The rheostat in the membrane: bcl-2 family proteins and apoptosis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Apoptosis, a mechanism for programmed cell death, has key roles in human health and disease. Many signals for cellular life and death are regulated by the BCL-2 family proteins and
converge at mitochondria, where cell fate is ultimately decided. The BCL-2 family includes both pro-life (e.g. BCL-XL) and pro-death (e.g. BAX, BAK) proteins. Previously, it was thought that
a balance between these opposing proteins, like a simple ‘rheostat’, could control the sensitivity of cells to apoptotic stresses. Later, this rheostat concept had to be extended, when it
became clear that BCL-2 family proteins regulate each other through a complex network of bimolecular interactions, some transient and some relatively stable. Now, studies have shown that the
apoptotic circuitry is even more sophisticated, in that BCL-2 family interactions are spatially dynamic, even in nonapoptotic cells. For example, BAX and BCL-XL can shuttle between the
cytoplasm and the mitochondrial outer membrane (MOM). Upstream signaling pathways can regulate the cytoplasmic–MOM equilibrium of BAX and thereby adjust the sensitivity of cells to apoptotic
stimuli. Thus, we can view the MOM as the central locale of a dynamic life–death rheostat. BAX invariably forms extensive homo-oligomers after activation in membranes. However, recent
studies, showing that activated BAX monomers determine the kinetics of MOM permeabilization (MOMP), perturb the lipid bilayer and form nanometer size pores, pose questions about the role of
the oligomerization. Other lingering questions concern the molecular mechanisms of BAX redistribution between MOM and cytoplasm and the details of BAX/BAK–membrane assemblies. Future studies
need to delineate how BCL-2 family proteins regulate MOMP, in concert with auxiliary MOM proteins, in a dynamic membrane environment. Technologies aimed at elucidating the structure and
function of the full-length proteins in membranes are needed to illuminate some of these critical issues. SIMILAR CONTENT BEING VIEWED BY OTHERS MECHANISMS OF BCL-2 FAMILY PROTEINS IN
MITOCHONDRIAL APOPTOSIS Article 12 July 2023 APOPTOTIC PRIMING IS DEFINED BY THE DYNAMIC EXCHANGE OF BCL-2 PROTEINS BETWEEN MITOCHONDRIA AND CYTOSOL Article Open access 18 May 2022 LINC
COMPLEX PROTEIN NESPRIN-2 HAS PRO-APOPTOTIC ACTIVITY VIA BCL-2 FAMILY PROTEINS Article Open access 15 January 2024 FACTS * BH3-only proteins (e.g. BID, BIM and PUMA) promote MOM integration
of BAX/BAK, leading to MOMP, which typically commits the cell to death. * The cytoprotective activity of BCL-XL is exerted through interactions with BAX/BAK and BH3-only proteins. *
Non-activated BAX and BCL-XL cycle between cytosolic and MOM-associated states. In some cells, they can cause each other to retrotranslocate from the MOM back to the cytoplasm. However, in
other situations, BCL-XL stabilizes BAX association with the MOM. * MOM-resident proteins, both identified and unidentified, have been proposed to facilitate the MOM permeabilizing function
of BAX. OPEN QUESTIONS * How do BAX and BAK permeabilize the MOM? * How do the pro-survival family members, e.g. BCL-XL, protect against cell death? * How do BCL-2 family proteins function
together in the MOM? * Which MOM proteins facilitate retrotranslocation and permeabilization and by what mechanisms? RECENT RESULTS * Membrane-inserted BAX monomers control the kinetics of
permeabilization, induce the formation of lipidic pores and significantly distort phospholipid bilayers. * BAX activation in the MOM triggers the assembly of a non-BAX ‘catalyst’ complex,
which then facilitates BAX pore formation. * Some of the initial conformational events in BAX and BAK activation have been identified by structural studies. BAX and BAK can both be activated
directly by certain BH3-only proteins. For BAK, this activation involves BH3 domain binding to the ‘canonical groove’, but for BAX, activator BH3 domain binding occurs at a different
surface. * Survival signals can promote BAX retrotranslocation from the MOM to the cytoplasm (reducing the amount of BAX in the MOM); on the other hand, the loss of survival signals causes
enhanced MOM accumulation of BAX and sensitizes cells to death signals. APOPTOSIS AND BCL-2 FAMILY PROTEINS Apoptosis is an active, intracellular cell death program that is common to nearly
all higher eukaryotic cell types.1 As a counterbalance to cellular proliferation, this process helps to maintain cellular homeostasis. Consequently, its deregulation can seriously affect
health. Abnormally suppressed apoptosis has key roles in many human diseases, including tumor development and tumor cell resistance to chemotherapy. The relevance of apoptosis to human
health has propelled it to the forefront of biomedical research, with the hope that understanding its molecular mechanism will translate into new therapeutic approaches. Early discoveries
concerning the BCL-2 oncogene product, in particular that it suppresses cell death, counteracts the pro-apoptotic action of mitochondria and represents a family of anti- and pro-apoptotic
proteins,2, 3, 4, 5, 6 provided some of the first insights into the molecular basis of programmed cell death and paved the way for subsequent studies that shape our current understanding of
apoptosis. It is now well established that the BCL-2 family proteins are the central regulators of the mitochondrial cell-intrinsic apoptotic pathway. Although many kinds of stresses can
induce apoptosis via diverse signaling pathways, these signals often converge at the mitochondrial outer membrane (MOM), where cell fate is ultimately decided. BCL-2 family proteins can have
functions at non-mitochondrial sites (e.g. the endoplasmic reticulum). Nevertheless, perhaps the most prominent roles of these proteins are to promote or inhibit MOM permeabilization
(MOMP), serving as a molecular ‘rheostat’ that regulates the mitochondrial pathway to apoptosis.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 MOMP induced by BAX and BAK allows
cytotoxic proteins to escape from the mitochondrial intermembrane space into the cytoplasm, leading to caspase activation and apoptosis. MOMP also leads to progressive mitochondrial
