Molecular population genetics of the malaria vector anopheles darlingi in central and south america
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT To analyze the genetic relatedness and phylogeographic structure of _Anopheles darlingi_ from 19 localities throughout Central and South America, we used a minimum spanning network,
diversity measures, differentiation, neutrality tests, and mismatch distribution with mitochondrial cytochrome oxidase subunit I (_COI_) sequences. All the Central American haplotypes were
separated by seven mutational steps from the South American haplotypes and the _F_ST distance-based neighbor-joining tree showed a primary division between Central and South America,
evidence for a putative gene pool division. More ancestral and diverse haplotypes were found in Amazonian and southern Brazil populations, suggesting that Central American populations may
have originated in South America. The patterns of the mtDNA haplotype diversity and five of six tests for equilibrium implicate demographic expansion in the South American populations as the
historical structure, but mismatch distribution depicts populations at mutation drift equilibrium (MDE). In South America, the departure from equilibrium was consistent with an expansion
that occurred during the Pleistocene. SIMILAR CONTENT BEING VIEWED BY OTHERS INTEGRATED TAXONOMY TO ADVANCE SPECIES DELIMITATION OF THE _ANOPHELES MACULIPENNIS_ COMPLEX Article Open access
28 December 2024 THE ORIGIN OF ISLAND POPULATIONS OF THE AFRICAN MALARIA MOSQUITO, _ANOPHELES COLUZZII_ Article Open access 26 May 2021 POPULATION GENOMIC EVIDENCE OF A PUTATIVE ‘FAR-WEST’
AFRICAN CRYPTIC TAXON IN THE _ANOPHELES GAMBIAE_ COMPLEX Article Open access 10 September 2024 INTRODUCTION Globally, there are approximately 273 million annual malaria cases, and greater
than 2.1 billion people are at risk of malaria (WHO/TDR, 2004). There are an estimated 960 000 cases of malaria reported in the Americas, and approximately 41% occur in Brazil (99% in the
Amazon region) (PAHO, 2002). One of the factors determining the degree of malaria endemicity in a susceptible geographic region is the species of mosquito vector present. Vector species and
population differences within species influence biting times, feeding and resting sites, and anthropophily (Lounibos and Conn, 2000), and these behaviors determine human mosquito contact.
The factors that affect the vector's capacity to transmit the plasmodium parasite vary with species and population, and include mosquito abundance, infection rate, anthropophily, and
longevity (Foster and Walker, 2002). In the Neotropics, relative abundances of important malaria vectors have changed temporally, such as _Anopheles albitarsis_ in southeastern Brazil
(Forattini et al, 1993), _Anopheles marajoara_ in northern Amazonian Brazil (Conn et al, 2002; Lehr, 2003) and _A. darlingi_ in western Amazonian Brazil (Soares Gil et al, 2003) and in the
city of Belém (Póvoa et al, 2003). _Anopheles albitarsis_ has emerged in southeastern Brazil possibly due to the development of irrigated land (Forattini et al, 1993). _Anopheles
darlingi_'s recent resurgence, in places such as Iquitos, Peru has been linked to increased malaria cases (Aramburu Guarda et al, 1999; Schoeler et al, 2003). The resurgence of _A.
darlingi_ is proposed to be a result of human migration and land use changes, which often result in invasion of its primary breeding sites along warm lowland rivers and subsequent increased
abundance (Charlwood, 1996; Conn et al, 1999). Such anthropogenic changes highlight important aspects of targeted malaria control: the possibility of altering locally important vectors, and
complicated interactions with human populations (Conn et al, 2002; Póvoa et al, 2003). _Anopheles darlingi_ is the most important malaria vector in the Amazon Basin (Deane, 1947, 1988). In
addition to high rates of infection, _A. darlingi_ is also a good malaria vector owing to its anthropophilic (Charlwood and Alecrim, 1989) and endophagic (feeds indoors) behavior
(Lourenço-de-Oliveira et al, 1989); although recently it is thought to have evolved to be more exophilic (rests outdoors) as a result of prolonged residual insecticide use in many regions
(Charlwood, 1996; Soares Gil et al, 2003). _Anopheles darlingi_ has an extensive distribution from southern Mexico to southern Brazil (Forattini, 1962). Although there are no documented
barriers to gene flow for _A. darlingi_, rDNA data that detected a fixed insertion/deletion in samples from Belize, but not in those from South America (Manguin et al, 1999), are suggestive.
_Anopheles albimanus_ and _A. pseudopunctipennis_, with distributions similar to _A. darlingi_, are differentiated between Central and South America (De Merida et al, 1995, 1999; Manguin et
al, 1995), possibly due to vicariance (Krzywinski and Besansky, 2003) or barriers to gene flow in Costa Rica and Panama (Molina-Cruz et al, 2004). Even though _A. darlingi_ is considered a
single species (reviewed in Manguin et al, 1999; Lounibos and Conn, 2000), it does exhibit heterogeneity in body size (Charlwood, 1996) and in some genetic markers such as polytene
chromosomes (Kreutzer et al, 1972), mtDNA (Freitas-Sibajev et al, 1995; Conn et al, 1999) and rDNA ITS2 sequences (Malafronte et al, 1999). The observed heterogeneity can affect important
behavioral determinants of vector efficiency, such as endophily (rests indoors) and possibly dispersal ability (range expansion) (Lounibos and Conn, 2000; Fairley et al, 2002). Within
species differentiation can also affect the efficacy of control techniques; for example, variation in biting times can affect usefulness of personal protection measures (Zimmerman and
Voorham, 1997). Owing to their relationship to human populations, many anopheline mosquito species are likely to violate mutation drift equilibrium (MDE) assumptions (Donnelly et al, 2001).
