Pre-diagnostic nsaid use but not hormone therapy is associated with improved colorectal cancer survival in women
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT BACKGROUND: Non-steroidal anti-inflammatory drugs (NSAIDs) and hormone therapy (HT) independently decrease the risk of colorectal cancer. However, their role in altering survival
after a colorectal cancer diagnosis is not well established. METHODS: We examined the association between the use of these common medications before diagnosis and colorectal cancer survival
among women in western Washington State diagnosed with incident colorectal cancer from 1997 to 2002. Cases were ascertained using the Surveillance, Epidemiology and End Results cancer
registry; mortality follow-up was completed through linkages to the National Death Index. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence
intervals (CIs). RESULTS: We observed no overall association between colorectal cancer survival and pre-diagnostic NSAID use. However, when stratified by tumour sub-site, NSAID use was
associated with a reduced risk of colorectal cancer mortality for women diagnosed with proximal (HR=0.55; 95% CI: 0.32–0.92), but not distal or rectal (HR=1.32; 95% CI: 0.83–2.10) tumours.
The usage of HT was not associated with colorectal cancer survival overall or by tumour sub-site. CONCLUSION: Usage of NSAIDs before diagnosis may be associated with improved colorectal
cancer survival among women diagnosed with proximal tumours. The usage of HT does not appear to have a function in altering colorectal cancer mortality. SIMILAR CONTENT BEING VIEWED BY
OTHERS HORMONE REPLACEMENT THERAPY AND CANCER MORTALITY IN WOMEN WITH 17 SITE-SPECIFIC CANCERS: A COHORT STUDY USING LINKED MEDICAL RECORDS Article Open access 24 June 2024 ACID-SUPPRESSIVE
MEDICATIONS AND RISK OF COLORECTAL CANCER: RESULTS FROM THREE LARGE PROSPECTIVE COHORT STUDIES Article Open access 16 June 2020 NON-ASPIRIN NON-STEROIDAL ANTI-INFLAMMATORY DRUGS IN
COLORECTAL CANCER: A REVIEW OF CLINICAL STUDIES Article Open access 28 June 2022 MAIN Non-steroidal anti-inflammatory drug (NSAID) use and hormone therapy (HT) use have each been
consistently shown to be associated with a significantly reduced risk of developing colorectal cancer (Giovannucci et al, 1994; Newcomb and Storer, 1995; Rossouw et al, 2002; Gambacciani et
al, 2003; Chlebowski et al, 2004; Chan et al, 2005; Bardia et al, 2007; Newcomb et al, 2007b; Baron, 2009; Cole et al, 2009). However, few studies have investigated the role of these common
medications in subsequent mortality after a diagnosis of colorectal cancer. Only three observational studies in the literature to date have addressed the relationship between NSAID use and
colorectal cancer survival (Fuchs and Heseltine et al, 2005; Chan et al, 2009; Zell et al, 2009). A study of stage III colorectal cancer patients (_n_=846 men and women combined) enrolled in
a randomized chemotherapy trial observed that consistent aspirin use was associated with 52% lower risk of either cancer recurrence or mortality (Fuchs and Heseltine et al, 2005). A report
from the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study, (_n_=840 women; 439 men) found that regular aspirin use after a diagnosis of colorectal cancer was associated
with a 29% reduced risk of colorectal cancer-specific mortality (Chan et al, 2009). Finally, a recent investigation in the California Teachers Study (CTS) cohort (_n_=621 women) observed
that regular NSAID use before diagnosis among women was associated with a 42% reduced rate of colorectal cancer mortality (Zell et al, 2009). Three previous studies have observed inverse
associations between regular HT use and the risk of death from colorectal cancer (Calle et al, 1995; Slattery et al, 1999; Mandelson et al, 2003), although the design of one study precluded
it from distinguishing between the effects of HT on reduced cancer incidence from those of HT on survival after diagnosis (Calle et al, 1995). In contrast, results from the Women's
Health Initiative and a recent large, population-based study of women with large bowel cancer both observed no association between HT use and colorectal cancer survival (Ritenbaugh et al,
2008; Newcomb et al, 2009). We investigated the association between both NSAID use and HT use before cancer diagnosis and subsequent death among female colorectal cases identified from the
population-based Surveillance, Epidemiology and End Results (SEER) registry in 13 counties of western Washington State. MATERIALS AND METHODS STUDY POPULATION Details of case ascertainment
have been published elsewhere (Newcomb et al, 2007a, 2007b). Briefly, eligible case subjects included women, aged between 50 and 74 years, residing in 13 counties in western Washington
State, who were diagnosed between 1997 and 2002 with incident, invasive colorectal cancer. Women aged between 20 and 49 years diagnosed during the same time period in the Puget Sound
counties (3 of the 13) were also eligible for inclusion. Cases were reported to the Cancer Surveillance System, a population-based registry that is part of the National Cancer
Institute's SEER program. Eligibility was limited to English-speaking subjects with available telephone numbers. With physician approval, the study subjects received an introductory
letter in the mail and were followed-up with a telephone call. A total of 1614 eligible women were identified. Of these cases, 100 were deceased, 151 were lost to follow-up before interview
and 181 refused to participate or did not complete the baseline interview, resulting in a final sample size of 1173 cases. Cases were interviewed and enrolled an average of 8.1 months
(s.d.=3.2) after colorectal cancer diagnosis. Analyses were restricted to Caucasian women (_n_=1051) because of sparse data among women of other races. The study was approved by the
Institutional Review Board of the Fred Hutchinson Cancer Research Center in accordance with assurances filed with and approved by the US Department of Health and Human Services. EXPOSURE AND
COVARIATE ASSESSMENT A structured 60-min telephone interview was used to obtain information from all cases on established and potential risk factors for colorectal cancer.
