Impact of uncontrolled freezing and long-term storage of peripheral blood stem cells at −80 °c on haematopoietic recovery after autologous transplantation. Report from two centres
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

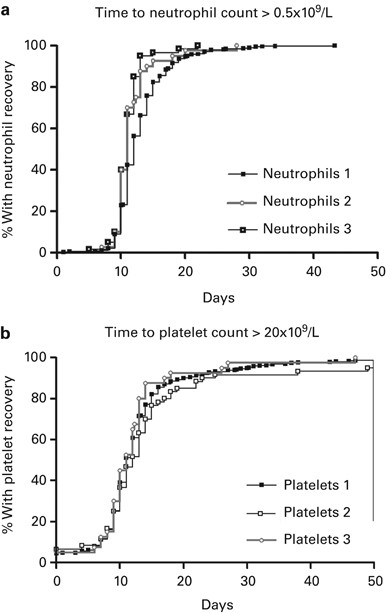
ABSTRACT Controlled-rate freezing and storage in vapour phase nitrogen are used by most transplantation teams for the cryopreservation and storage of peripheral blood haematopoietic stem
cells (PBSC). In this study, we analysed 666 autologous PBSC transplants after uncontrolled freezing and storage of PBSC at −80 °C. Statistical analysis showed that neutrophil recovery was
associated with both the infused CD34+ cell dose (_P_=0.01) and the post transplantation use of growth factors (_P_<0.001) and that platelet recovery was associated with the infused CD34+
cell dose (_P_<0.001) and with the diagnosis (_P_=0.02). We analysed three groups according to the duration of the cryopreservation period (less than 6 months, between 6 and 12 months or
more than 1 year). Haematopoietic recovery was not found to be adversely affected by longer storage at −80 °C. The haematopoietic recoveries of 50 pairs of sequential transplantations from
the same PBSC mobilization were analysed. Despite prolonged cryopreservation, there were no statistically significant differences in neutrophil (_P_=0.09) or platelet (_P_=0.22) recovery in
the second compared with the first transplant. In conclusion, the long-term storage of PBSC at −80 °C after uncontrolled-rate freezing is an easy and comparatively inexpensive
cryopreservation method that leads to successful haematopoietic recovery even after prolonged storage. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS LEUKOCYTAPHERESIS VARIABLES AND TRANSIT
TIME FOR ALLOGENEIC CRYOPRESERVED HPC: BETTER SAFE THAN SORRY Article 08 July 2022 NON-CRYOPRESERVED AUTOLOGOUS PERIPHERAL BLOOD STEM CELL TRANSPLANTATION FOR MULTIPLE MYELOMA AND LYMPHOMA
IN COUNTRIES WITH LIMITED RESOURCES: PRACTICE CONSIDERATIONS FROM THE WORLDWIDE NETWORK FOR BLOOD AND MARROW TRANSPLANTATION Article 07 October 2024 FRESH OR FROZEN GRAFTS FOR ALLOGENEIC
STEM CELL TRANSPLANTATION: CONCEPTUAL CONSIDERATIONS AND A SURVEY ON THE PRACTICE DURING THE COVID-19 PANDEMIC FROM THE EBMT INFECTIOUS DISEASES WORKING PARTY (IDWP) AND CELLULAR THERAPY
& IMMUNOBIOLOGY WORKING PARTY (CTIWP) Article 06 September 2023 REFERENCES * Tedder RS, FFuckerman MA, Goldstone AH, Hawkins AE, Fielding A, Briggs EM _et al_. Hepatitis B transmission
from contaminated cryopreservation tank. _Lancet_ 1995; 346: 137–140. Article CAS Google Scholar * Balint B, Ivanović Z, Petakov M, Taseski J, Jovcić G, Stojanović N _et al_. The
cryopreservation protocol optimal for progenitor recovery is not optimal for preservation of marrow repopulating ability. _Bone Marrow Transplant_ 1999; 23: 613–619. Article CAS Google
Scholar * Fleming KK, Hubel A . Cryopreservation of hematopoietic and non-hematopoietic stem cells. _Transfus Apher Sci_ 2006; 34: 309–315. Article CAS Google Scholar * Akkok CA, Liseth
K, Melve GK, Ersvaer E, Hervig T, Bruserud O . Is there a scientific basis for a recommended standardization of collection and cryopreservation of peripheral blood stem cell grafts?