dysfunction, which causes energy depletion and cell death even when caspases are inactive. This means that cells can recover from MOMP only under exceptional circumstances.22, 23, 24 Even in
cases where apoptosis is initiated by ligation of death receptors, through the extrinsic pathway, the upstream signals often require amplification by the mitochondrial pathway. Thus, MOMP
is frequently the decisive event preceding cell death. BCL-2 family proteins are distinguished by four conserved BCL-2 homology (BH1–BH4) domains. In particular, the BH3 domain is invariably
present and has an essential role in regulating cell death. BCL-2 family proteins engage in a complex network of heterodimeric interactions that collectively make life and death decisions
for the cell. Anti-apoptotic family members (e.g. BCL-2 and BCL-XL) protect the cell against apoptotic stimuli, whereas pro-death proteins (BAX and BAK – one or the other is needed for
apoptosis) actively kill the cell by inducing MOMP. Their functions are regulated by a third class of BCL-2 family members, the BH3-only proteins. One such BH3-only protein, BID, provides a
key link between the extrinsic and mitochondrial pathways to cell death because it is activated when extracellular stress cues, transmitted through cell surface death receptors, activate the
protease Caspase-8, which cleaves BID into an inactive N-terminal fragment and a critical pro-death C-terminal fragment (tBID). tBID and the other BH3-only proteins BIM and PUMA have two
main functions: first, they can form stable heterodimers with anti-apoptotic family members such as BCL-XL, neutralizing their protective activity, and second, they can transiently interact
with BAX and BAK, directly activating them.10, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 Other BH3-only proteins (e.g. BAD) can only suppress anti-apoptotic family members.
The anti-apoptotic family members act in two ways: to antagonize the multidomain proteins BAX and BAK and to sequester the BH3-only proteins. WHAT REGULATES THE INTERPLAY OF SOLUBLE
CYTOPLASMIC BCL-2 FAMILY PROTEINS WITH MEMBRANES? Structural and biochemical studies have identified some of the early events in BAX and BAK occurring upon interaction with BH3 domain
peptides.35, 36, 38, 39 However, interactions among full-length BCL-2 family proteins normally take place in the membrane environment. This is obviously true for BAK, which is permanently
membrane-resident. Nevertheless, some experimental alterations of the BAK molecule increase its solubility or decrease its MOM-association, even causing it to be inducibly MOM-translocated,
much like BAX.40 With regard to BAX, experiments in protein-free, cardiolipin-containing liposomes showed that tBID becomes recruited to the membrane before BAX,41 suggesting that initially,
MOM-associated tBID might promote BAX accumulation in the MOM by evoking a conformational change needed for membrane integration. The MOM protein Mtch2 has been proposed as a tBID-binding
receptor in the MOM.42 However, a recent study43 has shown that Mtch2 accelerates the separation of the N- and C-terminal fragments of Caspase-8-cleaved BID and promotes a complex process of
tBID integration in the membrane. This would then trigger BAX recruitment to the MOM and activation. However, activation by tBID and other BH3-only proteins appears not to be the only
trigger for BAX translocation. Recent studies in epithelial cells44 confirmed other studies in fibroblasts45, 46 showing that, under nonapoptotic conditions, BAX exists in equilibrium
between cytoplasmic and MOM-associated forms. A recent study by Schellenberg _et al._44 now shows that in epithelial cells, detachment from the extracellular matrix causes a loss of
adhesion-dependent survival signaling; somehow, this reduces the rate of BAX membrane dissociation, thereby shifting the equilibrium in favor of MOM localization. However, if cells are
allowed to reattach before they become committed to apoptosis, MOM-associated BAX can move back to the cytoplasm. This reversibility of MOM localization suggests that BAX can be stably
associated with mitochondria even in a non-activated state. Together, the retrotranslocation studies show that at least one form of BAX recruitment to the MOM does not appear to depend on
full apoptotic activation by BH3-only proteins. How BAX reversibly associates with the MOM is still not understood. As the BAX S184V mutant displays a lower rate of dissociation from the
MOM, Schellenberg _et al._44 suggest that the C-terminal _α_-helix of wt BAX can interact weakly with the membrane. _In vitro_, BAX does interact transiently with liposomes, causing it to
display the 6A7 epitope.47 This membrane-induced BAX conformational change is not inhibited by BCL-XL. Mixing BAX alone with liposomes does not lead to BAX oligomerization or even to
measurable BAX membrane association, as measured by a float-up assay. For stable BAX integration, full activation (e.g. by tBID) is required. In principle, membrane integration, which would
involve at least the hydrophobic C terminus and potentially other regions of this protein, is likely irreversible, as the energy barrier for removal of a transmembrane helix from the
membrane to water would be very high. Within cells, the MOM-association equilibrium could instead involve a reversible interaction of BAX with resident MOM proteins, especially as
adhesion-dependent signaling alters this equilibrium. We can well imagine that BAX (retro-)translocation and activation in whole cells involves multiple steps and still-unknown mechanisms.
Anoikis in mammary epithelial cells involves an hours-long interval between BAX translocation to mitochondria and BAX activation. In this setting, instead of the previously reported ability
of BCL-XL and BAX to retrotranslocate each other,45 BCL-XL stabilizes BAX association with the MOM. Perhaps this reflects co-integration of these two proteins, as in the ‘embedded together’
scenario previously described by Andrews _et al._14 Similarly, Llambi _et al._48 found that when BAX-induced MOMP is blocked by BCL-XL, both proteins become membrane-integrated. We do not
understand what determines co-integration _versus_ co-retrotranslocation. In particular, the structural details of how these proteins interact in the membrane environment are yet unknown.
However, one recent study dealt with the contribution of the C-terminal helix of BCL-XL, which is necessary for the ability of BCL-XL to retrotranslocate BAX.46 Interestingly, replacing the
C terminus of BCL-XL with the corresponding portion of BAX retained the property of the chimeric molecule to interact reversibly with the MOM, but eliminated the ability to retrotranslocate
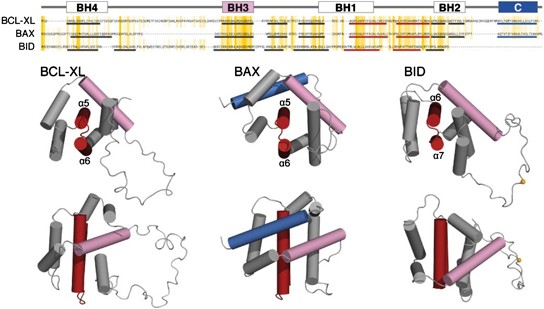
BAX. MOLECULAR BASIS FOR BCL-2 FAMILY PROTEIN FUNCTION The molecular structures of BCL-XL,49 BAX50 and BID,51, 52 all determined in solution by NMR, provided the initial framework for
understanding the functions and interactions of the BCL-2 family proteins. Despite their opposing functions, all three proteins adopt a common fold, with two central, more hydrophobic
_α_-helices forming a hairpin surrounded by six to eight more polar _α_-helices (Figure 1). This structure appears to be shared by all other pro- and anti-apoptotic BCL-2 family members.53,
54 The prototypical BCL-2 fold also resembles that of the pore-forming domains of bacterial toxins, suggestive of a common pore-forming function in which the two central helices (_α_5-_α_6
in BAX) are likely important for membrane insertion.55, 56, 57 The BH1, BH2 and BH3 domains form a hydrophobic groove on the protein surface that is conserved across both anti- and
pro-apoptotic BCL-2 family members and is crucial for mediating their interactions. In BCL-XL, this canonical groove (binding cleft) serves as the binding site for the BH3 domains of its
pro-apoptotic BCL-2 partners, including BAX/BAK and BID.58 This interaction produces a stable complex that is thought to protect against cell death by effectively sequestering the death
agonists. In the cytosolic form of BAX, however, the groove is normally occupied by BAX’s own hydrophobic C-terminal helix (_α_9), and certain BH3-only proteins (BID, BIM and perhaps PUMA)
appear to interact with a different BAX site, diametrically opposed to the canonical BH3-binding groove.35 This interaction is weak, but it is needed to trigger BAX activation. BAK lacks
this alternative site and appears to be activated by binding the BH3 domain of either BID or BIM to its canonical groove.38, 39, 59 Recent structural studies show that truncated BAXΔC and
BAKΔC (lacking the hydrophobic C-terminal helix) can bind activator BH3 peptides through their respective BH3-binding clefts.39, 60 BAXΔC treated with the detergent CHAPS and BID, BIM or BAX
BH3 peptides forms a domain-swapped dimer60 similar to that formed by BCL-XLΔC exposed to high pH or heat.61, 62 Dimers induced by the detergent octylmaltoside yielded indistinguishable
structures without the bound peptide. In these domain-swapped dimers, the _α_5–_α_6 helices of each monomer form one long extended helix and interact with the _α_6–_α_5 helices of the second
monomer, forming a planar surface that is proposed to engage the membrane. A mutant form of BAK, rendered soluble by mutagenesis of its C terminus, also binds BH3 peptides through its
surface cleft,38 as does a highly truncated form of BAX spanning only helices _α_2 to _α_5.60 BH3 ligand association with the BAX or BAK surface cleft is distinct from its interaction with a
diametrically opposed BH3 interaction site observed by Gavathiotis _et al._35 for intact, monomeric BAX in solution, suggesting that BAX and BAK may each be activated by direct interaction
with a BH3 ligand at distinct molecular sites, and that activation likely involves multiple steps.63 All of these studies suggest very interesting hypotheses about BCL-2 protein function.
However, because they were performed with extensively truncated proteins, in the presence of detergents and far removed from the natural environment of the phospholipid bilayer membrane –
conditions that can produce distortions in many integral membrane and membrane-associated proteins64 – it is unclear where and how these structures fit into the apoptosis pathways.