Interviewer-collected information included data on history of NSAID and exogenous hormone use, menstrual and reproductive history, smoking history, height and weight, history of colorectal
cancer screening, first-degree family history of cancer and demographic factors, such as age and race. For all women, only potential exposures that occurred before a reference date,
approximately 2 years before diagnosis, were considered in the analysis. The interview collected information on type and duration of NSAID use (aspirin or ibuprofen) and HT use (oestrogen
only or combined oestrogen and progestin). For NSAIDs, regular use was defined as use at least twice per week for 1 month or greater. Ever use was defined as regular use of any NSAID type at
any point in time before the reference date. Never users reported no use or less than the defined regular use threshold before the reference date. Duration of NSAID use was calculated using
the reported years of regular use of any type of NSAID medication; information on the reported frequency of use, measured in pills per day on average, was also collected. An NSAID-dose
variable used in Cox models was created using both the available duration and frequency information and included the following categories: ⩽1 time per day for ⩽2 years (dose 1); >1 time
per day for ⩽2 years (dose 2); ⩽1 time per day for >2 years (dose 3); and >1 time per day for >2 years (dose 4). For HT, ever use was defined as use of any preparation type for at
least 6 consecutive months at any time before the reference date. Never users reported no use or less than the defined ever use threshold before the reference date. Duration of HT use was
calculated using the reported years of use of any type of HT preparation. OUTCOME Vital status on all enrolled cancer cases was determined through linkages to the National Death Index to
obtain date and cause of death; cause of death was classified using ICD10 coding conventions. The National Death Index identifies known deaths with a high degree of sensitivity, validity and
completeness (Fillenbaum et al, 2009). The primary outcome of interest was death due to colorectal cancer. Time to death was evaluated from SEER-recorded date of colorectal cancer diagnosis
and National Death Index-recorded date of death. Patients alive at the time of their last known vital assessment were censored at that date, with the most recent vital status linkage
occurring in December 2009. Patients dying of causes other than colorectal cancer were censored at their recorded date of death. Sub-site and stage of the colorectal tumour at diagnosis were
defined using SEER records. Advanced disease was defined as colorectal cancer with distant metastasis at diagnosis; non-advanced disease included local and regional stage disease at
diagnosis. Sub-site of disease was categorized using ICD10 codes: proximal disease (C18.0–C18.5); distal disease (C18.6–C18.7); and rectal disease (C19.9–C20.9). STATISTICAL ANALYSIS
Kaplan–Meier survival curves were generated for both NSAID use (ever _vs_ never) and HT use (ever _vs_ never). The proportional hazards assumption was evaluated graphically as well as
statistically through inclusion of interaction terms between exposures and current time in Cox regression models (Ahmed et al, 2007). For both exposures investigated, the proportional
hazards assumption was not statistically violated for colorectal cancer-specific mortality. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95%
confidence intervals (CIs) for the association between pre-diagnostic NSAID use, HT use and colorectal cancer-specific mortality. To increase comparability to previous studies that excluded
metastatic disease (Chan et al, 2009), analyses were restricted to cases diagnosed with local or regional disease (_n_=933). Cox regression models included the following list of covariates
selected _a priori_: age at cancer diagnosis, body mass index at reference date, smoking status, family history of colorectal cancer, history of preventive screening and stage of disease at
diagnosis. Additionally, the regression models investigating each medication were adjusted for pre-diagnostic use of the other medication (i.e., NSAID use adjusted for pre-diagnostic HT
use). Preventive screening was defined as sigmoidoscopy or colonoscopy (endoscopy) screening that was received at least 2 years before the diagnosis of colorectal cancer. Categories of body
mass index were defined as the following in units of kg m−2: not overweight <25.0, overweight 25.0–29.9, obese ⩾30.0 (Clinical Guidelines on the Identification, Evaluation, and Treatment
of Overweight and Obesity in Adults, 1998). The multivariable Cox regression models were evaluated across strata of tumour sub-site (proximal _vs_ distal/rectal). Statistical interaction