_Cytotherapy_ 2011; 13: 1013–1024. Article Google Scholar * McCullough J, Haley R, Clay M, Hubel A, Lindgren B, Moroff G . Long-term storage of peripheral blood stem cells frozen and
stored with a conventional liquid nitrogen technique compared with cells frozen and stored in a mechanical freezer. _Transfusion_ 2010; 50: 808–819. Article Google Scholar * Stiff PJ,
Koester AR, Weidner MK, Dvorak K, Fisher RI . Autologous bone marrow transplantation using unfractionated cells cryopreserved in dimethylsulfoxide and hydroxyethyl starch without
controlled-rate freezing. _Blood_ 1987; 70: 974–978. CAS PubMed Google Scholar * Clark J, Pati A, McCarthy D . Successful cryopreservation of human bone marrow does not require a
controlled-rate freezer. _Bone Marrow Transplant_ 1991; 7: 121–125. CAS PubMed Google Scholar * Makino S, Harada M, Akashi K, Taniguchi S, Shibuya T, Inaba S _et al_. A simplified method
for cryopreservation of peripheral blood stem cells at −80 degrees C without rate-controlled freezing. _Bone Marrow Transplant_ 1991; 8: 239–244. CAS PubMed Google Scholar * Rosenfeld CS,
Gremba C, Shadduck RK, Zeigler ZR, Nemunaitis J . Engraftment with peripheral blood stem cells using noncontrolled-rate cryopreservation: comparison with autologous bone marrow
transplantation. _Exp Hematol_ 1994; 22: 290–294. CAS PubMed Google Scholar * Feremans WW, Bastin G, Moine FL, Ravoet C, Delville JP, Pradier O _et al_. Simplification of the blood stem
cell transplantation (BSCT) procedure: large volume apheresis and uncontrolled rate cryopreservation at −80 degrees C. _Eur J Haematol_ 1996; 56: 278–282. Article CAS Google Scholar *
Choi CW, Kim BS, Seo JH, Shin SW, Kim YH, Kim JS . Long-term engraftment stability of peripheral blood stem cells cryopreserved using the dump-freezing method in a −80 degrees C mechanical
freezer with 10% dimethyl sulfoxide. _Int J Hematol_ 2001; 73: 245–250. Article CAS Google Scholar * Kudo Y, Minegishi M, Itoh T, Miura J, Saito N, Takahashi H _et al_. Evaluation of
hematological reconstitution potential of autologous peripheral blood progenitor cells cryopreserved by a simple controlled-rate freezing method. _Exp Med_ 2005; 205: 37–43. Google Scholar
* Galmes A, Besalduch J, Bargay J, Matamoros N, Morey M, Novo A _et al_. A simplified method for cryopreservation of hematopoietic stem cells with −80 degrees C mechanical freezer with
dimethyl sulfoxide as the sole cryoprotectant. _Leuk Lymphoma_ 1995; 17: 181–184. Article CAS Google Scholar * Galmes A, Besalduch J, Bargay J, Matamoros N, Durán MA, Morey M _et al_.
Cryopreservation of hematopoietic progenitor cells with 5-percent dimethyl sulfoxide at −80 degrees C without rate-controlled freezing. _Transfusion_ 1996; 36: 794–797. Article CAS Google
Scholar * Galmes A, Besalduch J, Bargay J, Novo A, Morey M, Guerra JM _et al_. Long-term storage at −80 degrees C of hematopoietic progenitor cells with 5-percent dimethyl sulfoxide as the
sole cryoprotectant. _Transfusion_ 1999; 39: 70–73. Article CAS Google Scholar * Galmes A, Gutiérrez A, Sampol A, Canaro M, Morey M, Iglesias J _et al_. Long-term hematological
reconstitution and clinical evaluation of autologous peripheral blood stem cell transplantation after cryopreservation of cells with 5 and 10% dimethylsulfoxide at −80 degrees C in a
mechanical freezer. _Haematologica_ 2007; 92: 986–989. Article Google Scholar * Halle P, Tournilhac O, Knopinska-Posluszny W, Kanold J, Gembara P, Boiret N _et al_. Uncontrolled-rate
freezing and storage at −80 degrees C, with only 3.5-percent DMSO in cryoprotective solution for 109 autologous peripheral blood progenitor cell transplantations. _Transfusion_ 2001; 41:
667–673. Article CAS Google Scholar * Calvet L, Cabrespine A, Boiret-Dupré N, Merlin E, Paillard C, Berger M _et al_. Hematologic, immunologic reconstitution and outcome of 342 autologous
peripheral blood stem cell transplantations after cryopreservation in a −80 °C mechanical freezer and preserved less than 6 months. _Transfusion_ 2013; 53: 570–578. Article CAS Google
Scholar * Aird W, Labopin M, Gorin NC, Antin JH . Long-term cryopreservation of human stem cells. _Bone Marrow Transplant_ 1992; 9: 487–490. CAS PubMed Google Scholar * Motta MR, Benini
C, Bandini G, Gherlinzoni F, Miggiano MC, Calori E _et al_. Autologous bone marrow transplantation with marrow cryopreserved for ten years. _Bone Marrow Transplant_ 1993; 12: 177. CAS
PubMed Google Scholar * Spurr EE, Wiggins NE, Marsden KA, Lowenthal RM, Ragg SJ . Cryopreserved human haematopoietic stem cells retain engraftment potential after extended (5-14 years)
cryostorage. _Cryobiology_ 2002; 44: 210–217. Article Google Scholar * Watts MJ, Sullivan AM, Ings SJ, Barlow M, Devereux S, Goldstone AH _et al_. Storage of PBSC at −80 degrees C. _Bone
Marrow Transplant_ 1998; 21: 111–122. Article CAS Google Scholar * Katayama Y, Yano T, Bessho A, Deguchi S, Sunami K, Mahmut N _et al_. The effects of a simplified method for
cryopreservation and thawing procedures on peripheral blood stem cells. _Bone Marrow Transplant_ 1997; 19: 283–287. Article CAS Google Scholar * Kwiatkowski F, Girard M, Hacene K, Berlie
J . Sem: a suitable statistical software adaptated for research in oncology. _Bull Cancer_ 2000; 87: 715–721. CAS PubMed Google Scholar * Ayello J, Semidei-Pomales M, Preti R, Hesdorffer
C, Reiss RF . Effects of long-term storage at −90 degrees C of bone marrow and PBPC on cell recovery, viability, and clonogenic potential. _J Hematother_ 1998; 7: 385–390. Article CAS
Google Scholar * Muramaki M, Hara I, Miyake H, Yamada Y, Okada H, Kamidono S . Long-term cryopreservation of peripheral blood stem cells in patients with advanced germ cell tumors using the
dump-freezing method at −80 degrees C. _Oncol Rep_ 2003; 10: 1993–1998. PubMed Google Scholar * Hernandez-Navarro F, Ojeda E, Arrieta R, Rios-Rull P, Garcia-Bustos J, Quevedo E _et al_.