Cell-based and biophysical studies focused on BAK and BAX activation indicate that the BH3–groove interaction is essential for BAK65 and BAX66 oligomerization. However, the structures of the
domain-swapped dimers appear at odds with data on the interfaces in BAX oligomers formed during apoptosis66, 67, 68 and incompatible with a model in which helices _α_5 and _α_6 insert into
the membrane during permeabilization. The similarity of the BAXΔC structures with pro-survival proteins, together with the facts that BAX inserts in membranes can form membrane pores as a
monomer and is likely to unfold on the membrane (see below), indicate that swapped-dimer structures may not be directly involved in the activation pathway. However, they may represent some
off-pathway conformations, possibly related to the reversible retrotranslocation process. The latter would be an attractive possibility because the blockage of helices _α_5 and _α_6 through
the domain-swapping mechanism would prevent insertion of these helices into the membrane and thus prevent the subsequent, irreversible MOMP. Structural studies on full-length proteins in the
presence of a fully functional membrane bilayer environment will be needed to obtain a full picture of BAX/BAK activation. HOW DOES THE BCL-2 FAMILY REGULATE MOMP? For MOMP to occur, BAX
and BAK require direct activation by a ‘direct activator’ BH3-only protein, for example, BID, BIM or PUMA.34, 35, 36, 38, 39, 59, 69, 70 Anti-apoptotic BCL-2 family proteins can block MOMP
both by sequestering BH3-only proteins and by neutralizing BAX/BAK. This inhibitory cytoprotective function can in turn be neutralized by an excess of ‘indirect activator’ BH3-only proteins
(e.g. BAD), which bind the anti-apoptotic BCL-2 proteins, thereby liberating the pro-apoptotic direct activator BH3-only proteins. Some chemotherapeutic drugs currently under development
(e.g. ABT-263, Navitoclax) act in a similar manner to antagonize the interactions of BCL-XL and other anti-apoptotic relatives with their pro-apoptotic partners, thereby promoting
apoptosis.71, 72 After BAX activation via a transient hit-and-run interaction with a direct activator BH3-only protein such as tBID, BAX typically translocates from its soluble cytoplasmic
state to a MOM-integrated state. BAK is constitutively membrane-resident, but also becomes activated by BH3-only proteins in a similar manner. Activation of BAX and BAK leads to complete
membrane insertion of these proteins and then to MOMP, which generally commits the cell to death. The early stages of BAX and BAK activation have been analyzed elegantly by structure-guided
mutational analysis.35, 36, 38, 39 However, as the structures of membrane-inserted BAX and BAK are not known, the exact conformational changes in these proteins associated with MOMP activity
are still unclear. Indeed, the limited structural information that is available for the membrane-associated states of BCL-XL, BAX and tBID50, 73, 74, 75, 76, 77, 78 provide only a glimpse
of how BCL-2 proteins interact with membranes. tBID has been shown to adopt a stable helical conformation in lipids, with its helices parallel to the membrane surface and no transmembrane
helix insertion.75, 79 BAX-derived peptides corresponding to _α_5–_α_6 readily insert into membranes and mediate the formation of protein-lipidic pores.80, 81, 82, 83 The conformation of
BCL-XL in lipid bilayers may involve membrane embedding of the BCL-XL helical hairpin (_α_5-_α_6) as well as helix _α_1.73, 74 However, these results were obtained using truncated BCL-XL
lacking the hydrophobic C terminus (BCL-XLΔC), or using detergents to either solubilize or activate a conformational change, manipulations that can significantly distort protein structure
and function.64 Indeed, a number of recent reports have demonstrated the importance of studying membrane-associated proteins, including BCL-2 family proteins, in a lipid membrane
environment.84, 85 BAX MONOMERS ARE A DRIVING FORCE FOR MEMBRANE PERTURBATION Major conformational rearrangements and extensive homo-oligomerization of BAX and BAK are widely assumed to be
necessary for pore formation and MOMP.50 However, a detailed spatiotemporal molecular mechanism for these events remains elusive. Until recently, it has not been possible experimentally to
separate BAX/BAK oligomerization from the pore-formation activity involved in MOMP, and so researchers have assumed that oligomerization is a requirement. However, two independent
investigations have now converged on the notion that integrated BAX monomers are sufficient to perturb the lipid bilayer. In recent electron cryomicroscopy (cryo-EM) studies, Volkmann and
co-workers78 dissected the structural correlates of BAX membrane insertion, in a situation where oligomerization is physically impossible. This was accomplished through the use of
nanometer-scale phospholipid bilayer islands (nanodiscs), each capable of accommodating only a single BAX molecule.78 The three-dimensional reconstructions of these membrane assemblies
(Figure 2) demonstrate that in the presence of a BID BH3 peptide, an individual BAX molecule can insert into the nanodisc membrane, perturb the lipid bilayer and form a pore of ∼3.5 nm in
diameter. While the precise structure of this BAX pore will have to be determined, its monomeric state is dictated by the small, discrete size of the lipid bilayer nanodiscs, which can only
accommodate a single membrane-inserted BAX. Native gels, fluorescence and immunoblotting data all confirmed that BAX was monomeric in the nanodisc under the conditions used for imaging.
Interestingly, the three-dimensional structure of this full-length BAX-membrane assembly contained no discernible, compact, extra globular density that would account for a folded BAX monomer
globular structure associated with the nanodiscs (Figure 2). This is in stark contrast to other recent nanodisc studies where integrated proteins such as integrin receptors, anthrax toxin
or SNARE proteins are clearly visible.86, 87, 88 This observation suggests that BAX is unfolded on top of the nanodisc surface (Figure 3), presumably with its amphiphilic portions inserted
into the nanodiscs in a similar way to the ‘umbrella model’ proposed for colicin, where the outer helices of the globular structure rearrange to form the ‘umbrella’ resting on the membrane
surface, while the central hydrophobic helical hairpin (corresponding to _α_5–_α_6 in BAX) inserts across the membrane to form the ‘umbrella handle’.89, 90, 91 Such unfolding is consistent
with the finding that a substantial structural reorganization of BAX occurs during BH3-triggered activation.36 In contrast to these small BAX monomer-induced pores, Bax-dependent
permeabilization of the MOM involves a much greater permeability of the membrane, allowing the efflux of macromolecules such as Smac/DIABLO dimers or very large dextran molecules.10 How this
permeabilization occurs is a key question. Judging from the sizes of the released macromolecules, up to 2000 kD, we can infer that MOMP involves a large-scale rearrangement of the membrane.
Paradoxically, however, traditional EM methods did not reveal any alterations in the MOM.10 This suggested the possibility that MOMP arises not from the formation of fixed proteinaceous
pores but rather from pores that are lipidic (and not easily preserved during embedding for EM). Indeed, when Bax-induced pores in small unilamellar vesicles92 and in bacteria93 were later
imaged using gentler cryo-EM techniques, they were seen to be large and heterogeneous (25–100 nm), much like those produced by bacterial toxins. Similar large Bax-induced pores have now been
imaged in purified MOMs by cryo-EM (Newmeyer and Kuwana, personal communication). These and other observations80, 82, 94 argue that MOMP is the result of lipidic pore formation. Indeed, the
pores produced in nanodiscs by single Bax molecules are already lipidic in nature. The number of helices that can conceivably insert into the membrane from a single BAX molecule (four) is
too small to allow complete protein lining of a 3.5-nm diameter pore78 (Figure 3). The formation of lipidic pores induced by BAX monomers in the initial stages of MOMP is an attractive
alternative to models where the initial pores are protein-lined and formed by ordered BAX oligomers. The minimal protein requirement of monomer-induced pores potentially favors faster
induction of the pro-death signaling cascade, similar to the efficient process implemented by bacterial toxins. The joining of the initial monomer-induced lipidic pores at later stages of
MOMP to increase pore size can be achieved with far less conformational adaption than the joining of protein-lined pores would require. Independent evidence from biochemical kinetic analysis
now implies that Bax monomers are important for the formation even of large protein-conducting pores. Newmeyer and co-workers95 showed that MOMP kinetics are linearly dependent on BAX
concentration and show neither cooperativity nor saturability relative to BAX (Figures 4 and 5). This implies that BAX monomers, not oligomers, drive the kinetics of pore formation.
Together, the results of the nanodisc cryo-EM and kinetic experiments argue that monomeric membrane-inserted BAX is the key functional unit responsible for initiating MOM pore formation, MOM
destabilization and MOMP, leading to apoptosis. How do the small membrane perturbations produced by individual Bax monomers lead to the formation of supramolecular pores? The mechanism is
still unclear. One possibility is that an abundance of integrated BAX monomers in a local membrane region could produce enough curvature stress to destabilize the membrane. Several reports
have argued that curvature stress is the ultimate mechanism promoting the formation of protein-conducting membrane pores, and quite possibly the process can involve a cooperation between
protein and lipid components to sculpt the membrane.94, 96, 97, 98, 99, 100 Indeed, in cryo-EM images, Bax-induced pores in liposomes92 and MOM vesicles (Kuwana and Newmeyer, personal
communication) are frequently adjoined by negatively curved membrane regions. This strongly suggests a pore formation or enlargement mechanism involving curvature stress. An alternative
model was proposed by Martinez _et al._101 after observing a BAX-dependent stepwise opening of high-conductance membrane channels. Possibly, integrated BAX monomers could cluster together in
a quantum fashion to form ever-larger pores ringed by BAX molecules. These models need not be mutually exclusive. Cryo-EM imaging of extended lipid bilayer membranes sufficiently large to
accommodate several BAX molecules, together with additional structure determination approaches, will ultimately be needed to determine the membrane-associated structures of membrane-embedded
BAX oligomers at a high resolution. Imaging of colloidal gold-labeled BAX molecules in the MOM, if feasible, might also provide information about the organization of BAX molecules around
the lipidic pores. How BAX oligomerization might have a role in this process is still unclear. Based on the universal finding so far that BAX/BAK mutants blocking oligomerization also block
MOMP, it is tempting to conclude that oligomerization is required for pore formation. One common model is that the pores are enlarged through a process of Bax autoactivation, in which BAX
oligomers at the pore rim catalyze the integration of free BAX monomers and their addition to the ends of existing oligomers. However, to explain the linear response of MOMP kinetics to BAX
concentrations, the effect of oligomerization, if any, would have to be non-rate-limiting, that is, very rapid.95 This is formally possible. Nevertheless, a requirement for oligomerization
has not been proven. Although oligomerization is an inevitable byproduct of _α_-helix insertion, it might not contribute to membrane destabilization, but perhaps only to the autoactivation
of free Bax monomers. Oligomers could have a function different from pore formation, for example, to regulate mitochondrial dynamics. Alternatively, oligomers could be inert or even
inhibitory to pore formation. In this way, oligomers could act as a brake to limit the runaway amplification of a sub-threshold apoptotic signal. If so, cell death would require the
sustained production of integrated BAX/BAK monomers to promote pore formation and enlargement. As mentioned, BAX by itself can form pores in liposome membranes, upon activation by a
BH3-domain peptide or a BH3-only protein. However, to what extent this intrinsic activity explains MOM permeabilization in apoptotic cells remains unclear. Highlighting this uncertainty, the
recent study by Kushnareva _et al._95 showed that native MOMs are more responsive to low concentrations of BAX than liposomes and display more complex, biphasic permeabilization kinetics
(Figure 5). This enhanced BAX response requires the participation of heat-labile MOM proteins. Furthermore, these authors found that, in order to fit the measured kinetic curves, it was
necessary to consider a two-stage reaction scheme. In the first stage, activated BAX triggers the assembly of a non-Bax oligomeric complex. In the second stage, this complex catalyzes the
BAX-dependent formation of membrane pores. Interestingly, the catalyst formation reaction exhibited a membrane phase-like transition near 28 °C, suggesting that it involves a localized
membrane-remodeling event. The behavior of this catalyst complex is reminiscent of the membrane-distorting activities of Drp1 and Bif-1, two proteins previously proposed to have roles in