between NSAID use, HT use and tumour sub-site was investigated by the inclusion of interaction terms between the respective medication use (yes/no) and tumour sub-site (proximal/distal,
rectal) in regression models. All statistical analyses were performed using SAS software, version 9.1 (SAS Institute Inc., Cary, NC, USA); all _P_-values reported are two sided. RESULTS
After an average of 6.3 years of follow-up after cancer diagnosis and study enrolment, a total of 371 deaths from all causes and 274 deaths from colorectal cancer were ascertained. Among
women with non-advanced colorectal disease, 266 deaths from all causes and 149 deaths specifically due to colorectal cancer were identified. Approximately 50% of the women in the study
population reported ever using NSAIDs before their cancer diagnosis. Among women at least 50 years of age, the proportion of ever HT users was slightly higher than 55% (Table 1). Ever users
of NSAIDs, compared with never users, were more likely to have a family history of colorectal cancer, have a history of preventive screening, and to be obese. Although ever users of HT were
also more likely than never users to have a history of preventive screening, they were less likely to be obese and showed no substantive differences in previous family history of colorectal
cancer compared with never users. Greater proportions of both ever users of NSAIDs and ever users of HT were diagnosed with localised tumours. The HR for colorectal cancer survival after
diagnosis associated with pre-diagnostic NSAID use was 0.88 (95% CI: 0.62–1.24) (Table 2). We observed evidence of heterogeneity in the association of NSAID use before diagnosis with
colorectal cancer survival according to the sub-site of the diagnosed colorectal tumour (_P_-value for interaction=0.03). Pre-diagnostic NSAID use was significantly associated with improved
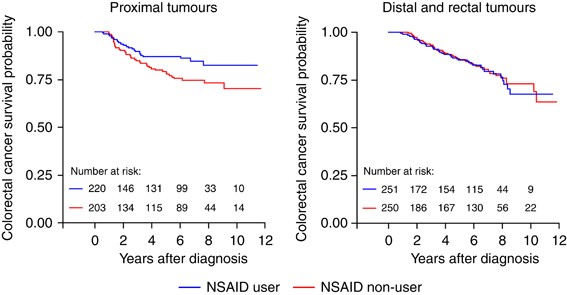
colorectal cancer survival among cases diagnosed with proximal disease (HR=0.55; 95% CI: 0.32–0.92). In contrast, we observed no apparent association with survival among women diagnosed with
left-sided disease (Table 2). This null association was observed among subgroups of left-sided disease, including cases diagnosed with distal tumours (HR=1.40; 95% CI: 0.62–3.19) and cases
diagnosed with rectal tumours (HR=1.25; 95% CI: 0.70–2.22). Colorectal cancer survival curves, based on NSAID use (ever _vs_ never), for both proximal tumours and distal/rectal tumours, are
presented in Figure 1. The statistically significant reduction in the risk of colorectal cancer mortality among proximal cases associated with pre-diagnostic NSAID use was observed to be
dose dependent (_P_-trend=0.01). Although women who reported use on average not more than once per day for 2 years or less (dose 1) were not observed to experience a reduced risk of
colorectal cancer mortality (HR: 1.06; 95% CI: 0.50–2.26), women who used NSAIDs more than once a day for greater than 2 years (dose 4) experienced approximately one quarter the risk of
dying of colorectal cancer (HR: 0.26; 95% CI: 0.07–0.88) compared with never users (data not shown). Estimates for aspirin and ibuprofen use were not observed to be of similar magnitude,
with a much stronger association present among regular ibuprofen users. However, the limited number of cases regularly using ibuprofen precludes any definitive conclusions, and these
type-specific differences should be investigated further in studies adequately power to examine associations within subgroups of NSAID type. The patterns of regular use for aspirin and
ibuprofen also differed substantially. Ibuprofen users appeared to be using at higher doses (pills per day) on a more regular basis than aspirin users (data not shown); the pattern of use
and not type of NSAID may, therefore, potentially account for observed differences. Pre-diagnostic HT use was not associated with the risk of colorectal cancer mortality among cases (HR:
0.95; 95% CI: 0.66–1.35). The association between pre-diagnostic HT use and colorectal cancer survival was consistently null among cases reporting use of oestrogen-only preparations as well
as cases reporting use of oestrogen plus progestin preparations. Additionally, the association between pre-diagnostic HT use and colorectal cancer survival was null among cases diagnosed
with both proximal tumours and those diagnosed with distal or rectal tumours. Longer durations of HT use were also not observed to alter the risk of colorectal cancer mortality after
diagnosis. Finally, we did not observe any significant heterogeneity in the association of HT with colorectal cancer survival according to NSAID use status (_P_-value for interaction=0.29).
DISCUSSION We observed an inverse association between NSAID use and death due to colorectal cancer among Caucasian women diagnosed with proximal disease. Women who regularly used NSAIDs
before diagnosis experienced approximately half the risk of colorectal cancer mortality compared to never users. These women experienced an approximate halving of risk of colorectal cancer
mortality compared with never users. This association was observed to be dose-dependent, with reductions in colorectal cancer mortality of greater magnitude associated with increasing
amounts of NSAID use. We did not, however, observe any evidence of an association between HT use before diagnosis and colorectal cancer survival, regardless of hormone preparation type,
duration of use or tumour characteristics at diagnosis. Previous studies provide evidence that NSAID use may be important for colorectal cancer survival after diagnosis (Fuchs and Heseltine
et al, 2005; Chan et al, 2009; Zell et al, 2009). The report from the California Teachers Study observed a dose-dependent improvement in colorectal cancer survival associated with NSAID use
before diagnosis (Zell et al, 2009). The NHS and Health Professionals Follow-up Study (Chan et al, 2009) reported that any association with improved survival was limited to post-diagnostic
NSAID use. However, cases from the NHS and Health Professionals Follow-up Study cohorts did not experience any significant survival benefit associated specifically with post-diagnostic use
if they also reported aspirin use before their colorectal cancer diagnosis (HR: 0.89; 95% CI: 0.59–1.35). These findings suggest that NSAID use both before and after diagnosis may have an
important role in altering colorectal cancer survival. Previous studies have not reported potential differences in the association between NSAID use and colorectal cancer mortality according
to tumour sub-site. The NHS and Health Professionals Follow-up Study examined potential differences according to whether the tumour was located in the colon _vs_ the rectum and observed no
heterogeneity. Our study is the first to report results according to proximal _vs_ distal or rectal tumour location, making comparison of our results to previous survival studies difficult.
However, previous literature has reported a site-specific effect for NSAIDs on the risk of developing incident colorectal cancer (Rosenberg et al, 1991; Smalley et al, 1999; Mahipal et al,
2006), with a stronger inverse association being observed for proximal disease. Our findings for HT are consistent with results from the Women's Health Initiative, as well as data from
a large population-based study of large bowel cancer, both of which showed no overall association between HT use and colorectal cancer survival (Ritenbaugh et al, 2008; Newcomb et al, 2009).
Previous studies that observed a statistically significant reduction in colorectal cancer mortality associated with HT often failed to account for important covariates, such as colorectal
cancer screening or NSAID use in their analyses (Slattery et al, 1999; Mandelson et al, 2003). We did in fact observe different prevalences of both screening and NSAID use among HT users
compared with never users in our study, highlighting the importance of accounting for these potential confounders in analyses to obtain an unbiased estimate of the effect of HT use before
diagnosis on colorectal cancer survival. There are several plausible mechanisms by which NSAID use before diagnosis could affect colorectal cancer mortality among women. Prostaglandin
synthases COX-1 and COX-2 are directly inhibited by NSAIDs, altering prostaglandin production and cellular inflammatory responses (Sheng et al, 1997; Brown and DuBois, 2005;
Rodriguez-Moranta and Castells, 2005; Wang and Dubois, 2010). This alteration of prostaglandin production results in the promotion of apoptosis, inhibition of angiogenesis and disruption of
processes crucial to tumour growth (Sheng et al, 2001; Chan, 2006; Wang and Dubois, 2006; Cha and DuBois, 2007; Greenhough et al, 2009). The anti-inflammatory effects of NSAID medications
may also impact upon colorectal cancer survival directly as inflammation has been linked to both colorectal cancer incidence and cancer progression (Coussens and Werb, 2002; Erlinger et al,
2004; Kim et al, 2008; Mantovani et al, 2008), and multiple markers of systemic inflammation have been linked specifically to colorectal cancer prognosis (Ishizuka et al, 2007; Shiu et al,
2008; Roxburgh et al, 2009). The use of NSAIDs before diagnosis may not only decrease the likelihood of an inflammatory tumour micro-environment but also may alter the type of tumour that
initially develops. The inhibition of the COX-2 pathway may be of particular importance; multiple studies have observed that COX-2 expression is a negative prognostic factor for patients
with colorectal cancer and results in high colorectal tumour loads in animal studies (Masunaga et al, 2000; Soumaoro et al, 2004; Yamac et al, 2005; Ogino et al, 2008). An earlier report
from the NHS observed that regular pre-diagnostic aspirin use resulted in a lower than expected incidence of tumours expressing high levels of COX-2 (Chan et al, 2007). Use of NSAIDs before
