Γ-aminobutyric acid transporter (gat1) overexpression in mouse affects the testicular morphology
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT _γ_-Aminobutyric acid and GABAergic receptors were previously reported to be distributed in reproductive systems besides CNS and predicted to participate in the modulation of
testicular function. _γ_-Aminobutyric acid transporter was implicated to be involved in this process. However, the potential role of _γ_-aminobutyric transporter in testis has not been
explored. In this study, we investigated the existence of mouse _γ_-aminobutyric acid transporter subtype I (mGAT1) in testis. Wild-type and transgenic mice, which overexpressing mGAT1 in a
variety of tissues, especially in testis, were primarily studied to approach the profile of mGAT1 in testis. Mice with overexpressed mGAT1 develop normally but with reduced mass and size of
testis as compared with wild-type. Testicular morphology of transgenic mice exhibited overt abnormalities including focal damage of the spermatogenic epithelium accompanied by capillaries
proliferation and increased diameter of seminiferous tubules lumen. Reduced number of spermatids was also found in some seminiferous tubules. Our results clearly demonstrate the presence of
GAT1 in mouse testis and imply that GAT1 is possibly involved in testicular function. SIMILAR CONTENT BEING VIEWED BY OTHERS SODIUM BUTYRATE PROMOTES SYNTHESIS OF TESTOSTERONE AND MEIOSIS OF
HYPERURICEMIC MALE MICE Article Open access 28 April 2025 CRISPR/CAS9-MEDIATED KNOCKOUT OF MCT8 REVEALS A FUNCTIONAL INVOLVEMENT OF MCT8 IN TESTIS AND SPERM DEVELOPMENT IN A RAT Article
Open access 07 July 2020 INTRATESTICULAR CREATINE MAINTAINS SPERMATOGENESIS BY DEFINING TIGHT JUNCTIONS Article Open access 28 December 2024 INTRODUCTION As the predominant inhibitory
neurotransmitter in the vertebrate, _γ_-aminobutyric acid (GABA) in central nervous system (CNS) was intensively studied. GABA actions are mediated by the ionotropic GABA_A_/GABA_C_
receptors, as well as the metabotropic GABA_B_ receptors. Recently, GABA was found in gonads and accessory reproductive organs, and a direct effect on steroidogenesis and sperm viability and
motility has been described1. The existence of peripheral-type benzodiazepine receptors (PBR) in testicular interstitial cells was characterized2. Glutamic acid and glutamate decarboxylase
(GAD) which produce GABA from glutamic acid, the main source of GABA via deamination, were also detected in testis1. All these studies mentioned above hinted at the physiological role of
GABA network in reproductive system. On the other side, the termination of GABA synaptic transmission is catalyzed by transporters which are ubiquitously distributed in various parts of
brain but has not yet been detected in reproductive system so far. Aanesen A. et al3 indicated that a high-affinity _γ_-aminobutyric acid transporter protein m ay be possibly involved in
GABA uptake in human spermatozoa, but without direct evidence. In the present study, we demonstrated, for the first time, that _γ_-aminobutyric acid transporter subtype 1 (mGAT1), which can
rapidly and specifically take up GABA into pre-synaptic terminal or surrounding glial cells in CNS, was also present in mouse testis. To approach the functional role of GAT1 in testis, a
model of transgenic mice over-expressing mGAT1 which have been identified previously (unpublished Data) was used. Here, we reported the influence of GAT1 on testicular morphology MATERIALS
AND METHODS _ANIMALS_ C57BL mice carrying mGAT1 cDNA have been constructed and identified (unpublished Data). Mouse GAT1 cDNA containing whole mRNA coding region, which was screened from the
mouse brain cDNA/_λ_ phage library, was cloned into pCDNA3 (EcoRI-ApaI) under the control of human cytomegalovirus (HCMV) promoter/enhancer. DNA constructs were microinjected into the
fertilized eggs of C57BL mice. Polymerase chain reaction (PCR) amplification and Southern blot hybridization of tail DNA samples verified the integration of variable copy numbers of
transgene into the genomes of founder mice and their progeny. Reverse transcription-polymerase chain reaction (RT-PCR) analysis and Nothern blot hybridization of mRNA samples from various