One result of this is inaccurate estimates of gene flow, which can confound potential choices of target populations for the introduction of refractory genes. Avise (2000) suggests that
closely related species in the same geographic region should have similar demographic histories due to their phylogenetic relatedness. In the northeastern Amazon, Lehr (2003) demonstrated
that _A. marajoara_, a close relative of _A. darlingi_ (Sallum et al, 2000), has undergone a recent population expansion, and is therefore not at MDE. In the present study, we analyze the
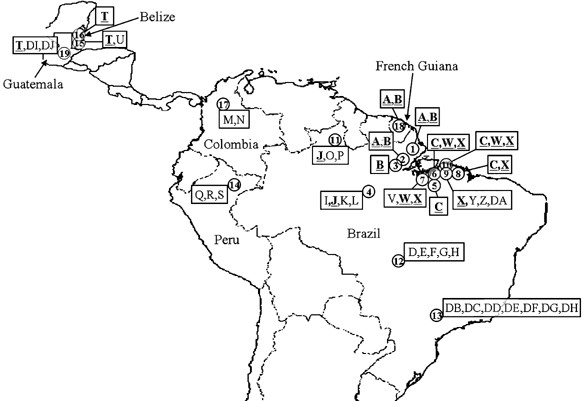
population structure of _A. darlingi_ from 19 localities throughout Central and South America, including Brazil, Peru, Belize, Colombia, French Guiana, and Guatemala. Using sequences of the
mitochondrial cytochrome oxidase subunit I (_COI_) gene, we test the hypothesis that _A. darlingi_ will have a similar demographic history to _A. marajoara_ in the northeastern Amazon
(Avise, 2000) and determine whether there is a division in the gene pool between Central and South America, as proposed by Krzywinski and Besansky (2003). MATERIALS AND METHODS MOSQUITO
COLLECTIONS Adult _A. darlingi_ were collected outdoors between 19:00 and 21:00 h by human landing catches and identified morphologically using the key of Deane et al (1946). The human
landing catch protocol was reviewed and approved by the Institutional Review Board of the New York State Department of Health and by the Biosafety Committee of the Instituto Evandro Chages,
Belém, Brazil. Table 1 depicts the 19 localities, longitude and latitude of each site, and number of mosquitoes sequenced. All mosquitoes were maintained in 95% ethanol at −80°C until use.
DNA from each specimen used herein has been retained as a frozen voucher at −80°C in the Conn Laboratory. DNA EXTRACTION AND SEQUENCING DNA was isolated from the head, thorax, or legs using
the DNeasy tissue kit, following standard DNeasy Tissue Handbook protocol for isolation of total DNA from animal tissues (Qiagen, CA, USA). A 1300 bp fragment of the _COI_ gene was amplified
using the forward primer UEA3 and the reverse primer UEA10 (Lunt et al, 1996). Each individual PCR reaction was performed using a Ready-To-Go-PCR bead (Amersham Pharmacia/Biotech, NJ, USA)
and run on a PTC-200 thermal cycler (BioRad, Inc.). The PCR products were cleaned with CentriSpin 40 columns (Princeton Separations, NJ, USA), and sent to the Wadsworth Center Molecular
Genetics Core for sequencing. The forward and reverse sequences were aligned using Sequencher 3.0 (Gene Codes Corp, MI, USA), grouped together by site and trimmed in PAUP, version 4.0
(Swofford, 2003), creating a 978 bp fragment of the _COI_ gene. Unique haplotypes were determined using MacClade, version 3.0 (Maddison and Maddison, 1997); identical sequences were
considered to be a single haplotype. PHYLOGENETIC RELATEDNESS The number of mutational steps necessary to link any two haplotypes with 95% confidence level was determined in ParsProb 1.1
(Posada et al, 2000). A minimum spanning network of the _A. darlingi_ haplotypes was created using TCS 1.12 (Clement et al, 2000). Phylogenetic relationships among the haplotypes were
estimated with PAUP using maximum likelihood and maximum parsimony (Swofford, 2003), and with Mr Bayes using a Bayesian approach (Rannala and Yang, 1996; Mau and Newton, 1997; Mau et al,
1999). _Anopheles albimanus_ was used as an outgroup (Sallum et al, 2000). Pairwise population estimates of _F_ST were computed using Arlequin 2.01 (Schneider et al, 2000), and the _F_ST
values were used as distance measures to create a neighbor-joining (NJ) tree using Mega V2.1 (Kumar et al, 2001). HISTORICAL DEMOGRAPHY The haplotype and nucleotide diversities were computed
in Arlequin 2.01 (Schneider et al, 2000). Nei's _G_ST was calculated to estimate the population differentiation based on differences in allele frequencies; Nei's _N_M was used to
estimate gene flow based on _G_ST (Nei, 1973). The tests of Tajima (1989) and Fu and Li (1993) were used to test the hypothesis that all mutations are selectively neutral (Kimura, 1983).
Tajima's _D_T (1989) is based on the differences between the number of segregating sites and the average number of nucleotide differences. The _D_ and _F_ tests, proposed by Fu and Li
(1993), are based on molecular polymorphism data. Fu's _F_S test (1997) and Strobeck's _S_ statistic (1987) assess the haplotype structure based on the haplotype frequency
distribution, and were used as additional tests of neutrality. These analyses were calculated using DnaSP, version 3 (Rozas and Rozas, 1999). The mismatch distribution (a frequency
distribution of the observed number of pairwise sequence differences) was performed to distinguish between a smooth unimodal distribution and a multimodal, or ragged, distribution (Slatkin
and Hudson, 1991; Rogers and Harpending, 1992; Rogers, 1995). The raggedness (_r_) statistic was calculated to quantify the smoothness of the mismatch distribution (Harpending et al, 1993).
The Mantel analysis was used to test the null hypothesis of the independence of the geographic and genetic distance by a pairwise matrix of geographical and genetic distances (estimated by
_F_ST). The mismatch distribution and Mantel test (Mantel, 1967) were calculated in Arlequin 2.01 (Schneider et al, 2000), and the raggedness test in DnaSP, version 3 (Rozas and Rozas,
1999). Significance of the Mantel test was determined by a permutation test of _n_=1000. RESULTS GENETIC VARIATION Of the 36 unique haplotypes detected, seven were shared (A, B, C, J, T, W,
and X); the remainder were unique to a single geographic location (Figure 1). All the sequences were A–T rich (combined frequency of 72.05%), which is expected within Insecta. Sixty-nine
transitions and seven transversions were identified, and there were four nonsynonymous mutations. A mutation at position 45 resulted in a methionine present in haplotype V (_A. darlingi_ in
ARA) in place of a valine in all other haplotypes. Another southern Amazonian mutation resulted in an isoleucine in place of a valine in haplotype W (_A. darlingi_ in ARA, BEL, and CAP). A
nonsilent mutation at position 193 produced a threonine in haplotype N (_A. darlingi_ in NEC) where an alanine is present in all other haplotypes; and a mutation in haplotype Q (_A.
