Atypical pattern of retardation on GDx-VCC and its effect on retinal nerve fibre layer evaluation in glaucomatous eyes
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

To assess the effect of atypical pattern of retardation (APR) on retinal nerve fibre layer (RNFL) measurements made by scanning laser polarimetry (SLP) with variable corneal compensation
(GDx-VCC) in glaucomatous eyes.
One eye each of 30 glaucomatous patients (average mean deviation (MD): −6.4±4.8) with APR on GDx-VCC retardation map were selected. In total, 34 glaucomatous, age- and severity-matched eyes
(average MD: −7.0±5.3) and 36 age-matched healthy subjects, both with a normal pattern of retardation (NPR) represented control groups. APR on retardation maps was characterized by
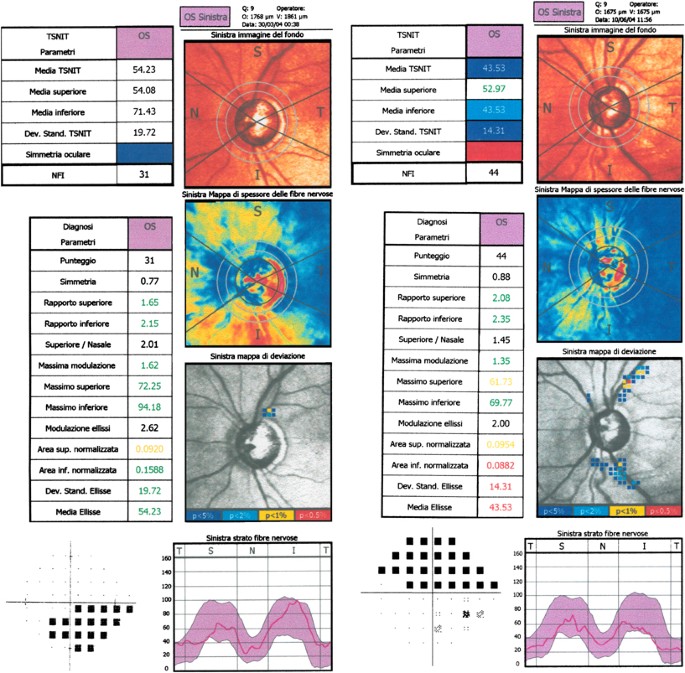
alternating peripapillary circumferential bands of low and high retardation, or high retardation areas arranged in a spokelike pattern, or high retardation nasal and temporal splotchy areas.
Typical scan score (TSS) was extracted for each included eye. GDx-VCC parameters (mean±SD) in the two groups of glaucomatous eyes were compared with healthy eyes' corresponding values
(Mann–Whitney U-test). Areas under receiver operating characteristic (AUROC) curves were generated to assess the APR effect on the parameters' diagnostic ability.
All parameters discriminated adequately between healthy and glaucomatous eyes with NPR (AUROCs ≥0.9 for nine parameters). On the contrary, considering healthy and glaucomatous eyes with APR,
four thickness parameters could not separate the two groups and AUROCs ≥0.85 appeared only for Inferior and Superior Ratio, NFI, Max Modulation.
APR may void the effect of custom compensation and provide spurious RNFL thickness measurements. When a printout of glaucomatous eyes with APR is evaluated, it is proper to rely on ratios,
modulation parameters, and NFI, since the diagnostic ability of thickness parameters is significantly reduced.
Peripapillary retinal nerve fibre layer (RNFL) thickness may be objectively assessed in vivo by scanning laser polarimetry (SLP). Polarized light crossing tissues with form birefringence
properties undergoes retardation linearly related to thickness in a primate model.1 The greater the retardation, the thicker the tissue. During the clinical course of glaucomatous optic
neuropathy, standard automated perimetry (SAP) detects a glaucoma-related visual field (VF) defect only after at least a 40% loss of retinal ganglion cells has already occurred.2, 3, 4
Instruments measuring optic disc topography and RNFL thickness may assist clinicians in early diagnosis and follow-up of glaucomatous optic neuropathy. Many reports showed that with respect
to SLP with fixed compensation (GDx-FCC), the use of variable compensation (GDx-VCC) improves significantly the structure–function relationship5 and diagnostic ability in glaucomatous
eyes.6, 7, 8, 9, 10, 11, 12, 13, 14 Moreover, early RNFL thinning in perimetrically unaffected fellow eyes of patients with unilateral glaucoma was detected.15 However, some eyes had to be
discarded from these studies because an atypical pattern of retardation (APR) appeared on the retardation map available in the printout.13, 14 Bagga and Greenfield16 recently described in a
great detail polarimetric features in these eyes. On average, APR was found to be associated with artefactual increase in RNFL thickness. The source of such high retardation is still
unknown, even if it probably arises from undefined subretinal structures.17 To our knowledge, no detailed description about polarimetric features in these eyes is available. The aim of this
study was to evaluate the APR effect on RNFL assessment on GDx-VCC in glaucomatous eyes.
We reviewed charts in the database of patients that underwent peripapillary RNFL evaluation on GDx-VCC at the Glaucoma Unit of Trieste University Eye Clinic between January and October 2004.
peripapillary RNFL thickness evaluation by means of GDx-VCC with image quality score ≥8, as provided by software;
SAP performed at ±1 month from SLP, showing a glaucomatous defect;
refractive error within the ±5 spherical dioptres range, with less than ±2 cylinder dioptres.
corneal or lens opacity interfering with clinical, SAP, or SLP examination;
significant peripapillary atrophy falling under ellipse measurement, tilted disc, uveitis, significant vitreous floaters, diffuse or localized retinal, or macular disease;
inability to perform reliable SAP (fixation losses, false-positive or negative rates >20%) or SLP (poor fixation, inattentive patients).
A sample of glaucomatous eyes with normal pattern of retardation (NPR) was selected, after adequate age- and severity matching with glaucomatous subjects and APR. A third sample of
age-matched normal subjects with NPR was selected. Any morphologic hint of APR in healthy eyes was a cause for exclusion from the study. One eye each of enrolled subjects was selected
randomly for inclusion, if both met eligibility criteria. Each patient underwent a comprehensive clinical examination. BCVA was measured on a standard ETDRS chart. Anterior segment was
evaluated on slit lamp, and gonioscopy and Goldmann applanation tonometry were performed. Optic disc was examined by stereo-biomicroscopy with a +78D lens, after pupil dilation. SAP was
performed with the Humphrey Field Analyzer (Humphrey Systems, Dublin, CA), 24-2 program, SITA standard strategy.
Healthy subjects had normal SAP (mean deviation (MD)—and pattern standard deviation (PSD)—within 95% confidence limits, Glaucoma Hemifield Test (GHT)—within normal limits), intraocular
pressure