tissues characterized the expression pattern of transgene. Age- and sex- matched wild-type C57BL mice were served as controls. Mice were housed in groups (< 4 per cage) in temperature and
humidity-controlled environment with a 12-h light/12-h dark rhythm. All mice had free access to food and water. _REVERSE TRANSCRIPTION-POLYMERASE CHAIN REACTION (RT-CR) ANALYSIS_ Testes
were removed from wild-type and homozygous transgenic mice after cervical dislocation. Whole RNA was extracted with Trizol reagent (GIBCO BRL) as detailed by the manufacturer. RNA integrity
was identified by formaldehyde-electrophoresis. RNA sample was thoroughly treated by incubating with RNA-Free DNase (5u/per_μ_g) for 45 min at 37°C before reverse-transcription performed
with GIBCO Kit. mGAT1 was amplified by PCR with 64°C as annealing temperature. PCR primers (5′ -end: 5′ -ACCAAGCTTAGGCTGCAAAGCTGCTG-3′; 3′ -end: 5′ -ACGCCTTTGAACATGGGCGCCAG- 3′) were
designed to match nucleotides from (−92) to (+375) of mouse GAT1 cDNA4. GAPDH mRNA was co-detected by PCR with primers (5′ -end: 5′ -ACGACCCCTTCATTGACC-3′; 3′ -end: 5′
-AGACACACACAGTAGACTCCACG-3′) which spanned 210 bp nucleotides within the coding sequence for GAPDH. The amount of RT-PCR product from GAPDH mRNA was refered to semiquantify the expression
level of mGAT1. RT procedure omitted RNA samples were directly amplified by PCR with 5-fold amounts of the same aliquot to demonstrate the amplified product was cDNA-based instead of genomic
DNA-based product. mGAT1 RT-PCR products from both wild-type and transgenic mice were purified and subsequently sequenced by using a primer specific to 5'-terminal sequence of mGAT1
cDNA. _IMMUNOFLUORESCENCE ANALYSIS_ Seven-month-old mice were anesthetized and perfused with chilled 95% ethanol. After perfusion, testes were removed and subsequently rinsed with 30%
sucrose in phosphate-buffered saline (PBS) and frozen in OCT. 20 _μ_m-thick frozen sections were cut and then rinsed in 0.01 M PBS. In staining procedure, cryostat sections were firstly
blocked with 10% goat serum plus 0.1% Triton X-100 in PBS and incubated with rabbit anti-mGAT1 antibody5 at 4°C overnight. After incubation, the slides were rinsed in PBS for 30 min and
exposed to biotinlated goat anti-rabbit IgG (Santa-Cruz) for 45 min at room temperature and then rinsed in PBS. Slides subsequently incubated with Fluorescent Extravidin (Santa-Cruz) for 45
min at room temperature. Slides were examined and photographed under Olympus fluorescence microscope. _HISTOLOGY ANALYSIS_ Seven-month-old mice were anesthetized and perfused with 4%
paraformaldehyde in PBS. After perfusion, testes were removed and then dehydrated through graded ethanol and cleared with xylene, subsequently embedded in paraffin. 5 _μ_m-thick sections
were cut on Leitz microtome. Slides were stained with haematoxylin and eosin, then examined and photographed under Leitz microscope. The diameters of the seminiferous tubules and their
lumens were measured by fitting a graticule of a calibrated Linear Scale in the × 10 eyepiece of Leitz microscope at objective lens × 40. Only circular and near circular tubules were
assessed. The height of the seminiferous epithelium was calculated by subtracting the lumen diameter from the tubule diameter. All tissue variables were assessed by viewing four randomly
chosen areas per section. Four sections per mouse from both wild-type (n=7) and transgenic mice (n=6) were measured. The mean data was analyzed statistically. Testicular morphology of both
wild-type and transgenic mice was represented by one section. _TESTICULAR WEIGHT_ Seven-month-old mice were sacrificed and dissected to remove testis. Testicular weight was assessed by
electron-balance. _DETERMINATION OF _ _3_ _H-GABA UPTAKE IN TESTIS_ Testicular cell suspension prepared by filtration with nylon membrane was pre-incubated in aCSF buffer (containing in mM:
NaCl 126.6; NaCO3 27.4; KCl 2.4; KH2PO4 0.49; CaCl2 1.2; MgCl2·6H2O 0.83; Na2HPO4 0.49; D-glucose 7.1; gassed with 95%O2/5%CO2; pH 7.2-7.4) for 5 min at 37°C. After that, uptake was
initiated by the addition of a mixture of cold and tritiated GABA (3H-GABA specific activity: 98ci/mM) and incubated further for 10 min. Low-[Na+] and high-[Na+] analysis of uptake in
