognostic factors for toxicity and survival for
patients enrolled in phase-I clinical trials. _Ann Oncol_ 11: 151–156 Article CAS Google Scholar * Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B,
Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Vollmer R, Wilding G (1999)
Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. _J Clin Oncol_ 17:
3461–3467 Article CAS Google Scholar * Cox DR (1972) Regression models and life tables. _J Roy Stat Soc (B)_ 34: 187–202 Google Scholar * Han C, Braybrooke J P, Deplanque G, Taylor M,
Mackintosh D, Kaur K, Samouri K, Ganesan TS, Harris AL, Talbot DC (2003) Comparison of prognostic factors in patients in phase I trials of cytotoxic drugs _vs_ new noncytotoxic agents. _Br J
Cancer_ 89: 1166–1171 Article CAS Google Scholar * Horstmann E, McCabe MS, Grochow L, Yamamoto S, Rubinstein L, Budd T, Shoemaker D, Emanuel EJ, Grady C (2005) Risks and benefits of
phase-1 oncology trials, 1991 through 2002. _N Engl J Med_ 352: 895–904 Article CAS Google Scholar * Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. _J Am
Stat Ass_ 53: 457–481 Article Google Scholar * Penel N, Vanseymortier M, Bonneterre ME, Clisant S, Dansin E, Vendel Y, Beuscart R, Bonneterre J (2008) Prognostic factors among cancer
patients with good performance status screened for phase I trials. _Invest New Drugs_ 98 (1): 53–58 Article Google Scholar * Roberts TG, Goulart BH, Squitieri L, Stallings SC, Halpern EF,
Chabner BA, Gazelle GS, Finkelstein SN, Clark JW (2004) Trends in the risk and benefits to patients with cancer participating in phase-I clinical trials. _JAMA_ 292: 2130–2140 Article CAS
Google Scholar * Sekine I, Yamamoto N, Kunitoh H, Ohe Y, Tamura T, Kodama T, Saijo N (2002) Relationship between objective responses in phase-I trials and potential efficacy of non-specific
cytotoxic investigational new drugs. _Ann Oncol_ 13: 1300–1306 Article CAS Google Scholar * Therasse P, Arbruck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van
Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. _J Natl Cancer Inst_ 92: 205–216 Article CAS Google
Scholar * Yamamoto N, Tamura T, Fukuoka M, Saijo N (1999) Survival and prognostic factors in lung cancer patients treated in phase-I trials: Japanese experience. _Int J Oncol_ 15: 737–741
CAS PubMed Google Scholar Download references AUTHOR INFORMATION Author notes * H-T Arkenau and D Olmos: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Drug
Development Unit, Royal Marsden Hospital and Institute of Cancer Research, Downs Road, Sutton, SM2 5PT, UK H-T Arkenau, D Olmos, J E Ang, J de Bono, I Judson & S Kaye Authors * H-T
Arkenau View author publications You can also search for this author inPubMed Google Scholar * D Olmos View author publications You can also search for this author inPubMed Google Scholar *
J E Ang View author publications You can also search for this author inPubMed Google Scholar * J de Bono View author publications You can also search for this author inPubMed Google Scholar
* I Judson View author publications You can also search for this author inPubMed Google Scholar * S Kaye View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHORS Correspondence to H-T Arkenau or S Kaye. RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons
Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Arkenau, HT., Olmos, D., Ang, J. _et al._ Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden
Hospital experience. _Br J Cancer_ 98, 1029–1033 (2008). https://doi.org/10.1038/sj.bjc.6604218 Download citation * Received: 09 November 2007 * Revised: 19 December 2007 * Accepted: 07
January 2008 * Published: 18 March 2008 * Issue Date: 25 March 2008 * DOI: https://doi.org/10.1038/sj.bjc.6604218 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative KEYWORDS * phase I trial * outcome * survival * prognostic factors