darlingi_ in IQ) resulted in an alanine in place of a threonine. There were no nonfunctional genes (ie, pseudogenes) as shown by the absence of stop codons, the prevalence of synonymous
substitutions, low pairwise divergence and clear electrophorograms. The two most common haplotypes were T (_n_=17) in Belize and Guatemala, and B (_n_=15) in northeastern Amazonian Brazil
(Table 2). Localities south of the Amazon region in Brazil have the highest haplotype diversities (ITB, MOJ, PEX, and DOU), and localities just northeast and southeast of the Amazon have the
most shared haplotypes within the populations (TAR, LI, ANT, TRP; TAI, BEL, ARA, PEB, and CAP) (Figure 1; Table 2). Populations in close geographic proximity have the greatest quantity of
shared haplotypes, and populations that are farther apart do not share haplotypes. Interestingly, localities directly northeast (TAR, LI, ANT, and TRP) and southeast of the Amazon (TAI, BEL,
ARA, PEB, MOJ, and CAP) do not share haplotypes, and localities northeast of the Amazon (TAR, LI, ANT, and TRP) only have two unique haplotypes (Table 2). Haplotypes in Peru and Colombia
are not shared among any other population, which could be due to their geographic separation from other localities sampled and (or) potential geographic or climatic barriers. In general,
these data would seem to support the isolation by distance model (Wright, 1951). Belize and Guatemala had low diversity with only four haplotypes identified from 21 mosquitoes analyzed.
Haplotype X is shared among the greatest number of populations in Brazil, followed by B and C (Table 2). Sequences for _A. darlingi_ and _A. albimanus_ used in this study have been deposited
in GenBank, accession numbers DQ298209 to DQ298244. PHYLOGEOGRAPHIC RELATEDNESS The minimum spanning network illustrates the mutational relationship of the _A. darlingi_ haplotypes (Figure
2). All haplotypes differed by less than 13 mutational steps, so they could be connected parsimoniously. The Central American haplotypes are separated by seven mutational steps from the
Colombian haplotypes (M and N), which are separated by an additional seven mutational steps from a Brazilian haplotype, DA. Many southern Amazonian Brazil haplotypes (X, C, W, V, Y, K, I)
differed by only one or two mutational steps, suggestive of a demographic expansion (Slatkin and Hudson, 1991; Fu, 1997). Haplotype X was the most common interior haplotype, so is most
likely the oldest haplotype (Castelloe and Templeton, 1994). The majority of haplotypes were tip alleles, and are considered as more recently derived and geographically restricted (Crandall
and Templeton, 1993; Castelloe and Templeton, 1994). The phylogenetic relationship among the haplotypes using the maximum-likelihood, maximum-parsimony, and Bayesian analyses were all very
poorly resolved and not informative because the sequence variation contains insufficient phylogenetic signal (data not shown). The _F_ST pairwise estimates of differentiation ranged from 0
to 1, and were used to create the NJ tree. Two primary clusters were found: (I) South America, and, (II) Central America plus NW Colombia (Figure 3). There appears to be a secondary division
within cluster I between the southern Amazon and southern South America (IA) and the Northern Amazon (IB). The pairwise comparisons of _F_ST within clusters I and II were 52.5 and 55%
significant, respectively, and the _F_ST comparisons between clusters were 100% significant (_F_ST values shown in Table 3) (Donnelly et al, 2004). Additional support for the primary
division is found from samples of the conserved nuclear _white_ gene that were cloned and sequenced for over 200 samples of _A. darlingi_ from most of the same localities as in the current
study (Mirabello and Conn, unpublished). HISTORICAL DEMOGRAPHY Nucleotide and haplotype diversities were used as measures of genetic diversity of _A. darlingi_. Haplotypes B and T had the
greatest haplotype frequency (Table 2). ITB, CAP, and PEX had the highest haplotype diversities. ANT, TAI, and CAY had no detectable diversity measures because only a single haplotype was
detected in each. Both of the diversity measures were high in ITB, DOU, and IQ. The NJ cluster with the greatest haplotype diversity was IA, and IB had the highest nucleotide diversity (also
having the greatest average number of nucleotide differences). Cluster II had low haplotype and nucleotide diversity measures. Nei's _G_ST and _N_M (1973) were used to examine the
pairwise genetic differentiation and gene flow, respectively, between the population clusters (IA, IB, and II) (Table 4). The highest level of genetic differentiation (_G_ST=0.3109) was
detected between Central America (II) and northern Amazon (IB) (Table 4). The estimates of gene flow (_N_M) were moderate among South America populations and between Amazonian and southern
South America and Central America. Only the gene flow estimate between Central America plus NW Colombia (II) and northern Amazon (IB) was below 1. Population comparisons with _N_M values
less than 1 are considered to have no gene flow (Nei, 1973, 1975). Tajima's (1989) _D_T and Fu and Li's (1993) _F_ and _D_ neutrality tests found that cluster I and IA have
significant negative _D_ and _F_ values, and nonsignificant negative _D_T values (Table 5). The results allow rejection of the neutral model in these regions, as a result of two possible
factors: (1) a relatively recent population expansion, which can raise the number of low-frequency variants, and (2) natural selection. The neutral model is also rejected in cluster IB,
where the _D_T, _D_, and _F_ values were all significantly positive (cluster II also had positive values, but nonsignificant), which suggests possible balancing selection or population
subdivision (Table 5). Fu's _F_S test (1997) and Strobeck's _S_ statistic (1987) determined that both cluster I and IA have significantly negative _F_S values and positive _S_ of
0.995 and 1.000, respectively, indicating possible population expansion. Fu (1997) states that _F_S is the most powerful test for detecting population expansion and genetic hitchhiking,
followed by Tajima's _D_T, and Fu and Li's _D_ and _F_ tests (Fu and Li, 1993). Cluster IB has significantly positive _F_S and a low _S_ statistic of 0.0001, indicating possible
background selection. Cluster II also had a positive _F_S and low _S_-value, but they were not statistically significant. Through a graphical illustration of the mismatch distribution it is
possible to determine if there is a smooth unimodal distribution following the Poisson distribution characteristic of a recent bottleneck or population expansion, or a multimodal
distribution indicating a population at MDE. In contrast to the previous demographic history analyses for _A. darlingi_, the mismatch distribution did not demonstrate the expected unimodal
distribution for all the localities together or for either primary or secondary clusters. The observed mismatch distribution for cluster I differed significantly from the simulated