Hematopoietic cell transplantation using plasma and DMSO without HES, with non-programmed freezing by immersion in a methanol bath: results in 213 cases. _Bone Marrow Transplant_ 1998; 21:
511–517. Article CAS Google Scholar * Perez-Oteyza J, Bornstein R, Corral M, Hermosa V, Alegre A, Torrabadella M _et al_. Controlled-rate versus uncontrolled-rate cryopreservation of
peripheral blood progenitor cells: a prospective multicenter study. Group for Cryobiology and Biology of Bone Marrow Transplantation (CBTMO), Spain. _Haematologica_ 1998; 83: 1001–1005. CAS
PubMed Google Scholar * Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L _et al_. An analysis of engraftment kinetics as a function of the CD34 content of peripheral
blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. _Blood_ 1995; 86: 3961–3969. CAS Google Scholar * Montanari M, Capelli D, Poloni
A, Massidda D, Brunori M, Spitaleri L _et al_. A. Long-term hematologic reconstitution after autologous peripheral blood progenitor cell transplantation: a comparison between controlled-rate
freezing and uncontrolled-rate freezing at 80 degrees C. _Transfusion_ 2003; 43: 42–49. Article Google Scholar * Feugier P, Bensoussan D, Girard F, Alla F, Schuhmacher A, Latger-Cannard V
_et al_. Hematologic recovery after autologous PBPC transplantation: importance of the number of postthaw CD34+ cells. _Transfusion_ 2003; 43: 878–884. Article Google Scholar Download
references ACKNOWLEDGEMENTS No source of support (grants, equipment, drugs) was required to conduct this study. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Laboratoire d’hématologie,
Centre Hospitalier de Jolimont, Haine Saint Paul, Belgium G Detry, B Husson & L Boon-Falleur * Service de Thérapie cellulaire et Hématologie Clinique, Université d’Auvergne, CHU Estaing,
Clermont-Ferrand, France L Calvet, A Cabrespine, J O Bay & O Tournilhac * Service d’hématologie, Centre Hospitalier de Jolimont, Haine Saint Paul, Belgium N Straetmans, C Ravoet, H
Petre & A Delannoy * Centre régional de cancérologie et thérapie cellulaire pédiatrique, Centre de Biothérapie d’Auvergne, CHU Estaing, Clermont-Ferrand, France C Paillard, E Merlin
& P Halle Authors * G Detry View author publications You can also search for this author inPubMed Google Scholar * L Calvet View author publications You can also search for this author
inPubMed Google Scholar * N Straetmans View author publications You can also search for this author inPubMed Google Scholar * A Cabrespine View author publications You can also search for
this author inPubMed Google Scholar * C Ravoet View author publications You can also search for this author inPubMed Google Scholar * J O Bay View author publications You can also search for
this author inPubMed Google Scholar * H Petre View author publications You can also search for this author inPubMed Google Scholar * C Paillard View author publications You can also search
for this author inPubMed Google Scholar * B Husson View author publications You can also search for this author inPubMed Google Scholar * E Merlin View author publications You can also
search for this author inPubMed Google Scholar * L Boon-Falleur View author publications You can also search for this author inPubMed Google Scholar * O Tournilhac View author publications
You can also search for this author inPubMed Google Scholar * A Delannoy View author publications You can also search for this author inPubMed Google Scholar * P Halle View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to G Detry. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Detry, G., Calvet, L., Straetmans, N. _et al._ Impact of uncontrolled freezing and
long-term storage of peripheral blood stem cells at −80 °C on haematopoietic recovery after autologous transplantation. Report from two centres. _Bone Marrow Transplant_ 49, 780–785 (2014).
https://doi.org/10.1038/bmt.2014.53 Download citation * Received: 30 July 2013 * Revised: 16 January 2014 * Accepted: 31 January 2014 * Published: 31 March 2014 * Issue Date: June 2014 *
DOI: https://doi.org/10.1038/bmt.2014.53 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative