Transparency, swelling and scarring in the corneal stroma
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT _Purpose_ This paper briefly reviews current explanations for corneal transparency and uses a well-developed model to try to explain the increased light scattering either
accompanying corneal swelling or following phototherapeutic keratectomy (PTK). _Methods_ The direct summation of fields (DSF) method was used to compute light transmission as a function of
wavelength. The method requires input of a number of structural parameters. Some of these were obtained from electron micrographs and others were calculated from X-ray diffraction data.
_Results_ By swelling sections of stroma cut from different depths in the tissue, we have shown that fluid entering the cornea causes more swelling in the posterior lamellae than in the
anterior lamellae. Furthermore, posterior lamellae can reach a higher final hydration than anterior lamellae. Collagen-free regions (‘lakes’) exist in corneas swollen _in vitro_and in
Fuch's dystrophy corneas, many of which may be caused by the death of cells. The DSF method shows that local fibril disordering, increased refractive index mismatch, and increased
corneal thickness together can account for a 20% increase in light scattering in a Fuch's dystrophy cornea at _H_=5.8 compared to the normal cornea. Additional scattering is probably
caused by ‘lakes’. The DSF method applied to PTK rabbit stroma with high levels of haze suggests that the newly deposited collagen is not the cause of the increased light scattering.
_Conclusions_ Fluid is not uniformly distributed within the corneal stroma when the cornea swells. Increased hydration of posterior lamellae may be because of known differences in the
glycosaminoglycans between the anterior and posterior stroma. Lamellar interweave in the anterior stroma probably limits the extent to which the constituent lamellae can swell. The DSF
method can be used to account for increased light scattering in oedematous corneas but cannot account for haze following PTK. INTRODUCTION One of the most remarkable properties of the cornea
is its ability to transmit almost all the incident light in the visible part of the spectrum. The reasons for corneal transparency have occupied scientists for many decades and despite
considerable advances in our understanding, to date there is still no universally accepted explanation. Even more perplexing are the causes of increased light scattering in the cornea during
wound healing or in some pathological situations. In this paper, we briefly review some of the theories put forward to explain corneal transparency and use the most well tested of these to
try to model the light scattering expected from oedematous corneas and from corneas following phototherapeutic keratectomy (PTK). CORNEAL TRANSPARENCY Any system where the attenuation of
light is only caused by scattering (in other words there are no other losses such as might be due, for example, to absorption) can be described by where _F_t is the percentage of the
incident light transmitted without scattering, _α_s is the scattering attenuation coefficient, and _t_ is the thickness in the direction of the light path. In the case of a corneal lamella
consisting of parallel collagen fibrils, the scattering attenuation coefficient can be written as the product _ρσ_, where _ρ_ is the number of fibrils per unit area in a cross-section (often
called the bulk fibril number density or simply the number density) and _σ_ is the scattering cross-section. Over the years there have been many models put forward to explain transparency;
the difference between these models essentially depends on the mathematical formulation of the scattering cross-section term. Here we describe the most important of these models. All must
consider the structure of the cornea, that is, the size and shape of the stromal constituents and their refractive indices since each of these factors influences the amount of light
scattered by the structure. In particular, the refractive index of the collagen fibrils, the refractive index of the extrafibrillar material, and the ratio of these two refractive indices,
all play a major role in determining the extent of light scattered by the stroma. The simplest model1 proposes that all corneal components have a _uniform_ refractive index (which is
equivalent to a zero value for the scattering cross-section). This essentially means that light cannot distinguish between fibrils and the material between them, hence it can propagate
directly through the tissue unscattered. This model is generally rejected, partly because it fails to explain two important properties of the cornea, birefringence and transparency loss when
the structure is distorted. Also, recent X-ray diffraction data have unambiguously confirmed earlier evidence for a difference in the refractive indices of the collagen fibrils and of the
extrafibrillar material.2 Most modern models are based on the lattice theory put forward by Maurice.3 By approximating the collagen fibril to perfect, infinitely long cylinders, an estimate
of the scattering from an individual fibril can be calculated. The refractive index difference between the fibrils and interfibrillar matrix means that each fibril scatters a small amount of