wild-type was carried out separately to demonstrate that the GABA uptake capacity is sodium-dependent which is a characteristic possessed by GABA transporter. In this experiment, the
different final concentration of GABA used was 0.04_μ_M and 40_μ_M respectively in a medium containing 4.2 nM tritiated GABA. Modified aCSF buffer was served as Low-[Na+] buffer, in which
NaCl was substituted by LiCl with the same concentration. For kinetic analysis of uptake in both wild-type and transgenic mice, the amount of 3H-GABA used was kept constant and the different
GABA used concentrations (0.04–40_μ_M) were obtained by adding different amount of unlabelled GABA. After 10 min incubation, uptake was terminated by vacuum filtration through filters. The
GABA content of the filters was assayed by liquid scintillation counting, taking dilution factors into account. The same aliquot of testicular cell suspension was lysised with 2 mol/L NaOH
to quantify the protein concentration. Uptake was expressed as fmol/mg protein. For kinetic data analysis, linear-fitting was done to calculate Km and Vmax values according to
Michaelis-Menten equation (V=Vmax[S]/(Km +[S])). _STATISTICAL ANALYSIS_ Mean value and significant difference were analyzed by analysis of variance and student's t-test. RESULTS _GAT1
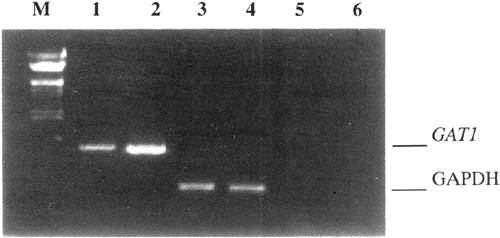
MRNA DISTRIBUTION IN TESTIS OF WILD-TYPE AND TRANSGENIC MICE_ The distribution of GAT1 mRNA in testis of seven-month-old wild-type mice was revealed by RT-PCR amplification and sequence
analysis. With specific primers matching to mGAT1, a 468 base-pair fragment was amplified (Fig 1, Lane 1). Simultaneously, we obtained a corresponded size product from brain mRNA via PCR
with the same primers (Data not shown). Further sequence analysis indicates that the product from both brain and testis possessed identical sequence except that two nucleotides [G(+105 in
brain GAT1 mRNA)->A (+105 in testis GAT1 mRNA)] with synonymous amino acid mutation (Gln >Gln) which might be introduced by low-fidelity Taq polymerase (Data not shown). With results
mentioned above, we concluded that GAT1 was authentically expressed in mouse testis. Transgene expression level in testis was assessed by semiquantative analysis. In RNA extraction from
testis of transgenic mice, the _GAT1_ mRNA level was apparently higher (Fig 1, Lane 2) than that in wild-type mice (Fig 1, Lane 1), because the amount of PCR product reflected both the
endogenous and transgenic GAT1 expression levels in transgenic mice. GAPDH mRNA levels, which were served as endogenous reference (Fig 1, Lane 3, 4) to monitor quantity and integrity of RNA
in both samples, remained approximately identical. No amplification products formed from reverse-transcrption process omitted samples (Fig 1, Lane 5, 6) verified that the specific product
was derived from mRNA other than genomic DNA. Above results indicate that _GAT1_ was over-expressed in the testis of transgenic mice as compared with wild-type mice. _GAT1 PROTEIN
LOCALIZATION IN TESTIS OF WILD-TYPE ANDTRANSGENIC MICE_ To locate _GAT1_ in specific testicular cell types, in situ immunofluorescence experiments were performed (Fig 2). High-affinity
anti-mGAT1 antibody specifically against the C-terminal region of GAT15 was developed to probe the GAT1 in seminiferous tubules. Moderate immunoreactivity was found in elongated spermatids
and spermatozoa within seminiferous epithelium. Spermatozoa within the lumen of tubule was also weakly labeled in wild-type mice (Fig 2A, C). Thus, expression of GAT1 was apparently confined
to spermatids and spermatozoa. Evaluation of immunofluorescence intensity by photoimager analysis indicated that the testis sections of transgenic mice (Fig 2B, D) possessed more intensive
immunoreactivity than that of wild-type mice. To our surprise, the GAT1-positive signal was also limited to the spermatids and spermatozoa, which was identical to the expression pattern of
endogenous GAT1. It might reflect that the expression pattern of transgene directed by human cytomegalovirus (HCMV) promoter possess the cell-type specific characteristic. In addition, no
spermatozoa within the lumen of seminiferous tubules was labeled in wild-type testis. _TRANSGENIC MICE OVER-EXPRESSING GAT1 LEADS TO ABNORMALITIES OF TESTICULAR MORPHOLOGY_ Abnormalities of