diagnosis may lead to the development of tumours expressing lower levels of COX-_2_, resulting in the diagnosis of tumours that are less aggressive and have a molecular profile that improves
survival. Proximal colorectal tumours have a distinct molecular phenotype compared with distal or rectal tumours (Richman and Adlard, 2002; Azzoni et al, 2007; Benedix et al, 2010) and may,
therefore, represent a different form of colorectal disease. For example, variation in expression of the NSAID-targeted COX-2 enzyme according to the location of colorectal tissue has been
described previously (Dimberg et al, 1999; Wiese et al, 2003; Nasir et al, 2004), and may be a potential explanation for our observed tumour sub-site difference. Additionally, tumours that
feature a high-degree of CpG island methylation are more likely to be found in the proximal colon, particularly among women (Jass, 2007). Chronic inflammation has previously been
hypothesised to accelerate the process of methylation in patients with ulcerative colitis (Issa et al, 2001), providing a possible aetiologic link between inflammatory processes and proximal
colorectal tumours. Although the exact molecular mechanisms behind this ‘proximal effect’ are not presently clear, its implications have growing importance in light of recent reports that
have shown that proximal tumours have inherently poorer prognosis than more distal tumours (Wray et al, 2009; Benedix et al, 2010). This study's strengths included having a large,
population-based sample of women and the collection of detailed exposure information for both NSAIDs and HT, including data on type, duration and frequency of NSAID medication used, as well
as information on HT preparation type and duration of use. Follow-up of all enrolled colorectal cancer cases was complete and standardized. The inclusion of important covariates in our
regression models allowed us to generate measures of association that were not biased by potentially strong confounders such as screening. Additionally, the availability of data on tumour
characteristics at diagnosis allowed us to examine differences in hypothesised associations across tumour sub-site. Limitations of the study include the availability of only pre-diagnostic
information for NSAIDs and HT. It seems likely that pre-diagnostic medication use is correlated with post-diagnostic use, and future studies to examine these exposures independently are
warranted. Information on medication use was collected after diagnosis, introducing the potential for incorrect recall, although this likely affected all cases equally and as such would
result in non-differential misclassification and bias of the observed association to the null. Only limited treatment information was available in this population; however, SEER-reported
first-course treatment type mirrored stage of disease at diagnosis for the majority of cases, and we accounted for extent of disease in our analyses. We did observe that after accounting for
stage of disease at diagnosis, women diagnosed with rectal tumours were slightly more likely to receive treatment (data not shown). Finally, we were unable to enroll all cases immediately
after diagnosis. Failure to enroll cases in a timely manner can result in patient loss that may lead to a lack of representativeness of the study cohort (Coghill et al, 2009). However, we
did not experience a high percentage of patient loss, as we were able to enroll approximately 80% of cases within 10 months of their diagnosis. In addition to colorectal cancer-specific
survival, we attempted to explore the association between the use of these common medications before diagnosis and all-cause mortality. We observed HRs similar in direction to those reported
for colorectal cancer mortality, with no significant associations observed. However, the vast majority of deaths in our cohort were due to colorectal cancer, limiting our ability to
investigate the relationship with mortality from other causes. Despite the inverse association consistently observed between NSAIDs and HT in relation to colorectal cancer incidence in
women, our results suggest that this same relationship did not uniformly extend to colorectal cancer mortality. Pre-diagnostic NSAID use was associated with significantly improved colorectal
cancer survival among women diagnosed with proximal tumours. However, HT was not associated with colorectal cancer survival. Future studies should examine molecular characteristics of
diagnosed tumours, including COX-2 expression and methylation status among regular NSAID users, and the potential interaction of NSAID use history with patient characteristics, such as
genetic polymorphisms. Studies of the use of these common medications in relation to survival after a diagnosis of colorectal cancer should also be conducted among multi-ethnic cohorts of
women. The practical implication of this study is that regular NSAID use earlier in life in persons at risk of colorectal cancer may not only prevent disease but also may prevent more
aggressive disease. This should be considered when weighing the risks and benefits of NSAID use as a chemopreventive therapy. CHANGE HISTORY * _ 29 MARCH 2012 This paper was modified 12
months after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Ahmed FE, Vos PW, Holbert D (2007) Modeling survival in colon cancer: a
methodological review. _Mol Cancer_ 6: 15 Article Google Scholar * Azzoni C, Bottarelli L, Campanini N, Di Cola G, Bader G, Mazzeo A, Salvemini C, Morari S, Di Mauro D, Donadei E,
Roncoroni L, Bordi C, Sarli L (2007) Distinct molecular patterns based on proximal and distal sporadic colorectal cancer: arguments for different mechanisms in the tumorigenesis. _Int J
Colorectal Dis_ 22: 115–126 Article Google Scholar * Bardia A, Ebbert JO, Vierkant RA, Limburg PJ, Anderson K, Wang AH, Olson JE, Vachon CM, Cerhan JR (2007) Association of aspirin and
nonaspirin nonsteroidal anti-inflammatory drugs with cancer incidence and mortality. _J Natl Cancer Inst_ 99: 881–889 Article CAS Google Scholar * Baron JA (2009) Aspirin and NSAIDs for
the prevention of colorectal cancer. _Recent Cancer Res_ 181: 223–229 Article CAS Google Scholar * Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H (2010) Comparison of 17
641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. _Dis Colon Rectum_ 53: 57–64 Article Google Scholar *
Brown JR, DuBois RN (2005) COX-2: a molecular target for colorectal cancer prevention. _J Clin Oncol_ 23: 2840–2855 Article CAS Google Scholar * Calle EE, Miracle-McMahill HL, Thun MJ,
Heath Jr CW (1995) Estrogen replacement therapy and risk of fatal colon cancer in a prospective cohort of postmenopausal women. _J Natl Cancer Inst_ 87: 517–523 Article CAS Google Scholar
* Cha YI, DuBois RN (2007) NSAIDs and cancer prevention: targets downstream of COX-2. _Annu Rev Med_ 58: 239–252 Article CAS Google Scholar * Chan AT, Giovannucci EL, Meyerhardt JA,
Schernhammer ES, Curhan GC, Fuchs CS (2005) Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. _JAMA_ 294: 914–923 Article CAS Google Scholar
* Chan AT, Ogino S, Fuchs CS (2007) Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. _N Engl J Med_ 356: 2131–2142 Article CAS Google Scholar * Chan AT,
Ogino S, Fuchs CS (2009) Aspirin use and survival after diagnosis of colorectal cancer. _Jama_ 302: 649–658 Article CAS Google Scholar * Chan TA (2006) Prostaglandins and the colon
cancer connection. _Trends Mol Med_ 12: 240–244 Article CAS Google Scholar * Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, Rosenberg CA, Taylor VM,
Harris R, Chen C, Adams-Campbell LL, White E (2004) Estrogen plus progestin and colorectal cancer in postmenopausal women. _N Engl J Med_ 350: 991–1004 Article CAS Google Scholar *
Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults (1998) _The Evidence Report_. National Institutes of Health, National Heart, Lung,
and Blood Institute: Bethesda, MD * Coghill AE, Newcomb PA, Potter JD (2009) Aspirin use, colorectal cancer survival, and loss to follow-up. _Jama_ 302: 2549; author reply 2549–2550 Article
CAS Google Scholar * Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, Chaussade S, Baron JA (2009) Aspirin for the chemoprevention of colorectal adenomas: meta-analysis
of the randomized trials. _J Natl Cancer Inst_ 101: 256–266 Article CAS Google Scholar * Coussens LM, Werb Z (2002) Inflammation and cancer. _Nature_ 420: 860–867 Article CAS Google
Scholar * Dimberg J, Samuelsson A, Hugander A, Soderkvist P (1999) Differential expression of cyclooxygenase 2 in human colorectal cancer. _Gut_ 45: 730–732 Article CAS Google Scholar *
Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ (2004) C-reactive protein and the risk of incident colorectal cancer. _JAMA_ 291: 585–590 Article CAS Google Scholar * Fillenbaum GG,
Burchett BM, Blazer DG (2009) Identifying a national death index match. _Am J Epidemiol_ 170: 515–518 Article Google Scholar * Fuchs C, Heseltine DL _et al_ (2005) Influence of regular
aspirin use on survival for patients with stage III colon cancer: findings from intergroup trial CALGB 89803. _J Clin Oncol_ 23: 3530 Article Google Scholar * Gambacciani M, Monteleone P,
Sacco A, Genazzani AR (2003) Hormone replacement therapy and endometrial, ovarian and colorectal cancer. _Best Pract Res Clin Endocrinol Metab_ 17: 139–147 Article CAS Google Scholar *
Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC (1994) Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. _Ann Intern Med_ 121:
241–246 Article CAS Google Scholar * Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A (2009) The COX-2/PGE2 pathway: key roles in the hallmarks of cancer
and adaptation to the tumour microenvironment. _Carcinogenesis_ 30: 377–386 Article CAS Google Scholar * Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K (2007) Inflammation-based
prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. _Ann Surg_ 246: 1047–1051 Article Google Scholar * Issa JP, Ahuja N, Toyota M, Bronner
MP, Brentnall TA (2001) Accelerated age-related CpG island methylation in ulcerative colitis. _Cancer Res_ 61: 3573–3577 CAS PubMed Google Scholar * Jass JR (2007) Classification of
colorectal cancer based on correlation of clinical, morphological and molecular features. _Histopathology_ 50: 113–130 Article CAS Google Scholar * Kim S, Keku TO, Martin C, Galanko J,
Woosley JT, Schroeder JC, Satia JA, Halabi S, Sandler RS (2008) Circulating levels of inflammatory cytokines and risk of colorectal adenomas. _Cancer Res_ 68: 323–328 Article CAS Google
Scholar * Mahipal A, Anderson KE, Limburg PJ, Folsom AR (2006) Nonsteroidal anti-inflammatory drugs and subsite-specific colorectal cancer incidence in the Iowa women's health study.