light. However, if the fibrils are packed in a lattice arrangement, correlation in their relative positions leads to destructive interference of light scattered away from the forward
direction, all the light energy going into the constructive interference in the forward direction. However, both electron microscopy and X-ray diffraction do not show the presence of this
regular packing of collagen fibrils.4,5 Table 1 shows these two models alongside the other main models, which are all based on Maurice's early work. Hart and Farrell4 showed that only
short-range order in the positions of the collagen fibrils is necessary for the required destructive interference of scattered photons. Results from X-ray diffraction showed that the type of
short-range order in the packing seen in electron micrographs is indeed what is found in the tissue.5 Feuk6 developed a long-range order model based on small, random displacements of the
fibrils from ideal lattice sites. Twersky,7 assuming that the fibrils were arranged as in a two-dimensional fluid, expressed the distribution explicitly in terms of the volume fraction
occupied by the fibrils. Benedek8 considered the problem from the point of view of fluctuations in the fibril number density. These concepts were explored quantitatively by Vaezy and Clark,9
who examined fluctuations in the spatial arrangement of the collagen fibrils using Fourier methods. Recently, Ameen _et al_10 used photonic band structure methods to explain light
transmission through corneal lattices. Space is too limited to go into these models in greater detail, so, for a fuller account, the reader is directed to reviews by Farrell and McCally11
and Freegard.12 A more generalised mathematical review of transparency in biological tissues is by Tuchin.13 By way of a summary, it has been pointed out by Farrell and McCally11 that all
currently viable transparency theories agree with three points: * 1 each fibril is an ineffective scatterer; * 2 despite this, the large number of fibrils requires that destructive
interference of scattered light must occur; and * 3 the cornea is thin. DIRECT SUMMATION OF FIELDS METHOD In 1986, Freund _et al_14 published a method to compute light scattering from the
cornea, following on from the theoretical principles previously advanced by Hart and Farrell.4 The technique can be used to predict transmission by an arbitrary short-range order
distribution of different-sized fibrils. A full account of the approach, called the direct summation of fields (DSF) method, is found in the original papers.14,15 It is a statistical
technique in which the scattering from each individual fibril is computed, then the effects of interference are included and summed for the whole tissue using a method called ensemble
averaging. It is worth mentioning at this stage that, in this method, transmission is computed as a function of wavelength, ignoring the lamellar structure of the stroma and also the
presence of stromal cells. With these assumptions in mind, however, the DSF method has been tested and found to give reliable results in a number of situations.16,17,18 In order to use DSF
to compute the expected light transmission, it is necessary to measure a number of structural and physical properties of the stroma. The refractive index of the hydrated collagen fibrils,
the refractive index of the interfibrillar matrix, and the ratio of these have previously been obtained using X-ray diffraction measurements in a number of different species.2 The relative
positions of the individual fibrils, and the diameter of each fibril, are obtained from electron micrographs. There is a problem in using measurements from electron microscopy in that it has
been shown that a number of microscope preparation protocols result in considerable shrinkage of fibril diameters and, particularly, interfibril spacings.19 To overcome this problem, we
have used X-ray diffraction (a technique where corneas can be examined without the need for any processing20) to measure the mean values of these parameters in the same tissue as used for
microscopy, and then scaled the data from electron micrographs so as to compensate for this shrinkage. Table 2 gives the values for several of these parameters for human corneas. The corneas
were obtained from the Eye Bank in culture medium, and were dehydrated to close to physiological hydration using polyethylene glycol.21 It should be noted that the value for the fibril
number density is lower than previously reported, partly owing to the scaling procedure. Fibril diameters and spacings were measured by image analysis of selected electron micrographs where
collagen fibrils were sectioned in cross-section. The positions and diameters of collagen fibrils in a human cornea were obtained after image analysis of these micrographs and scaled using
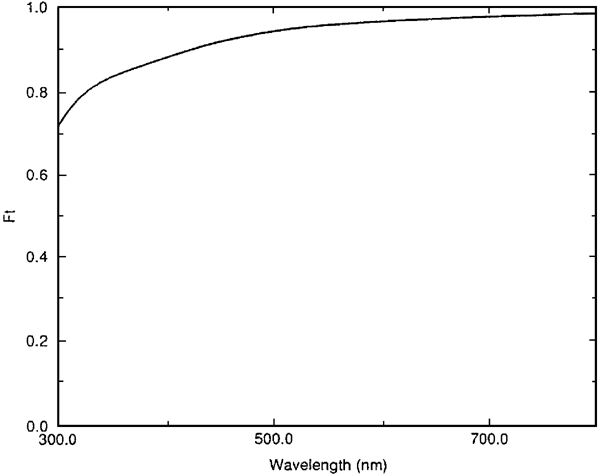
X-ray data from the same samples as described above. By taking the corneal thickness as 0.52 mm and combining these data with the refractive index data in Table 2, the DSF method was used to
predict the transmission as a function of wavelength. As expected (Figure 1), transmission was predicted to exceed 90% throughout most of the visible spectrum. In their original paper4 and
later16,15,11 Farrell and co-workers made only limited reference to how changes in the individual parameters might affect transparency in abnormal conditions. It is of interest to examine