testicular morphology in transgenic mice over-expressing _GAT1_ were revealed by histological observation. The testis of transgenic mice indicated many focal damages, in which some
seminiferous tubules contained mostly spermatogenic cells while others were very vacuolated with reduced spermatogenic epithelium (Fig 3D, E, F). Although the diameter of seminiferous tubule
showed no difference (P > 0.05) between transgenic mice and wild-type, the height of seminiferous epithelium in testis of transgenic mice reduced significantly (P < 0.05), accompanied
by conspicuously expanded lumen (P < 0.01) (Tab 1). Compared with wild-type which generally showed a full complement of spermatogenic cells including spermatozoa within the seminiferous
tubules, the reduction of seminiferous epithelium in transgenic mice indicated a depletion of spermatogenic cells. Furthermore, transgenic mice had fewer spermatids and spermatozoa per
seminiferous tubule cross section than did the wild-type mice (Data not shown) (Fig 3F, C). In the testis of mice over-expressing GAT1, the interstitial region was featured with abnormally
proliferated capillaries. Leydig cells were present within the interstitium, yet in marked contrast to the wild-type mice, some Leydig cells occasionally contained a lot of lipid droplets in
the cytoplasm. No morphological change was observed in sertoli cells of transgenic mice. The effect of GAT1 over-expression on the mass and size of testis was also assessed statistically.
The mean testicular mass of transgenic mice was reduced significantly (P < 0.01) compared with that of wild-type (Fig 4A). Testicular size was also apparently reduced in short axis but
not in long axis (Fig 4B). Especially, testis of transgenic mice featured the loss of rigidity and elasticity which could be grossly evaluated by press with fingers, a difference which might
be resulted from the alteration of testicular morphology. DISCUSSION _γ_-Aminobutyric acid has been previously found to be widely distributed in periphery besides nervous system and was
suggested to possess comprehensive physiological role in these organs. Recently, a number of neurotransmitters are reported to be involved in the modulation of testicular function, including
a stimulatory action of _γ_-amino-butyric acid on steroidogenesis in the rat testis1. Coexistence of GABAA and GABAB receptors in testicular interstitial cells was also indicated2. It is
known that in CNS, neurotransmitter actions are mediated by specific receptors and terminated by high-affinity transporters, constituting together a complicated network. Based on above
mentioned findings, it is logical to infer that _γ_-aminobutyric acid transporter will be present in testis. Our results clearly demonstrated the constitutive expression of _γ_-aminobutyric
acid transporter subtype 1 (GAT1) in mouse testis, which confined to spermatids and spermatozoa. We also primarily assessed the influence of increased amount of GAT1 on the testicular
morphology with a transgenic model. The most striking feature of testis caused by GAT1 over-expression is the focal damage of the spermatogenic epithelium accompanied by capillaries
proliferation which may be implicated in the onset of testicular pathologies that cause male infertility6. The reproductive performance of male of homozygous transgenic mice mated with a
wild-type female was grossly observed. Transgenic mice with age under 2 mon showed no difference to age-matched wild-type in the number of pregnancies. But in mice older than 2-3 mon, the
numbers of pregnancies were apparently lower when compared with the results from wild-type mice. This progressive reduction in pregnancy efficiency indicated that appropriate expression of
GAT1 may be required for the maintenance of normal testicular function. To investigate whether _γ_-amino-butyric acid transporters are involved in the GABA uptake process in testis, we
performed 3H-GABA uptake experiments in the presence of low and high level of sodium concentration. As shown in Fig 5, it is clearly demonstrated that GABA uptake capacity is