MOMP.99, 102, 103, 104 However, Kushnareva _et al._95 found no evidence supporting a role for these proteins in Bax-induced permeabilization of isolated MOM vesicles. Chemical compounds
(mDivi-1 analogs) that had been shown to inhibit Drp1’s yeast ortholog, Dnm1, also inhibit MOMP in mammalian cells,105 and some mDivi-1 analogs blocked BAX-induced pore formation in rat
liver and Xenopus MOM vesicles. Kushnareva _et al._95 were unable to detect Drp1 in their MOM preparations, suggesting that the catalyst protein whose existence was inferred by their studies
is different from Drp1. Although Bif-1 was weakly detectable in rat liver MOMs, it was below the limits of immunoblot detection in Xenopus egg MOM vesicles, which display the same type of
MOMP kinetics as rat liver MOM vesicles. Naturally, these results do not exclude the possibility that Drp1 or Bif-1 participate in MOMP within cells, as these proteins could be recruited
from the cytoplasm. However, the cell-free studies argue that Drp1 and Bif-1 are not essential for MOMP and that another unidentified MOM-resident protein catalyzes BAX-induced MOMP. In
general, the auxiliary roles of non-BCL-2 family proteins in MOMP remain to be clarified. Nevertheless, the simplest hypothesis is that such proteins facilitate BAX’s intrinsic pore-forming
function. HOW DOES BCL-XL PROTECT AGAINST CELL DEATH? BCL-XL protects cells both by interacting with BAX to prevent its membrane integration and by interacting with BID and other BH3-only
proteins, to sequester their pro-death activity. The C terminus of BCL-XL contains a clear MOM-targeting motif and shares sequence homology with those of BAX, BAK and other MOM-targeted
BCL-2 proteins. The lipid bilayer membrane strongly influences BCL-2 protein–protein interactions,20, 48, 84, 106 and several lines of evidence show that the hydrophobic C terminus of BCL-XL
is important for regulating both the interaction of BCL-XL with its partners and its membrane integration. However, most structural studies have focused on truncated forms of BCL-XL lacking
the hydrophobic C terminus, to promote solubility, and little is known about how BCL-XL interacts with the membrane or with membrane-associated BAX and BID. Solid-state NMR studies suggest
an ‘umbrella’ model of BCL-XL membrane integration,74 as proposed for colicins.89, 90, 91 However, the studies with BCL-XL were performed with truncated protein, and the presence of the C
terminus is likely to have a significant effect on the membrane-associated conformation of this protein. The structure of BAX is the only one determined for an intact, multidomain BCL-2
protein including the hydrophobic C terminus.50 In solution, this membrane-targeting module folds into BAX’s BH3-binding cleft, suggesting that it may act as a regulatory switch for
intermolecular interactions. Recent structural studies with moderately truncated forms of anti-apoptotic BCL-W indicate that it also may adopt a similar conformation.107, 108 Thus, the C
terminus of BCL-XL is also likely to interact with the BH3-binding cleft, with important consequences for the interactions of BCL-XL with its partners and with membranes. Recently, BCL-XL
and BAX have been reported to cycle between cytosolic and MOM-associated states, and each protein can cause the other to retrotranslocate from the MOM back to the cytoplasm.45, 46 The
mechanism of retrotranslocation is still unknown. The simplest hypothesis is that the mutual membrane repulsion of BCL-XL and BAX results from the intrinsic interactions of these proteins in
the membrane. Although the presence of an activating BH3-only protein would be required for the full integration of BAX in the membrane, even the unactivated BAX molecule can interact
weakly with the membrane, perhaps through its C terminus.47, 109 If so, retrotranslocation may then result from competitive displacement of this weak membrane interaction of the Bax C
terminus by interaction with the hydrophobic groove of a BCL-XL molecule (which would be exposed upon the transient interaction of this BCL-XL molecule with the membrane). Afterwards, the
resulting BAX–BCL-XL heterodimer would dissociate from the membrane and then further dissociate into soluble monomers. Similarly, retrotranslocation of BCL-XL could involve a similar
displacement of its C-terminal weak membrane interaction by the hydrophobic groove of Bax. In the presence of a BH3-only protein such as tBID, the situation is different, as BAX and BCL-XL
can become irreversibly membrane-integrated. In this situation, how BCL-XL acts is still unclear, as in some cases BCL-XL can block the membrane integration of BAX, while in other cases,
BCL-XL and BAX can both be membrane-integrated. For example, in the nanodisc cryo-EM study, Volkmann _et al._78 found that BCL-XL abolished the integration of BAX into nanodisc membranes in
the presence of BID BH3 peptides. This agrees with other work10, 34, 95, 110, 111, 112 showing that BCL-XL or BCL-2 can block membrane recruitment and integration of BAX, even in the
presence of a BAX-activating signal. In other studies, membrane integration of both BCL-XL and BAX (i.e. the ‘embedded together’ scenario14) was observed.44, 48 Perhaps co-integration
requires a balanced stoichiometry of BCL-XL and BAX.113 Alternatively, it could require the participation of a MOM protein that facilitates insertion of BAX and BCL-XL, similar to the
recently reported role of Mtch2 in facilitating the membrane integration of tBID.43 OUTLOOK Understanding the process of BAX/BAK-dependent membrane pore formation remains a Holy Grail of the
apoptosis field. Recent publications significantly advance our understanding of the mitochondrion-dependent intrinsic pathway to apoptosis, but also emphasize the need for high-resolution
structural studies aimed at determining the molecular conformations and interactions of key BCL-2 family proteins without truncations and within a membrane environment. This kind of detailed
molecular understanding is needed to define the intrinsic activities of this protein family and also to facilitate analysis of the roles of cofactor proteins, once these are identified. The
evidence is overwhelming that the membrane environment is critical for preserving the structural and functional integrity of both integral and membrane-associated proteins. This is entirely
consistent with Anfinsen’s hypothesis that protein conformation ‘is determined by the totality of inter-atomic interactions and hence by the amino acid sequence in a given environment’.109
For membrane-associated proteins such as the BCL-2 family proteins, the ‘given environment’ of the phospholipid bilayer is essential for preserving native structure and function.