the theoretical effects of changing each of these parameters, keeping the rest constant, so as to gauge to which parameters light transmission is most sensitive. It should be remembered that
here we are testing a single model for transparency, which has certain implicit assumptions about the tissue. Note also that this is a theoretical situation; in practice, many of the
parameters are related, and a change in one is often accompanied by a change in one or more of the others. With these caveats, Figure 2a shows that increasing the fibril radius to 20 nm
reduces transmission, particularly in the blue end of the spectrum, whereas reducing the radius has the opposite effect. So why not have small collagen fibrils in humans, as is found in
fish? The answer is probably down to tissue mechanics — larger fibrils mean a stronger cornea. The independent effect of the fibril number density on light scattering is difficult to assess,
as altering the fibril number density by increasing the separation of the fibrils simultaneously changes the effects of interference. However, we find that keeping the relative positions of
the fibrils constant but moving the fibrils apart (Figure 2b) leads to less scattering (greater transmission). An important point to realise, however, is that, contrary to what is often
asserted, increased interfibril spacings (for example, when the cornea swells) are not _per se_ responsible for the increased light scattering that accompanies oedema. From Equation (1) it
is clear that light scattering will increase with increased corneal thickness (assuming the increased thickness is because of extra tissue mass rather than oedema). However, Figure 2c shows
that this effect is relatively small, the corneal thickness could almost double without seriously increasing scattering. This presumably accounts for why, for example, a bovine cornea has
similar transparency to a thinner human cornea. Light transmission through the cornea is very sensitive to an increased mismatch in the refractive indices of the collagen and the
extrafibrillar matrix. Theoretically, there are two ways of varying their ratio, either keep the refractive index of the fibrils constant and vary that of the matrix, or _vice versa_. Both
have a similar effect (Figure 2d). If the ratio is one, there is total transmission throughout the spectrum. This is the uniform refractive index condition. As we increase the ratio,
transmission reduces, once again, particularly at the blue end of the spectrum. In conclusion, the DSF method can be used to demonstrate that light scattering in the cornea will increase if:
* 1 order in the spatial arrangement of the fibrils is destroyed; * 2 fibril diameters increase; * 3 fibril number density increases; * 4 there is an increased refractive index imbalance
between the hydrated fibrils and the extrafibrillar matrix; * 5 corneal thickness increases. So far, we have imagined the cornea as a structure made only of collagen fibrils and
extrafibrillar matrix. Of course, there are a large number of keratocytes in the stroma, which gradually reduce in density from the anterior to the posterior stroma.22,23 Maurice3 believed
that there were insufficient of these to contribute significantly to scattering. Besides, they are relatively thin in the direction of the light path through the cornea. More recently,
Jester _et al_24 have suggested that these cells contain special proteins called corneal crystallins, which produce a uniform refractive index in the cells and may match the refractive index
of the cytoplasm to that of the surrounding matrix. This, together with the dimensions of the cells, renders them weak scatterers (except for their nuclei, which are readily visible in the
confocal microscope). However, if keratocytes change their shape or spill their contents, a different situation ensues, and they are capable of becoming very efficient scatterers.25,26
CORNEA OEDEMA An understanding of structure and transparency changes when the cornea swells is dependent on our knowledge of where imbibed water is situated, both at the level of the tissue
as a whole and within the lamellae themselves. In many animals, the anterior stroma is less ordered,15 less hydrated,27,28,29 has a higher keratocyte density,22,23 has a lower keratan
sulphate (KS) to chondroitin/dermatan sulphate (DS) ratio,28 and is less easily swollen29,30,31 than the posterior stroma. We have examined four frozen human corneas (two at physiological
hydration and two swollen in culture medium). These were sectioned at 100 _μ_m intervals from the anterior to posterior using a Mikrom sliding microtome. All sections were weighed and then
placed in dH2O. At fixed intervals, each section was reweighed and then returned to the dH2O to continue swelling until a constant weight was reached. The hydration of each section was
calculated for both the physiological and the swollen corneas. The results (Figure 3) confirm results from other species27,28,29 and show that hydration increases with tissue depth in both
the physiological and the swollen human corneas. This may be related to the gradual increase in the KS/DS ratio with depth, since KS is known to show greater water absorption than DS.32,33
However, as Bron34 has pointed out it is possible that there is a differential loss of proteoglycans between anterior and posterior stroma as the cornea swells and this, if it happens, would
affect the swelling at different stromal depths. The corneal sections were immersed in distilled water until they essentially stopped swelling. The final hydrations achieved were plotted as
a function of tissue depth (Figure 4). We found that the maximum achievable hydration increases as a function of depth. This means that the posterior stroma is capable of swelling much more
than the anterior. It is likely that anterior swelling is limited by lamellar interweaving and insertions into Bowman's layer, a phenomenon that may have considerable importance in
maintaining the correct shape of the cornea.31,34 Information on the distribution of imbibed water _within_ the lamellae has been obtained using X-ray diffraction methods. When the denuded
cornea swells, there is a linear relation between the fibril separation _squared_ and the hydration.35 Interfibrillar centre-to-centre spacings were determined for bovine corneas as a
function of hydration using X-ray diffraction, and the results are shown in Figure 5 (line a). By extrapolating this line to _H_=0, it is possible to plot a theoretical graph of the expected
interfibril spacing on the assumption that all the water entering the stroma has gone towards separating the constituent fibrils.5,21 This theoretical plot is shown in Figure 5 (line b).
The shading around the theoretical plot is the uncertainty owing to the uncertainty in determining the spacing at _H_=0 from the experimental data. The interesting point is that at a given
hydration, the interfibril spacing is lower than it should be considering the amount of water in the stroma. This must mean that some of the water is not between fibrils, and thus must be in
fibril-free regions. Some of these regions are probably places occupied by cells that have died post mortem. If we assume that keratocytes occupy 15% of the stromal volume, we can take this
into account in the theoretical calculation, and the match between theory and experiment becomes much better (Figure 6). So it appears that fibril-free regions (‘lakes’) form in swollen
corneas, and that when cells die, the spaces previously occupied by them might themselves become ‘lakes’ that could contribute to an increase in light scattering. LIGHT SCATTERING IN
OEDEMATOUS CORNEAS A full survey of the literature probing the causes of scattering when the cornea swells is beyond the scope of this article. From a theoretical standpoint, ‘lakes’ would
add a term to the total scattering cross-section that would vary as _B_/_λ_2.37,38 By measuring transmission as corneas swelled, Farrell _et al_ were able to compute the scattering
cross-section and demonstrated that it has a 1/_λ_2 dependence, as predicted by the presence of ‘lakes’.35 Lakes are not seen in the normal human cornea, but they are present in bullous
keratopathy and Fuch's dystrophy corneas.39 Some fibril-free regions appear to be because of matrix disorder (Figure 7), while others might reflect the presence of dead cells. The
question is, to what extent does the intralamellar disordering lead to light scattering? In principle, the summation of scattered fields approach could again be used to compute the
theoretical effects of disordering and/or lakes. The required structural information (fibril positions, diameters, number density) can be obtained from electron micrographs scaled according
to the X-ray diffraction measurements as described previously. However, as the cornea swells, the extra water will change the refractive indices of the interfibrillar matrix and also,
possibly, of the collagen fibrils themselves. However, we know from previous studies21 that collagen fibrils do not swell appreciably above physiological hydration, so their refractive index
is independent of tissue hydration and stays constant at 1.416. As water or electrolyte enters the interfibrillar matrix, it dilutes it and the refractive index falls. The amount by which
it falls can be estimated by measuring the change in the refractive index of the stroma as a function of tissue thickness or hydration (Figure 8) and then applying Gladstone and Dales's
law of refractive indices to the system.2 Since the imbibed fluid does not enter the fibrils themselves,21 this fall in the refractive index as the stroma swells leads to an increase in the
ratio of the refractive index of the fibrils to that of the interfibrillar matrix and to a corresponding increase in light scattering. We found that between physiological hydration and
_H_=3.8, there was a 0.15% reduction in the refractive index of the matrix and a corresponding 0.1% increase in the ratio of the refractive indices of the fibrils and the matrix (S Khan, S
Dennis, and K Meek, unpublished results). Between physiological hydration and _H_=5.8, these percentages were 0.59% and 0.58%, respectively. Armed with this quantitative information, it is
now possible to apply the summation of scattered fields method to Fuch's dystrophy corneas. The result is shown in Figure 9. It can be appreciated that the intralamellar disruption in
the spatial arrangement of fibrils, the increased mismatch in the refractive indices, and the increased thickness of the stroma together lead to the overall reduction in light transmission.
PHOTOTHERAPEUTIC KERATECTOMY It is well known that haze develops following laser ablation to the anterior stroma. The definition of haze is difficult because of the different methods used to
measure it, and even less certain is the origin of the haze.40 Various authors have ascribed the observed haze to irregularities in the epithelium,41,42 to subepithelial deposits,43 to the
presence of vacuoles,44 to the deposition of poorly organised collagen,45,46 or to the presence of activated keratocytes.25,26 However, none of these suggestions have been experimentally or
theoretically shown to account for increased light scattering. We have used PTK in rabbits to predict the percentage transmission of visible light through the newly deposited collagen using
the DSF method and hence to see if this collagen could account for the observed haze. All experimental procedures were carried out in accordance with the ARVO Resolution on the Use of
animals in Ophthalmic and Vision Research. PTK took place at St Thomas' Hospital London using an Omnimed excimer laser (Summit Technology, Boston, MA, USA) with a wavelength of 193 nm.
The pulse energy resulted in a radiant exposure of 180 mJ/cm2 at a pulse frequency of 10 Hz. The beam shape was circular with a fixed diameter of 6.0 mm. Wounds were allowed to heal for up
to 19 months. We used an objective measurement for corneal haze developed by Lohmann _et al_47 in which haze was determined using a slit-lamp-mounted charged-coupled device (CCD) system. The
results of the haze measurements are shown in Figure 10 and confirm that both a transitory haze (which peaks after a month) and a more persistent or late developing haze (which remains for
many months) occur. The question we address here is to what extent the persistent haze can be ascribed to the nature of the newly deposited collagen. After 8 months of healing, the rabbit
corneas had laid down a layer of newly deposited collagen that had almost compensated for the amount removed (approximately 100 _μ_m). Apart from the most superficial layer, most of this
collagen had formed a lamellar structure, although the order in the fibril packing was visibly poor. Micrographs were taken at different depths and typical ones were used in the DSF method
to predict light transmission. In this case, however, we had no information about refractive indices in the new matrix, so we made the assumption that these were normal. Figure 11 shows that
despite the fact that the fibril diameters and organisation had not returned to normal, only a very small drop in light transmission is predicted. This is probably due, in large part, to
the fact that the newly deposited layer extended to only about 100 _μ_m. The thinness of this collagenous layer, therefore, counteracts the increased scattering caused by the poor
organisation of the new collagen. It appears, therefore, that newly deposited collagen is not the cause of persistent haze following PTK. Electron microscopy of our rabbit corneas showed a
qualitative correlation between haze, the number of activated keratocytes, and the smoothness of the subepithelial basement lamina. We therefore believe that either or both of these
contribute more to post-PTK persistent haze than does the newly deposited collagen. CONCLUSIONS Despite the considerable effort that has been put into understanding corneal transparency,
there is still no universally accepted explanation and no model that has been thoroughly tested. Some progress has been made, particularly with respect to our understanding of what factors
govern corneal fibril size49,50 and organisation, including the roles of the ambient ions51 and of proteoglycans. The recent availability of gene-targeted mice with null mutations for
selected proteoglycans52,53,54 now makes it possible to correlate the structural effect of selected deletions with tissue transparency. For example, it is interesting that lumican-null mice
have cloudy corneas,52 decorin-null mice have clear corneas,53 and keratocan-null mice have mostly clear corneas.54 These and similar tissues open the possibility to greatly increase our
understanding of the causes of light scattering from abnormal corneas in the near future. REFERENCES * Smith JW . The transparency of the corneal stroma. _Vis Res_ 1969; 9: 393–396. Article
Google Scholar * Leonard DW, Meek KM . Refractive indices of the collagen fibrils and extrafibrillar material in the corneal stroma. _Biophys J_ 1997; 72: 1382–1387. Article CAS Google
Scholar * Maurice DM . The structure and transparency of the corneal stroma. _J Physiol_ 1957; 136: 263–286. Article CAS Google Scholar * Hart RW, Farrell RA . Light scattering in the
cornea. _J Opt Soc Am_ 1969; 59: 766–774. Article CAS Google Scholar * Sayers Z, Koch MHJ, Whitburn SB, Meek KM, Elliott GF, Harmsen A . Synchrotron X-ray diffraction study of the corneal
stroma. _J Mol Biol_ 1982; 160: 593–607. Article CAS Google Scholar * Feuk T . On the transparency of the stroma in the mammalian cornea. _IEEE Trans Biomed Eng_ 1970; BME17: 1866–1890.
Google Scholar * Twersky V . Transparency of pair-correlated, random distributions of small scatterers, with applications to the cornea. _J Opt Soc Am_ 1975; 65: 524–530. Article CAS
Google Scholar * Benedek GB . Theory and transparency of the eye. _Appl Opt_ 1971; 10: 459–473. Article CAS Google Scholar * Vaezy S, Clark JI . Quantitative analysis of the
microstructure of the human cornea and sclera using 2-D Fourier methods. _J Microsc_ 1994; 175: 93–99. Article CAS Google Scholar * Ameen DB, Bishop MF, McMullen T . A lattice model for
computing the transmissivity of the cornea and sclera. _Biophys J_ 1998; 75: 2520–2531. Article CAS Google Scholar * Farrell RA, McCally RL . Corneal transparency. In: Albert DM, Jakobiec
FA (eds). _Principles and Practice of Ophthalmology_. WB Saunders: Philadelphia, PA, 2000, pp 629–643. Google Scholar * Freegard TJ . The physical basis of transparency of the normal
cornea. _Eye_ 1997; 11: 465–471. Article Google Scholar * Tuchin VV . Light scattering study of tissues. _Phys Usp_ 1997; 40: 495–515. Article Google Scholar * Freund DE, McCally RL,
Farrell RA . Direct summation of fields for light scattering by fibrils with applications to normal corneas. _Appl Opt_ 1986; 25: 2739–2746. Article CAS Google Scholar * Freund DE,
McCally RL, Farrell RA, Cristol SM, L'Hernault NL, Edelhauser HF . Ultrastructure in anterior and posterior stroma of perfused human and rabbit corneas: relation to transparency.
_Invest Ophthalmol Vis Sci_ 1995; 36: 1508–1523. CAS PubMed Google Scholar * Freund DE, McCally RL, Farrell RA . Light scattering tests of structure in normal and swollen rabbit corneas.
_Johns Hopkins APL Tech Dig_ 1991; 12: 137–143. Google Scholar * Leonard DW . The ultrastructure of the corneal stroma and its implications for transparency. PhD thesis, The Open
University, Milton Keynes, UK, 1996. * Rawe IM, Leonard DW, Meek KM, Zabel RW . X-ray diffraction and transmission electron microscopy of Morquio Syndrome Type A cornea: a structural
analysis. _Cornea_ 1997; 16: 369–376. Article CAS Google Scholar * Fullwood NJ, Meek KM . A synchrotron X-ray study of the changes occurring in the corneal stroma during processing for
electron microscopy. _J Microsc_ 1993; 169: 53–60. Article CAS Google Scholar * Meek KM, Quantock AJ . The use of X-ray scattering techniques to determine corneal ultrastructure. _Prog
Ret Eye Res_ 2001; 20: 95–137. Article CAS Google Scholar * Meek KM, Fullwood NJ, Cooke PH, Elliott GF, Maurice DM, Quantock AJ _et al_. Synchrotron X-ray diffraction studies of the
cornea with implications for stromal hydration. _Biophys J_ 1991; 60: 467–474. Article CAS Google Scholar * Patel S, McLaren J, Hodge D, Bourne W . Normal human keratocyte density and
corneal thickness measurement by using confocal microscopy _in vivo_. _Invest Ophthalmol Vis Sci_ 2001; 42: 333–339. CAS Google Scholar * Moller-Pedersen T, Ehlers N . A three-dimensional
study of the human corneal keratocyte density. _Curr Eye Res_ 1995; 14: 459–464. Article CAS Google Scholar * Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD _et al_.
The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. _J Cell Sci_ 1999; 112: 613–622. CAS PubMed Google Scholar * Moller-Pedersen T, Cavanagh HD, Petroll WM,
Jester JV . Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. _Ophthalmology_ 2000; 107:
1235–1245. Article CAS Google Scholar * Moller-Pedersen T . The cellular basis of corneal transparency and haze development (Abstract). _Ophthalmic Res_ 2002; 34: 4. Google Scholar *
Turss R, Friend J, Reim M, Dohlman CH . Glucose concentration and hydration of the corneal stroma. _Ophthalmic Res_ 1971; 2: 253–260. Article CAS Google Scholar * Castoro JA, Bettelheim
AA, Bettelheim FA . Water gradients across bovine cornea. _Invest Ophthalmol Vis Sci_ 1988; 29: 963–968. CAS PubMed Google Scholar * Lee D, Wilson G . Non-uniform swelling properties of
the corneal stroma. _Curr Eye Res_ 1981; 1: 457–461. Article CAS Google Scholar * Kikkawa Y, Hirayama K . Uneven swelling of the corneal stroma. _Invest Ophthalmol_ 1970; 9: 735–741. CAS
PubMed Google Scholar * Müller L, Pels E, Vrensen GFJM . The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. _Br J Ophthalmol_ 2001; 85:
437–443. Article Google Scholar * Plessy B, Bettelheim FA . Water vapor sorption of keratan sulfate. _Mol Cell Biochem_ 1975; 6: 85–91. Article CAS Google Scholar * Bettelheim FA,
Plessy B : The hydration of proteoglycans of bovine cornea. _Biochim Biophys Acta_ 1975; 381: 203–214. Article CAS Google Scholar * Bron AJ . The architecture of the corneal stroma. _Br J
Ophthalmol_ 2001; 85: 379–383. Article CAS Google Scholar * Goodfellow JM, Elliott GF, Woolgar AE . X-ray diffraction studies of the corneal stroma. _J Mol Biol_ 1978; 119: 237–252.
Article CAS Google Scholar * Huang Y, Meek KM . Swelling studies on the cornea and sclera: the effects of pH and ionic strength. _Biophys J_ 1999; 77: 1655–1665. Article CAS Google
Scholar * Farrell RA, McCally RL, Tatham PER . Wavelength dependencies of light scattering in normal and cold swollen rabbit corneas and their structural implications. _J Physiol (London)_
1973; 233: 589–612. Article CAS Google Scholar * Farrell RA, McCally RL . On corneal transparency and its loss with swelling. _J Opt Soc Am_ 1976; 66: 342. Article CAS Google Scholar *
Quantock AJ, Meek KM, Brittain P, Ridgway AE, Thonar EJ . Alteration of the stromal architecture and depletion of keratan sulphate proteoglycans in oedematous human corneas: histological,
immunochemical and X-ray diffraction evidence. _Tissue Cell_ 1991; 23: 593–606. Article CAS Google Scholar * Corbett MC, Marshall J . Corneal haze after photorefractive keratectomy, a
review of etiological mechanisms and treatment options. _Lasers Light_ 1996; 7: 173–196. Google Scholar * Tuft SJ, Marshall J, Rothery S . Stromal remodelling following photorefractive
keratectomy. _Lasers Ophthalmol_ 1987; 1: 177–183. Google Scholar * Marshall J, Trokel S, Rothery S, Krueger RR . Long term healing of the central cornea after photorefractive keratectomy
using an excimer laser. _Ophthalmology_ 1988; 95: 1411–1421. Article CAS Google Scholar * Hanna KD, Pouliquen Y, Waring GO, Savoldelli M, Cotter J, Morton K _et al_. Corneal stromal wound
healing after 193 nm excimer laser ablation. _Arch Ophthalmol_ 1989; 107: 895–901. Article CAS Google Scholar * Rawe IM, Zabel RW, Tuft SJ, Chen V, Meek KM . A morphological study of
rabbit corneas after laser keratectomy. _Eye_ 1992; 6: 637–642. Article Google Scholar * Fantes FE, Hanna KD, Waring III GO, Pouliquen Y, Thompson KP, Savoldelli M . Wound healing after
excimer laser keratomileusis in monkeys. _Arch Ophthalmol_ 1990; 108: 665–675. Article CAS Google Scholar * Wu WCS, Stark WJ, Green WR . Corneal wound healing after 193 excimer laser
keratectomy. _Arch Ophthalmol_ 1991; 109: 1426–1432. Article CAS Google Scholar * Lohmann CP, Fitzke F, O'Brart D, Muir MK, Timberlake G, Marshall J . Corneal light scattering and
visual performance in myopic individuals with spectacles, contact lenses, or excimer laser photorefractive keratectomy. _Am J Ophthalmol_ 1993; 155: 444–453. Article Google Scholar *
Connon CJ, Marshall J, Patmore AL, Brahma A, Meek KM . Persistent haze and disorganisation of anterior stromal collagen appear unrelated following phototherapeutic keratectomy (PTK). _J
Refract Surg_ 2003; 19: 1–11. Google Scholar * Adachi E, Hayashi T . _In vitro_ formation of hybrid fibrils of type V collagen and type I collagen. Limited growth of type I collagen into
thick fibrils by type V collagen. _Connect Tissue Res_ 1986; 14: 257–266. Article CAS Google Scholar * Birk DE . Type V collagen: heterotypic type I/V collagen interactions in the
regulation of fibril assembly. _Micron_ 2001; 32: 223–237. Article CAS Google Scholar * Kostyuk O, Nalovina O, Mubard T, Reginio JW, Meek KM, Quantock AJ _et al_. Transparency of the
bovine corneal stroma at physiological hydration and its dependence on concentration of the ambient anion. _J Physiol_ 2002; 243(2): 633–642. Article Google Scholar * Chakravarti S,
Petroll WM, Hassell JR, Jester JV, Lass JH, Paul J _et al_. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. _Invest Ophthalmol
Vis Sci_ 2000; 41: 3365–3373. CAS PubMed PubMed Central Google Scholar * Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV . Targeted disruption of decorin leads to
abnormal collagen fibril morphology and skin fragility. _J Cell Biol_ 1997; 136: 729–743. Article CAS Google Scholar * Saika S, Shiraishi A, Liu CY, Funderburgh JL, Kao CW, Converse RL
_et al_. Role of lumican in the corneal epithelium during wound healing. _J Biol Chem_ 2000; 275: 2607–2612. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank
Professor John Marshall and Anne Patmore for carrying out the PTK and making the haze measurements shown in Figure 10. We also thank Mr Nick Hawksworth for supplying postoperative
pathological corneas, Dr Val Smith for supplying normal human corneas from the Bristol Eye Bank, and Dr S Akhtar for assistance with electron microscopy. We are grateful to the staff at the
SRS Daresbury laboratory for their ongoing help with the data collection. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Cardiff Institute of Tissue Engineering and Repair and Department of
Optometry and Vision Sciences, Cardiff University, Cardiff, UK K M Meek, C J Connon, S Dennis & S Khan * The Open University, Oxford Research Unit, Oxford, UK D W Leonard Authors * K M
Meek View author publications You can also search for this author inPubMed Google Scholar * D W Leonard View author publications You can also search for this author inPubMed Google Scholar *
C J Connon View author publications You can also search for this author inPubMed Google Scholar * S Dennis View author publications You can also search for this author inPubMed Google
Scholar * S Khan View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to K M Meek. ADDITIONAL INFORMATION This work was
funded by the Wellcome Trust and is currently funded by the Medical Research Council RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Meek, K., Leonard,
D., Connon, C. _et al._ Transparency, swelling and scarring in the corneal stroma. _Eye_ 17, 927–936 (2003). https://doi.org/10.1038/sj.eye.6700574 Download citation * Received: 28 February
2003 * Accepted: 28 February 2003 * Published: 20 November 2003 * Issue Date: 01 November 2003 * DOI: https://doi.org/10.1038/sj.eye.6700574 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative KEYWORDS * corneal transparency * stroma * swelling * oedema * phototherapeutic keratectomy