sodium-dependent, which is one of the essential characteristic possessed by transporter. Km value determined by kinetic analysis (Data not shown) is also consistent with reported range (1
mM-4mM) 7. However, preliminary experiment indicated that testicular GABA uptake showed no significant difference between wild-type and transgenic mice which over-expressing GAT1 in testis
(Data not shown). One of opinion suggested that the sodium-dependent GABA uptake in mouse testis may be also attributed to other GABA transporter subtypes other than _GAT1_. Other
possibility is that the failure of increased uptake capacity in testis with over-expressed m_GAT1_ may be resulted from the morphology alteration as a compensatory effect. This may also
imply that GAT1 has possibly other functions in testis. In conclusion, the present study demonstrates the existence of GAT1 in mouse, it's over-expression results in alteration on
testicular morphology. REFERENCES * Frungieri MB, Gonzalez Galvar SI, Calandra RS . Influence of photoinhibition of GABA and glutamic acid levels, and on glutamate decarboxylase activity in
the testis and epididymis of the golden hamster. _Int J Andral_ 1996; 19(3):171–8. Article CAS Google Scholar * Ritta MN, Campos MB, Calandra RS . Coexistence of g-aminobutyric acid type
A and type B receptors in testicular interstitial cells. _J Neurochem_ 1991; 56(4):1236–40. Article CAS Google Scholar * Aanesen A, Fried G, Andersson E, Gottlieb C . Carrier-mediated
g-aminobutyric acid uptake in human spermatozoa indicating the presence of a high-affinity g-aminobutyric acid transporter protein. _Biol Reprod_ 1996; 54:841–6. Article CAS Google Scholar
* Tam Dominic, Anthony CW, Lihe Guo . Cloning and sequencing of GABA transporter complementary. _Cell Research_ 1994; 4(1):109–16. Article Google Scholar * Guo Qiang Cai, Jian Fei, Yan
Ping Xul et al. Nuclear proteins from liver and kidney bind a 37 bp sequence in the 5′upstream region of the mGAT1 gene. _Neuroreport_ 1998; 9(18):4059–62. PubMed Google Scholar * Markey
CM, Jequier AM, Meyer GT, Martin GB . Testicular morphology and androgen profiles following testicular ischaemia in rams. _J Repro Ferti_ 1994; 101:643–50. Article CAS Google Scholar *
Krogsgaard-Larsen P, Falch E, Larssen OM, Schousboe A . GABA uptake inhibitors: relevance to antiepileptic drug research. _Epilepsy Res_ 1987; 1:77–93. Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS The authors are very grateful to Dr. Cai Guoqiang for his generous gift of anti-mGAT1 antibody. We thank prof. Wang Da for invaluable assistance with histology
techniques. We also thank prof. Yan Yuanchang for his beneficial scientific interpretation and Miss Gao Lu for her technical assistance on this manuscript. This work was supported by grants
from National Science Foundation (No. 39630140). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Shanghai Institute of Cell Biology, Chinese Academy of Science, Shanghai, 200031, China Ying
Hua MA, Jia Hua HU, Xiao Gang ZHOU, Jian FEI & Li He GUO * Shanghai Institute of Physiology, Chinese Academy of Science, Shanghai, 200031, China Zhen Tong MEI Authors * Ying Hua MA View
author publications You can also search for this author inPubMed Google Scholar * Jia Hua HU View author publications You can also search for this author inPubMed Google Scholar * Xiao Gang
ZHOU View author publications You can also search for this author inPubMed Google Scholar * Zhen Tong MEI View author publications You can also search for this author inPubMed Google Scholar
* Jian FEI View author publications You can also search for this author inPubMed Google Scholar * Li He GUO View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHORS Correspondence to Jian FEI or Li He GUO. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE MA, Y., HU, J., ZHOU, X. _et al._
_γ_-Aminobutyric acid transporter (GAT1) overexpression in mouse affects the testicular morphology. _Cell Res_ 10, 59–69 (2000). https://doi.org/10.1038/sj.cr.7290036 Download citation *
Received: 24 January 2000 * Revised: 15 February 2000 * Accepted: 16 February 2000 * Issue Date: 01 March 2000 * DOI: https://doi.org/10.1038/sj.cr.7290036 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative KEYWORDS * γ-aminobutyric acid transporter * testicular morphology * mouse