_Cancer Epidemiol Biomarkers Prev_ 15: 1785–1790 Article CAS Google Scholar * Mandelson MT, Miglioretti D, Newcomb PA, Harrison R, Potter JD (2003) Hormone replacement therapy in relation
to survival in women diagnosed with colon cancer. _Cancer Causes Control_ 14: 979–984 Article Google Scholar * Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related
inflammation. _Nature_ 454: 436–444 Article CAS Google Scholar * Masunaga R, Kohno H, Dhar DK, Ohno S, Shibakita M, Kinugasa S, Yoshimura H, Tachibana M, Kubota H, Nagasue N (2000)
Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. _Clin Cancer Res_ 6: 4064–4068 CAS PubMed Google Scholar * Nasir
A, Kaiser HE, Boulware D, Hakam A, Zhao H, Yeatman T, Barthel J, Coppola D (2004) Cyclooxygenase-2 expression in right- and left-sided colon cancer: a rationale for optimization of
cyclooxygenase-2 inhibitor therapy. _Clin Colorectal Cancer_ 3: 243–247 Article CAS Google Scholar * Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, Hall D, Hopper JL,
Jass J, Le Marchand L, Limburg P, Lindor N, Potter JD, Templeton AS, Thibodeau S, Seminara D (2007a) Colon Cancer Family Registry: an international resource for studies of the genetic
epidemiology of colon cancer. _Cancer Epidemiol Biomarkers Prev_ 16: 2331–2343 Article Google Scholar * Newcomb PA, Chia VM, Hampton JM, Doria-Rose VP, Trentham Dietz A (2009) in relation
to survival from large bowel cancer. _Cancer Causes Control_ 20: 409–416 Article Google Scholar * Newcomb PA, Storer BE (1995) Postmenopausal hormone use and risk of large-bowel cancer. _J
Natl Cancer Inst_ 87: 1067–1071 Article CAS Google Scholar * Newcomb PA, Zheng Y, Chia VM, Morimoto LM, Doria-Rose VP, Templeton A, Thibodeau SN, Potter JD (2007b) Estrogen plus
progestin use, microsatellite instability, and the risk of colorectal cancer in women. _Cancer Res_ 67: 7534–7539 Article CAS Google Scholar * Ogino S, Kirkner GJ, Nosho K, Irahara N,
Kure S, Shima K, Hazra A, Chan AT, Dehari R, Giovannucci EL, Fuchs CS (2008) Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. _Clin Cancer Res_ 14:
8221–8227 Article CAS Google Scholar * Richman S, Adlard J (2002) Left and right sided large bowel cancer. _BMJ_ 324: 931–932 Article Google Scholar * Ritenbaugh C, Stanford JL, Wu L,
Shikany JM, Schoen RE, Stefanick ML, Taylor V, Garland C, Frank G, Lane D, Mason E, McNeeley SG, Ascensao J, Chlebowski RT (2008) Conjugated equine estrogens and colorectal cancer incidence
and survival: the Women's Health Initiative randomized clinical trial. _Cancer Epidemiol Biomarkers Prev_ 17: 2609–2618 Article CAS Google Scholar * Rodriguez-Moranta F, Castells A
(2005) Mechanisms of colon cancer prevention with and beyond COX-2 inhibition. _Curr Top Med Chem_ 5: 505–516 Article CAS Google Scholar * Rosenberg L, Palmer JR, Zauber AG, Warshauer ME,
Stolley PD, Shapiro S (1991) A hypothesis: nonsteroidal anti-inflammatory drugs reduce the incidence of large-bowel cancer. _J Natl Cancer Inst_ 83: 355–358 Article CAS Google Scholar *
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J (2002) Risks and benefits of estrogen plus
progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. _JAMA_ 288: 321–333 Article CAS Google Scholar *
Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC (2009) The relationship between the local and systemic inflammatory responses and survival in patients undergoing curative surgery
for colon and rectal cancers. _J Gastrointest Surg_ 13: 2011–2018; discussion 2018–2019 Article Google Scholar * Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD,
DuBois RN (1997) Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. _J Clin Invest_ 99: 2254–2259 Article CAS Google Scholar * Sheng H, Shao J,
Washington MK, DuBois RN (2001) Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. _J Biol Chem_ 276: 18075–18081 Article CAS Google Scholar * Shiu YC, Lin JK,