distribution of a demographic expansion model (Figure 4). The distribution for all the localities throughout Central and South America showed a multimodal distribution typical of populations
at MDE. The raggedness statistic for clusters I and IB are both very small (0.0174 and 0.0105, respectively), suggesting a population expansion. Cluster IB has a large _r_-value (0.7395),
indicating a population at equilibrium. Time since the population expansion can be estimated from _t_=_τ_/2_μ_, where _μ_ is the mutation rate per site per generation (Slatkin and Hudson,
1991). The Drosophila mutation rate of 10−8/site/year (Powell et al, 1986) and 10 generations/year (Walton et al, 2000) were used in the calculation. The estimate of _τ_, from the raggedness
calculation, is 4.951 for cluster I, and 3.176 for cluster IA. Therefore, the time to expansion for _A. darlingi_ in South America is approximately 253 119 years ago (95% CI, 85 554–402
419), and in Amazonian and southern South America is approximately 162 372 years ago (95% CI, 54 882–258 144). Both expansion times are during the late Pleistocene. The Mantel analysis to
test the null hypothesis of the independence of the geographic and genetic distance between each population was conducted to test for isolation by distance. The correlation was not
significant (_R_2=0.011, _P_=0.489). DISCUSSION In a review of evolutionary studies Donnelly et al (2002) claim that the genetic population structure of primary malaria vectors is shallow
with a weak effect of distance on differentiation, and this has been supported by studies of the major African vectors _A. gambiae_ (Lehmann et al, 1996) and _A. arabiensis_ (Donnelly and
Townson, 2000). Our analyses detected considerable population structure, and isolation by distance was detected by Conn et al (1999) for _A. darlingi_ in South America. A similar pattern was
also found in neotropical vectors _A. aquasalis_ (Fairley et al, 2002) and _A. albimanus_ (De Merida et al, 1999), although in a more recent study in the latter species the effect of
distance on differentiation was weak (Molina-Cruz et al, 2004). In our study of _A_. _darlingi_, the inference of range expansion was well supported but isolation by distance was not.
Isolation by distance and the low nucleotide diversity observed in Central American populations of _A. albimanus_ and low nucleotide diversity in _A. darlingi_ could be due to small
effective migration rates and effective population size, and/or genetic drift (De Merida et al, 1999; Molina-Cruz et al, 2004). The nucleotide diversity estimates for _A. gambiae_ are also
low, which is consistent with a large panmictic population (Besansky et al, 1997; Lehmann et al, 1998). These differences may be due to lack of isolation by distance in the African
_Anopheles_, as compared to the Neotropical _Anopheles_, and their high dispersal ability, large effective population size, and/or recent range expansion (corresponding to the human
population expansion during the arrival of agriculture in West Africa) (Lounibos and Conn, 2000; Donnelly et al, 2001, 2002). The _N_M estimates between Central America and the northern
Amazon indicated little or no recurrent gene flow (_N_M<1). Contemporary gene flow estimates are important in malaria vectors because they are used to predict the spread of genes,
essential information for effective introduction of transgenes for _Plasmodium_ resistance (Cohuet et al, 2005; Tripet et al, 2005). No gene flow combined with high genetic differentiation
may be the result of vicariance or obstruction by natural barriers (ie, topography or habitat that a migrating individual cannot cross) existing between Central America and northern Amazon.
_Anopheles albimanus_ data suggest that a single barrier exists within Central America, which may be the mountain range that crosses Costa Rica and Western Panama (Molina-Cruz et al, 2004).
The phylogeographic break between Central American plus NW Colombia and South American _A. darlingi_ is consistent with studies of other Neotropical taxa, such as Neotropical butterflies
(Brower 1994), toads (Slade and Moritz, 1998), bats (Hoffmann and Baker, 2003) and trees (Dick et al, 2003). However, Manguin et al (1999) observed substantial gene flow among populations of
_A. darlingi_ throughout its geographic range using four nonmitochondrial markers (isozyme, RAPD-PCR, ITS2, and morphology). These conflicting results may be due to the differences in
mitochondrial and nuclear DNA inheritance and evolutionary histories which can affect estimates of gene flow (Presa et al, 2002). Mitochondrial DNA evolves relatively rapidly, it is
maternally inherited, and has an extremely low level of recombination as compared to the nuclear genome (Avise, 2000). Manguin et al (1999) found that _A. darlingi_ from Belize differed from
all the South American populations by a three-base deletion in the rDNA ITS2 marker. There is no evidence of clinal variation, and the range of _A. darlingi_ in Central America is narrow
(Manguin et al, 1999). Therefore, Manguin et al (1999) suggested the differentiation is either due to a recent introduction event that may have been caused by humans, or an extrinsic factor.
One interpretation of our data is a possible introduction event from Colombian _A. darlingi_ populations into Central America. More ancestral and diverse haplotypes were observed in
Amazonian and southern Brazil populations, so they are likely older, and Colombian haplotypes are, of the South American samples examined in this study, most closely related to the Central
American haplotypes. In general, older populations have a higher diversity than younger populations (Kambhampati and Rai, 1991; Molina-Cruz et al, 2004). In our study, the older populations
with the highest diversity are ITB, CAP and PEX, and cluster IA (Amazonian and southern South America). According to Avise (2000), high haplotype diversity relative to the nucleotide
diversity suggests a population bottleneck followed by a rapid population expansion and buildup of mutations; nearly all the populations (except TAI and PEB) of _A. darlingi_ in cluster IA,
and all the localities together fit this criterion. We hypothesized that the Amazonian populations of _A. darlingi_ had undergone a population expansion similar to _A. marajoara_ in northern
Brazil (Lehr, 2003), based on regional patterns determined for many organisms reviewed in Avise (2000). The large proportion of shared haplotypes and lack of unique haplotypes in the Amazon
region in Brazil, and most of the demographic history analyses support an expansion in Amazonian and southern South America populations of _A. darlingi_. However, mismatch analysis shows a
multimodal pattern characteristic of populations at MDE. Harpending (1994) states that a population expansion that is too recent, for example at the end of the Pleistocene epoch, would not
result in a smooth unimodal mismatch distribution. MDE is thought to be achieved when the effective population (_N_e) size has stayed constant for 2_N_e–4_N_e generations (Nei and Li, 1976).
_A. darlingi_ population size probably fluctuates due to its dependence on specific climatic and environmental conditions, which have changed dramatically over the last millennia (Prance,
1982; Absy et al, 1991; Cavalli-Sforza et al, 1994; Nicholson, 1995; Donnelly et al, 2001). The estimated expansion time for _A. marajoara_ was much more recent (36 400 years ago in the
eastern Amazon; Lehr, 2003) as compared to _A. darlingi_ (estimated time to expansion is >100 000 years). This difference may reflect the cycles of Amazonian savannah contraction and
re-expansion, and thus habitat availability owing to the differences in habitat preference between _A. marajoara_ (breeding sites associated with savannah and agricultural habitat; Conn et
al, 2002; Lehr, 2003) and _A. darlingi_ (primary breeding sites along warm lowland rivers; Rozendaal, 1990; Roberts et al, 2002). If _A. darlingi_, like other primary vectors (eg, _A.
gambiae_) has only recently become a human pest, it would be expected to show a recent population expansion. However, the historical human population expansion in the Amazon region is
predicted to have occurred between 10 000 (Roosevelt et al, 1991) and 2500 years BP (Willis et al, 2004), and a rapid decline in indigenous human population size occurred after colonial
contact in the 18th–19th centuries (Willis et al, 2004). Brazilian government settlement policies, still in effect today, have caused nonindigenous human populations in many areas of the
Amazon in Brazil to expand significantly during the 20th century (Cruz Marques, 1987; Alecrim, 1992; Schmink and Wood, 1992). Our estimates support a very different history for _A. darlingi_
in South America compared with _A. gambiae_ in subSaharan Africa, despite both being primary vectors. _Anopheles darlingi_ being a more opportunistic feeder (Charlwood, 1996) is not nearly
as dependent on human blood meals compared with _A. gambiae_ (White, 1974; Coluzzi et al, 1979; Besansky et al, 2004). Therefore its populations are more likely to fluctuate based on
climatic conditions (that would influence breeding site availability) compared with _A. gambiae_'s noteworthy dependence on human population densities (Coluzzi, 1982; Donnelly et al,
2001). ACCESSION CODES ACCESSIONS GENBANK/EMBL/DDBJ * DQ298209 * DQ298244 REFERENCES * Absy ML, Cleef AM, Fournier M, Martin L, Servant M, Sifeddine A _et al_ (1991). Mise en évidence de
quatre phases d'ouverture de la forèt dense dans le sud-est de l'Amazonie au cours des 60 000 dernières anéés. Première comparison d'autres régions tropicales. _CR Acad Sci
Paris t.312_ SÉRIE II: 673–678. Google Scholar * Alecrim WD (1992). Malaria, prospecting activities and government policies in the Amazon Region. _Rev Inst Med Trop São Paulo_ 34: S48.
PubMed Google Scholar * Aramburu Guarda J, Ramal Asayag C, Witzig R (1999). Malaria re-emergence in the Peruvian Amazon region. _Emerg Infect Dis_ 5: 209–215. Article CAS PubMed PubMed
Central Google Scholar * Avise JC (2000). _Phylogeography. The History and Formation of Species_. University Press: Massachusetts. Book Google Scholar * Besansky NJ, Hill CA, Costantini
C (2004). No accounting for taste: host preference in malaria vectors. _Trends Parasitol_ 20: 249–251. Article PubMed Google Scholar * Besansky NJ, Lehmann JA, Fahey GT, Fontenille D,
Braack LEO, Hawley WA, Collins FH (1997). Patterns of mitochondrial DNA variation within and between African malaria vectors, _Anopheles gambiae_ and _A. arabiensis_, suggest extensive gene
flow. _Genetics_ 147: 1817–1828. Article CAS PubMed PubMed Central Google Scholar * Brower AVZ (1994). Rapid morphological radiation and convergence among races of the butterfly
_Heliconius erato_ inferred from patterns of mitochondrial DNA evolution. _PNAS USA_ 91: 6491–6495. Article CAS PubMed PubMed Central Google Scholar * Castelloe J, Templeton AR (1994).
Root probabilities for intraspecific gene trees under neutral coalescent theory. _Mol Phyl Evol_ 3: 102–113. Article CAS Google Scholar * Cavalli-Sforza LL, Menozzi P, Piazza A (1994).
_The History and Geography of Human Genes_. Princeton University Press: New Jersey. Google Scholar * Charlwood JD (1996). Biological variation in _Anopheles darlingi_ root. _Mem Inst
Oswaldo Cruz_ 91: 391–398. Article CAS PubMed Google Scholar * Charlwood JD, Alecrim WA (1989). Capture-recapture studies with the South American malaria vector _Anopheles darlingi_
Root. _Ann Trop Med Parasitol_ 83: 569–576. Article CAS PubMed Google Scholar * Clement M, Posada D, Crandall KA (2000). TCS: a computer program to estimate gene genealogies. _Mol Ecol_
9: 1657–1659. Article CAS PubMed Google Scholar * Cohuet A, Dia I, Simard F, Raymond M, Rousset F, Antonio-Nkondjio C _et al_ (2005). Gene flow between chromosomal forms of the malaria
vector _Anopheles funestus_ in Cameroon, Central Africa, and its relevance in malaria fighting. _Genetics_ 169: 301–311. Article CAS PubMed PubMed Central Google Scholar * Coluzzi M
(1982). Spatial distribution of chromosomal inversions and speciation in anopheline mosquitoes. In: Bargozzi C (ed) _Mechanisms of Speciation_. Alan R Liss., Inc.: New York. pp 143–153.
Google Scholar * Coluzzi M, Sabatini A, Petrarca V, Di Deco MA (1979). Chromosomal differentiation and adaptation to human environments in the _Anopheles gambiae_ complex. _Trans R Soc Trop
Med Hyg_ 73: 483–497. Article CAS PubMed Google Scholar * Conn JE, Rosa-Freitas MG, Luz SLB, Momen H (1999). Molecular population genetics of the primary neotropical malaria vector
_Anopheles darlingi_ using mtDNA. _J Am Mosq Control Assoc_ 15: 468–474. CAS PubMed Google Scholar * Conn JE, Wilkerson RC, Segura NO, De Souza RTL, Schlichting CD, Wirtz RA, Povoa MM
(2002). Emergence of a new neotropical malaria vector facilitated by human migration and changes in land use. _Am J Trop Med Hyg_ 66: 18–22. Article PubMed Google Scholar * Crandall KA,
Templeton AR (1993). Empirical tests of some predictions from coalescent theory with applications to intraspecific phylogeny reconstruction. _Genetics_ 134: 959–969. Article CAS PubMed
PubMed Central Google Scholar * Cruz Marques A (1987). Human migration and the spread of malaria in Brazil. _Parasit Today_ 3: 166–170. Article CAS Google Scholar * De Merida AM, De
Mata MP, Molina E, Porter CH, Black IV WC (1995). Variation in ribosomal DNA intergenic spacers among populations of _Anopheles albimanus_ in South and Central America. _Am J Trop Med Hyg_
53: 469–477. Article CAS PubMed Google Scholar * De Merida AM, Palmieri M, Yurrita M, Molina A, Molina E, Black IV WC (1999). Mitochondrial DNA variation among _Anopheles albimanus_
populations. _Am J Trop Med Hyg_ 6: 230–239. Article Google Scholar * Deane LM (1947). Observaçoes sobre a malaria na Amazonia brasileira. _Rev Soc Bras Med Trop_ 24: 13–20. Google Scholar
* Deane LM (1988). Malaria studies and control in Brazil. _Am J Trop Med Hyg_ 33: 223–230. Article Google Scholar * Deane LM, Causey OR, Deane MP (1946). An illustrated key by adult
female characteristics for identification of thirty-five species of Anopheline from the northeast and Amazon regions of Brazil, with notes on the malaria vectors (Dipeta: Culicidae). _Am J
Trop Med_ 18: 1–18. Google Scholar * Dick CW, Abdul-Salim K, Bermingham E (2003). Molecular systematics reveals cryptic Tertiary diversification of a widespread tropical rainforest tree.
_Am Naturalist_ 162: 691–703. Article Google Scholar * Donnelly MJ, Licht MC, Lehmann T (2001). Evidence for recent population expansion in the evolutionary history of the malaria vectors
_Anopheles arabiensis_ and _Anopheles gambiae_. _Mol Biol Evol_ 18: 1353–1364. Article CAS PubMed Google Scholar * Donnelly MJ, Pinto J, Girod R, Besansky NJ, Lehmann T (2004).
Revisiting the role of introgression _vs_ shared ancestral polymorphisms as key processes shaping genetic diversity in the recently separated sibling species of the _Anopheles gambiae_
complex. _Heredity_ 92: 61–68. Article CAS PubMed Google Scholar * Donnelly MJ, Simard Frédéric, Lehmann T (2002). Evolutionary studies of malaria vectors. _Trends Parasitol_ 18: 75–80.
Article PubMed Google Scholar * Donnelly MJ, Townson H (2000). Evidence for extensive genetic differentiation among populations of the malaria vector _Anopheles arabiensis_ in eastern
Africa. _Insect Mol Biol_ 9: 357–367. Article CAS PubMed Google Scholar * Fairley TL, Povoa MM, Conn JE (2002). Evaluation of the Amazon River delta as a barrier to gene flow for the
regional malaria vector, _Anopheles aquasalis_ (Diptera:Culicidae) in northeastern Brazil. _J Med Entomol_ 39: 861–869. Article CAS PubMed Google Scholar * Forattini OP (1962). _Parte
Geral, Diptera, Anophelini_, Entomologica Medica, Vol 1. Faculdade de Higiene e Saúde Pública, Universidade de São Paulo: São Paulo, Brasil. pp 622. Google Scholar * Forattini OP, Kakitani
I, Massad E, Marucci D (1993). Studies on mosquitoes (Diptera: Culicidae) and anthropic environment. 3-Survey of adult stages at the rice irrigation system and the emergence of _Anopheles
albitarsis_ in southeastern Brazil. _Revista de Saude Publica_ 27: 313–325. Article CAS PubMed Google Scholar * Foster WA, Walker ED (2002). Mosquitoes (Culicidae). In: Mullen G, Durden
L (eds) _Medical and Veterinary Entomology_. Academic Press: New York. Chapter 12, pp 204–256. Google Scholar * Freitas-Sibajev MG, Conn J, Mitchell SE, Cockburn AF, Seawright JA, Momen H
(1995). Mitochondiral DNA and morphological analyses of _Anopheles darlingi_ populations from Brazil (Diptera: Culicidae). _Mosq Syst_ 27: 79–99. Google Scholar * Fu YX (1997). Statistical
tests of neutrality of mutations against population growth, hitchhiking and background selection. _Genetics_ 147: 915–925. Article CAS PubMed PubMed Central Google Scholar * Fu YX, Li
WH (1993). Statistical tests of neutrality of mutations. _Genetics_ 133: 693–709. Article CAS PubMed PubMed Central Google Scholar * Harpending HC (1994). Signature of ancient
population growth in a low-resolution mitochondrial DNA mismatch distribution. _Hum Biol_ 66: 591–600. CAS PubMed Google Scholar * Harpending HC, Sherry ST, Rogers AR, Stoneking M (1993).
The genetic structure of ancient human populations. _Curr Anthropol_ 34: 483–496. Article Google Scholar * Hoffmann FG, Baker RJ (2003). Comparative phylogeography of short-tailed bats
(Carollia: Phyllostomidae). _Mol Ecol_ 12: 3403–3414. Article CAS PubMed Google Scholar * Kambhampati S, Rai KS (1991). Mitochondrial DNA variation within and among populations of the
mosquito _Aedes albopictus_. _Genome_ 34: 288–292. Article CAS PubMed Google Scholar * Kimura M (1983). _The Neutral Theory of Molecular Evolution_. Cambridge University Press:
Cambridge, England. Book Google Scholar * Kreutzer RD, Kitzmiller JB, Ferreira E (1972). Inversion polymorphism in the salivary gland chromosomes of _Anopheles darlingi_ Root. _Mosq News_
32: 555–565. Google Scholar * Krzywinski J, Besansky NJ (2003). Molecular systematics of _Anopheles_: from subgenera to subpopulations. _Annu Rev Entomol_ 48: 111–139. Article CAS PubMed
Google Scholar * Kumar S, Tamura K, Jakobsen IB, Nei M (2001). MEGA2: molecular evolutionary genetics analysis software. _Bioinformatics_ 17: 1244–1245. Article CAS PubMed Google
Scholar * Lehmann T, Hawley WA, Gerbert H, Collins FH (1998). The effective population size of _Anopheles gambiae_ in Kenya: implications for population structure. _Mol Biol Evol_ 15:
264–276. Article CAS PubMed Google Scholar * Lehmann T, Hawley WA, Kamau L, Fontenille D, Simard F, Collins FH (1996). Genetic differentiation of _Anopheles gambiae_ populations from
East and west Africa: comparison of microsatellite and allozyme loci. _Heredity_ 77: 192–200. Article CAS PubMed Google Scholar * Lehr MA (2003). Phylogenetics and Phylogeography of a
Neotropical Anopheline Cryptic Species Complex. MS Thesis, University of Vermont. * Lounibos LP, Conn JE (2000). Malaria vector heterogeneity in South America. _Am Entomologist_ 46: 238–249.
Article Google Scholar * Lourenço-de-Oliveira R, Guimarães AEG, Arie M, Fernandes da Silva T, Castro MG, Motta MA, Deane LM (1989). Anopheline species, some of their habits and relation
to malaria in endemic areas of Rondonia State, Amazon region of Brazil. _Mem Inst Oswaldo Cruz_ 84: 501–514. Article PubMed Google Scholar * Lunt DH, Zhang DX, Szymura JM, Hewitt GM
(1996). The insect cytochrome oxidase I gene: evolutionary patterns and conserved primers for phylogenetic studies. _Insect Mol Biol_ 5: 153–165. Article CAS PubMed Google Scholar *
Maddison WP, Maddison DR (1997). _MacClade: Analysis of Phylogeny and Character Evolution_ Version 3.07. Sinauer Associates: Massachusetts. Google Scholar * Malafronte RS, Marrelli MT,
Marinotti O (1999). Analysis of ITS2 DNA sequences from Brazilian _Anopheles darlingi_ (Diptera: Culicidae). _J Med Entomol_ 36: 631–634. Article CAS PubMed Google Scholar * Manguin S,
Roberts DR, Peyton EL, Fernandez-Salas I, Barreto M, Fernandez Loayza R _et al_ (1995). Biochemical systematics and population genetic structure of _Anopheles pseudopunctipennis_, vector of
malaria in Central and South America. _Am J Trop Med Hyg_ 53: 362–377. Article CAS PubMed Google Scholar * Manguin S, Wilkerson RC, Conn JE, Rubio-Palis Y, Donoff-Burg JA, Roberts DR
(1999). Population structure of the primary malaria vector in South America, _Anopheles darlingi_, using isozyme, random amplified polymorphic DNA, internal transcribed spacer 2, and
morphologic markers. _Am J Trop Med Hyg_ 60: 364–376. Article CAS PubMed Google Scholar * Mantel N (1967). The detection of disease clustering and a generalized regression approach.
_Cancer Res_ 27: 209–220. CAS PubMed Google Scholar * Mau B, Newton M (1997). Phylogenetic inference for binary data on dendrograms using Markov chain Monte Carlo. _J Comput Graph Stat_
6: 122–131. Google Scholar * Mau B, Newton M, Larget B (1999). Bayesian phylogenetic inference via Markov chain Monte Carlo methods. _Biometrics_ 55: 1–12. Article CAS PubMed Google
Scholar * Molina-Cruz A, de Merida AM, Mills K, Rodriguez F, Schoua C, Yurrita MM _et al_ (2004). Gene flow among _Anopheles albimanus_ populations in Central America, South America, and
the Caribbean assessed by microsatellites and mitochondrial DNA. _Am J Trop Med Hyg_ 71: 350–359. Article CAS PubMed Google Scholar * Nei M (1973). Analysis of gene diversity in
subdivided populations. _Proc Natl Acad Sci USA_ 70: 3321–3323. Article CAS PubMed PubMed Central Google Scholar * Nei M (1975). _Molecular Population Genetics and Evolution_, Vol 40.
North Holland Publishing Company: North-Holland. Google Scholar * Nei M, Li WH (1976). The transient distribution of allele frequencies under mutation pressure. _Genet Res_ 28: 205–214.
Article CAS PubMed Google Scholar * Nicholson SE (1995). Environmental change within the historical period. In: Goudie AS, Adams WM, Orme A (eds) _The Physical Geography of Africa_.
Oxford University Press: Oxford, England. pp 60–75. Google Scholar * Pan American Health Organization (2002). _Status of Malaria Programs in the Americas_, CSP26/INF/3. Pan American Health
Organization, Washington, DC. * Posada D, Crandall KA, Templeton AR (2000). GeoDis: a program for the cladistic nested analysis of the geographical distribution of genetic haplotypes. _Mol
Ecol_ 9: 487–488. Article CAS PubMed Google Scholar * Póvoa MM, Conn JE, Schlichting CD, Amaral JC, Segura MN, Da Silva AN _et al_ (2003). Malaria vectors, epidemiology, and the
re-emergence of _Anopheles darlingi_ in Belém, Pará, Brazil. _J Med Entomol_ 40: 379–386. Article PubMed Google Scholar * Powell JR, Caccone A, Amato GD, Yoon C (1986). Rate of nucleotide
substitution in _Drosophila_ mitochondrial DNA and nuclear DNA are similar. _Proc Natl Acad Sci USA_ 83: 9090–9093. Article CAS PubMed PubMed Central Google Scholar * Prance GT (1982).
A review of the phylogeographic evidences for Pleistocene climate changes in the Neotropics. _Ann Mo Bot Gard_ 69: 594–624. Article Google Scholar * Presa P, Pardo BG, Martínez P,
Bernatchez L (2002). Phylogeographic congruence between mtDNA and rDNA ITS markers in Brown Trout. _Mol Biol Evol_ 9: 2161–2175. Article Google Scholar * Rannala B, Yang Z (1996).
Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. _J Mol Evol_ 43: 304–311. Article CAS PubMed Google Scholar * Roberts DR, Manguin S,
Rejmankova E, Andre R, Harbach RE, Vanzie E _et al_ (2002). Spatial distribution of adult _Anopheles darlingi_ and _Anopheles albimanus_ in relation to riparian habitats in Belize, Central
America. _J Vector Ecol_ 27: 21–30. PubMed Google Scholar * Rogers AR (1995). Genetic evidence for a pleistocene population explosion. _Evolution_ 49: 608–615. Article PubMed Google
Scholar * Rogers AR, Harpending H (1992). Population growth makes waves in the distribution of pairwise differences. _Mol Biol Evol_ 9: 552–569. CAS PubMed Google Scholar * Roosevelt AC,
Housley RA, Imazio da Silveira M, Maranca S, Johnson R (1991). Eighth millennium pottery from a prehistoric shell midden in the Brazilian Amazon. _Science_ 254: 1621–1624. Article CAS
PubMed Google Scholar * Rozas J, Rozas R (1999). DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. _Bioinformatics_ 15: 174–175.
Article CAS PubMed Google Scholar * Rozendaal JA (1990). Observations on the distribution of anophelines in Suriname with particular reference to the malaria vector _Anopheles darlingi_.
_Mem Inst Oswaldo Cruz_ 85: 221–234. Article CAS PubMed Google Scholar * Sallum MAS, Schultz, TR, Wilkerson RC (2000). Phylogeny of Anophelinae (Diptera Culicidae) based on
morphological characters. _Ann Entomol Soc Am_ 93: 745–775. Article Google Scholar * Schmink M, Wood CH (1992). _Contested Frontiers in Amazonia_. Columbia University Press: New York.
Google Scholar * Schneider S, Roessli D, Excoffier L (2000). _Arlequin: A Software for Population Genetic Data_. Genetics and Biometry Laboratory, University of Geneva: Switzerland. Google
Scholar * Schoeler GB, Flores-Mendoza C, Fernandez R, Davila JR, Zyzak M (2003). Geographical distribution of _Anopheles darlingi_ in the Amazon Basin region of Peru. _J Am Mosq Control
Assoc_ 19: 286–296. PubMed Google Scholar * Slade RW, Moritz C (1998). Phylogeography of Bufo marinus from its natural and introduced ranges. _Proc R Soc Lond Ser B – Biological Sciences_
265: 769–777. Article CAS Google Scholar * Slatkin M, Hudson RR (1991). Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. _Genetics_
129: 555–562. Article CAS PubMed PubMed Central Google Scholar * Soares Gil LH, Alves FP, Zieler H, Salcedo JMV, Durlacher RR, Cunha RPA _et al_ (2003). Seasonal malaria transmission
and variation of anopheline density in two distinct endemic areas in Brazilian Amazônia. _J Med Entomol_ 40: 636–641. Article Google Scholar * Strobeck C (1987). Average number of
nucleotide differences in a sample from a single subpopulation: a test for population subdivision. _Genetics_ 117: 149–153. Article CAS PubMed PubMed Central Google Scholar * Swofford
DL (2003). _PAUP (Phylogenetic Analysis Using Parsimony) and Other Methods_, Version 4. Sinauer Associates: Massachusetts. Google Scholar * Tajima F (1989). Statistical method for testing
the neutral mutation hypothesis by DNA polymorphisms. _Genetics_ 123: 585–595. Article CAS PubMed PubMed Central Google Scholar * Tripet F, Dolo G, Lanzaro GC (2005). Multilevel
analyses of genetic differentiation in _Anopheles gambiae_ s.s. reveal patterns of gene flow important for malaria-fighting mosquito projects. _Genetics_ 169: 313–324. Article CAS PubMed
PubMed Central Google Scholar * Walton C, Handley JM, Tun-Lin W, Collins FH, Harbach RE, Baimai V et al (2000). Population structure and population history of _Anopheles dirus_ mosquitoes
in Southeast Asia. _Mol Biol Evol_ 17: 962–974. Article CAS PubMed Google Scholar * White GB (1974). _Anopheles gambiae_ complex and disease transmission in Africa. _Trans R Soc Trop Med
Hyg_ 68: 278–301. Article CAS PubMed Google Scholar * WHO/TDR (2004). _Disease Watch: Malaria_. World Health Organization: Geneva. * Willis KJ, Gillson L, Brncic TM (2004). How ‘virgin’
is virgin rainforest? _Science_ 304: 402–403. Article CAS PubMed Google Scholar * Wright S (1951). The genetical structure of populations. _Ann Eugenics_ 15: 323–354. Article CAS
Google Scholar * Zimmerman RH, Voorham J (1997). The use of impregnated bed nets and other materials for the control of malaria in the Americas. _Pan Am J Public Health_ 2: 18–25. Article
CAS Google Scholar Download references ACKNOWLEDGEMENTS We are particularly grateful to Drs Robert H Gilman, Ranulfo González, John P Grieco, Sylvie Manguin, Marinete M Póvoa, Norma
Padilla, and Richard C Wilkerson for providing samples. We thank members of the Conn laboratory for technical help and advice. We appreciate the comments of two anonymous reviewers who
improved this manuscript. This study was funded by National Institutes of Health grants AI R2940116 and AI R0154139 to JEC. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Biomedical Sciences, Division of Immunology and Infectious Disease, University at Albany, State University of New York, 1400 Washington Avenue, Albany, 12222, NY, USA L Mirabello & J E
Conn * New York State Department of Health, Wadsworth Center, Griffin Laboratory, 5668 State Farm Road, Slingerlands, 12159, NY, USA J E Conn Authors * L Mirabello View author publications
You can also search for this author inPubMed Google Scholar * J E Conn View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to J E Conn. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mirabello, L., Conn, J. Molecular population genetics of the malaria vector
_Anopheles darlingi_ in Central and South America. _Heredity_ 96, 311–321 (2006). https://doi.org/10.1038/sj.hdy.6800805 Download citation * Received: 24 March 2005 * Accepted: 15 November
2005 * Published: 01 March 2006 * Issue Date: 01 April 2006 * DOI: https://doi.org/10.1038/sj.hdy.6800805 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
KEYWORDS * _Anopheles darlingi_ * malaria vector * population genetics * mtDNA * range expansion