Huang CJ, Jiang JK, Wang LW, Huang HC, Yang SH (2008) Is C-reactive protein a prognostic factor of colorectal cancer? _Dis Colon Rectum_ 51: 443–449 Article Google Scholar * Slattery ML,
Anderson K, Samowitz W, Edwards SL, Curtin K, Caan B, Potter JD (1999) Hormone replacement therapy and improved survival among postmenopausal women diagnosed with colon cancer (USA). _Cancer
Causes Control_ 10: 467–473 Article CAS Google Scholar * Smalley W, Ray WA, Daugherty J, Griffin MR (1999) Use of nonsteroidal anti-inflammatory drugs and incidence of colorectal cancer:
a population-based study. _Arch Intern Med_ 159: 161–166 Article CAS Google Scholar * Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K (2004) Cyclooxygenase-2
expression: a significant prognostic indicator for patients with colorectal cancer. _Clin Cancer Res_ 10: 8465–8471 Article CAS Google Scholar * Wang D, Dubois RN (2006) Prostaglandins
and cancer. _Gut_ 55: 115–122 Article CAS Google Scholar * Wang D, Dubois RN (2010) The role of COX-2 in intestinal inflammation and colorectal cancer. _Oncogene_ 29: 781–788 Article CAS
Google Scholar * Wiese FW, Thompson PA, Warneke J, Einspahr J, Alberts DS, Kadlubar FF (2003) Variation in cyclooxygenase expression levels within the colorectum. _Mol Carcinog_ 37: 25–31
Article CAS Google Scholar * Wray CM, Ziogas A, Hinojosa MW, Le H, Stamos MJ, Zell JA (2009) Tumor subsite location within the colon is prognostic for survival after colon cancer
diagnosis. _Dis Colon Rectum_ 52: 1359–1366 Article Google Scholar * Yamac D, Celenkoglu G, Coskun U, Akyurek N, Akcali Z, Dursun A, Koybasioglu F (2005) Prognostic importance of COX-2
expression in patients with colorectal cancer. _Pathol Res Pract_ 201: 497–502 Article CAS Google Scholar * Zell JA, Ziogas A, Bernstein L, Clarke CA, Deapen D, Largent JA, Neuhausen SL,
Stram DO, Ursin G, Anton-Culver H (2009) Nonsteroidal anti-inflammatory drugs: effects on mortality after colorectal cancer diagnosis. _Cancer_ 115: 5662–5671 Article Google Scholar
Download references ACKNOWLEDGEMENTS This work was supported by the National Cancer Institute at the National Institutes of Health (Grants R01 CA076366 and U24 CA074794). AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Fred Hutchinson Cancer Research Center, Cancer Prevention Program, 1100 Fairview Ave N, M4-B402, Seattle, WA 98109, USA, A E Coghill, P A Newcomb, V M Chia, Y
Zheng, K J Wernli, M N Passarelli & J D Potter Authors * A E Coghill View author publications You can also search for this author inPubMed Google Scholar * P A Newcomb View author
publications You can also search for this author inPubMed Google Scholar * V M Chia View author publications You can also search for this author inPubMed Google Scholar * Y Zheng View author
publications You can also search for this author inPubMed Google Scholar * K J Wernli View author publications You can also search for this author inPubMed Google Scholar * M N Passarelli
View author publications You can also search for this author inPubMed Google Scholar * J D Potter View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to P A Newcomb. RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons
Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Coghill, A., Newcomb, P., Chia, V. _et al._ Pre-diagnostic NSAID use but not hormone therapy is associated with improved colorectal cancer survival in women. _Br J
Cancer_ 104, 763–768 (2011). https://doi.org/10.1038/sj.bjc.6606041 Download citation * Received: 05 July 2010 * Revised: 02 November 2010 * Accepted: 11 November 2010 * Published: 08
February 2011 * Issue Date: 01 March 2011 * DOI: https://doi.org/10.1038/sj.bjc.6606041 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS *
colorectal cancer survival * COX-2 * epidemiology * proximal tumours * NSAIDs * hormone therapy