ABBREVIATIONS * MOM: mitochondrial outer membrane * MOMP: MOM permeabilization REFERENCES * Kerr JF, Wyllie AH, Currie AR . Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. _Br J Cancer_ 1972; 26: 239–257. CAS PubMed PubMed Central Google Scholar * Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM . Cloning of the
chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. _Science_ 1984; 226: 1097–1099. CAS PubMed Google Scholar * Vaux DL, Cory S, Adams JM . Bcl-2 gene
promotes haemopoietic cell survival and cooperates with c-Myc to immortalize pre-B cells. _Nature_ 1988; 335: 440–442. CAS PubMed Google Scholar * Boise LH, Gonzalez-Garcia M, Postema CE,
Ding L, Lindsten T, Turka LA _et al_. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. _Cell_ 1993; 74: 597–608. CAS PubMed Google Scholar *
Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ . Bcl-2 functions in an antioxidant pathway to prevent apoptosis. _Cell_ 1993; 75: 241–251. CAS PubMed Google Scholar *
Newmeyer DD, Farschon DM, Reed JC . Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. _Cell_ 1994; 79:
353–364. CAS PubMed Google Scholar * Korsmeyer SJ . Regulators of cell death. _Trends Genet_ 1995; 11: 101–105. CAS PubMed Google Scholar * Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer
DD . The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. _Science_ 1997; 275: 1132–1136. CAS PubMed Google Scholar * Yang J, Liu X, Bhalla K,
Kim CN, Ibrado AM, Cai J _et al_. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. _Science_ 1997; 275: 1129–1132. CAS PubMed Google Scholar * Kuwana
T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R _et al_. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. _Cell_ 2002; 111:
331–342. CAS PubMed Google Scholar * Kuwana T, Newmeyer DD . Bcl-2-family proteins and the role of mitochondria in apoptosis. _Curr Opin Cell Biol_ 2003; 15: 691–699. CAS PubMed Google
Scholar * Danial NN, Korsmeyer SJ . Cell death: critical control points. _Cell_ 2004; 116: 205–219. CAS PubMed Google Scholar * Adams JM, Cory S . Bcl-2-regulated apoptosis: mechanism
and therapeutic potential. _Curr Opin Immunol_ 2007; 19: 488–496. CAS PubMed PubMed Central Google Scholar * Leber B, Lin J, Andrews DW . Embedded together: the life and death
consequences of interaction of the Bcl-2 family with membranes. _Apoptosis_ 2007; 12: 897–911. CAS PubMed PubMed Central Google Scholar * Chipuk JE, Green DR . How do BCL-2 proteins
induce mitochondrial outer membrane permeabilization? _Trends Cell Biol_ 2008; 18: 157–164. CAS PubMed PubMed Central Google Scholar * Degterev A, Yuan J . Expansion and evolution of
cell death programmes. _Nature reviews_ 2008; 9: 378–390. CAS PubMed Google Scholar * Reed JC . Bcl-2-family proteins and hematologic malignancies: history and future prospects. _Blood_
2008; 111: 3322–3330. CAS PubMed PubMed Central Google Scholar * Youle RJ, Strasser A . The BCL-2 protein family: opposing activities that mediate cell death. _Nat Rev Mol Cell Biol_
2008; 9: 47–59. CAS PubMed Google Scholar * Yao Y, Marassi FM . BAX and BAK caught in the act. _Mol Cell_ 2009; 36: 353–354. CAS PubMed PubMed Central Google Scholar * Leber B, Lin J,
Andrews DW . Still embedded together binding to membranes regulates Bcl-2 protein interactions. _Oncogene_ 2010; 29: 5221–5230. CAS PubMed PubMed Central Google Scholar * Martinou JC,
Youle RJ . Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. _Dev Cell_ 2011; 21: 92–101. CAS PubMed PubMed Central Google Scholar * Colell A, Ricci JE, Tait S,
Milasta S, Maurer U, Bouchier-Hayes L _et al_. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. _Cell_ 2007; 129: 983–997.
CAS PubMed Google Scholar * Lartigue L, Kushnareva Y, Seong Y, Lin H, Faustin B, Newmeyer DD . Caspase-independent mitochondrial cell death results from loss of respiration, not cytotoxic
protein release. _Mol Biol Cell_ 2009; 20: 4871–4884. CAS PubMed PubMed Central Google Scholar * Tait SW, Parsons MJ, Llambi F, Bouchier-Hayes L, Connell S, Munoz-Pinedo C _et al_.
Resistance to caspase-independent cell death requires persistence of intact mitochondria. _Dev Cell_ 2010; 18: 802–813. CAS PubMed PubMed Central Google Scholar * Wang K, Yin XM, Chao
DT, Milliman CL, Korsmeyer SJ . BID: a novel BH3 domain-only death agonist. _Genes Dev_ 1996; 10: 2859–2869. CAS PubMed Google Scholar * Gross A, Yin XM, Wang K, Wei MC, Jockel J,
Milliman C _et al_. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. _J Biol
Chem_ 1999; 274: 1156–1163. CAS PubMed Google Scholar * Li H, Zhu H, Xu CJ, Yuan J . Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. _Cell_
1998; 94: 491–501. CAS PubMed Google Scholar * Luo X, Budihardjo I, Zou H, Slaughter C, Wang X . Bid a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in
response to activation of cell surface death receptors. _Cell_ 1998; 94: 481–490. CAS PubMed Google Scholar * Eskes R, Desagher S, Antonsson B, Martinou JC . Bid induces the
oligomerization and insertion of Bax into the outer mitochondrial membrane. _Mol Cell Biol_ 2000; 20: 929–935. CAS PubMed PubMed Central Google Scholar * Korsmeyer SJ, Wei MC, Saito M,
Weiler S, Oh KJ, Schlesinger PH . Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. _Cell Death Differ_ 2000; 7:
1166–1173. CAS PubMed Google Scholar * Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T _et al_. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and
BAK-mediated mitochondrial apoptosis. _Mol Cell_ 2001; 8: 705–711. CAS PubMed Google Scholar * Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA _et al_. A distinct pathway
remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. _Dev Cell_ 2002; 2: 55–67. CAS PubMed Google Scholar * Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds
MG _et al_. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. _Mol Cell_ 2005; 17: 393–403. CAS PubMed Google Scholar
* Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR _et al_. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane
permeabilization both directly and indirectly. _Mol Cell_ 2005; 17: 525–535. CAS PubMed Google Scholar * Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG _et al_. BAX
activation is initiated at a novel interaction site. _Nature_ 2008; 455: 1076–1081. CAS PubMed PubMed Central Google Scholar * Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD .
BH3-triggered structural reorganization drives the activation of proapoptotic BAX. _Mol Cell_ 2010; 40: 481–492. CAS PubMed PubMed Central Google Scholar * Du H, Wolf J, Schafer B,
Moldoveanu T, Chipuk JE, Kuwana T . BH3 domains other than Bim and Bid can directly activate Bax/Bak. _J Biol Chem_ 2011; 286: 491–501. CAS PubMed Google Scholar * Leshchiner ES, Braun
CR, Bird GH, Walensky LD . Direct activation of full-length proapoptotic BAK. _Proc Natl Acad Sci USA_ 2013; 110: E986–E995. CAS PubMed PubMed Central Google Scholar * Moldoveanu T,
Grace CR, Llambi F, Nourse A, Fitzgerald P, Gehring K _et al_. BID-induced structural changes in BAK promote apoptosis. _Nat Struct Mol Biol_ 2013; 20: 589–597. CAS PubMed PubMed Central
Google Scholar * Ferrer PE, Frederick P, Gulbis JM, Dewson G, Kluck RM . Translocation of a Bak C-terminus mutant from cytosol to mitochondria to mediate cytochrome C release: implications
for Bak and Bax apoptotic function. _PLoS One_ 2012; 7: e31510. CAS PubMed PubMed Central Google Scholar * Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B _et al_.
Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. _Cell_ 2008; 135: 1074–1084. CAS PubMed Google Scholar * Zaltsman Y,
Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH _et al_. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. _Nat Cell Biol_ 2010; 12: 553–562. CAS
PubMed PubMed Central Google Scholar * Shamas-Din A, Bindner S, Zhu W, Zaltsman Y, Campbell C, Gross A _et al_. tBid undergoes multiple conformational changes at the membrane required for
Bax activation. _J Biol Chem_ 2013; 288: 22111–22127. CAS PubMed PubMed Central Google Scholar * Schellenberg B, Wang P, Keeble JA, Rodriguez-Enriquez R, Walker S, Owens TW _et al_. Bax
exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. _Mol Cell_ 2013; 49: 959–971. CAS PubMed PubMed Central Google Scholar * Edlich F,
Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C _et al_. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. _Cell_ 2011; 145: 104–116. CAS PubMed PubMed Central
Google Scholar * Todt F, Cakir Z, Reichenbach F, Youle RJ, Edlich F . The C-terminal helix of Bcl-x(L) mediates Bax retrotranslocation from the mitochondria. _Cell Death Differ_ 2013; 20:
333–342. CAS PubMed Google Scholar * Yethon JA, Epand RF, Leber B, Epand RM, Andrews DW . Interaction with a membrane surface triggers a reversible conformational change in Bax normally
associated with induction of apoptosis. _J Biol Chem_ 2003; 278: 48935–48941. CAS PubMed Google Scholar * Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL _et
al_. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. _Mol Cell_ 2011; 44: 517–531. CAS PubMed PubMed Central Google Scholar * Muchmore SW, Sattler M,
Liang H, Meadows RP, Harlan JE, Yoon HS _et al_. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. _Nature_ 1996; 381: 335–341. CAS PubMed Google Scholar *
Suzuki M, Youle RJ, Tjandra N . Structure of Bax: coregulation of dimer formation and intracellular localization. _Cell_ 2000; 103: 645–654. CAS PubMed Google Scholar * Chou JJ, Li H,
Salvesen GS, Yuan J, Wagner G . Solution structure of BID, an intracellular amplifier of apoptotic signaling. _Cell_ 1999; 96: 615–624. CAS PubMed Google Scholar * McDonnell JM, Fushman
D, Milliman CL, Korsmeyer SJ, Cowburn D . Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. _Cell_ 1999; 96: 625–634. CAS
PubMed Google Scholar * Fesik SW . Insights into programmed cell death through structural biology. _Cell_ 2000; 103: 273–282. CAS PubMed Google Scholar * Petros AM, Olejniczak ET, Fesik
SW . Structural biology of the Bcl-2 family of proteins. _Biochim Biophys Acta_ 2004; 1644: 83–94. CAS PubMed Google Scholar * Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik
SW _et al_. Bcl-x(L) forms an ion channel in synthetic lipid membranes. _Nature_ 1997; 385: 353–357. CAS PubMed Google Scholar * Sobko AA, Kotova EA, Antonenko YN, Zakharov SD, Cramer WA
. Effect of lipids with different spontaneous curvature on the channel activity of colicin E1: evidence in favor of a toroidal pore. _FEBS Lett_ 2004; 576: 205–210. CAS PubMed Google
Scholar * Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B _et al_. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. _EMBO J_
2005; 24: 2096–2103. CAS PubMed PubMed Central Google Scholar * Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M _et al_. Structure of Bcl-xL-Bak peptide complex:
recognition between regulators of apoptosis. _Science_ 1997; 275: 983–986. CAS PubMed Google Scholar * Dai H, Smith A, Meng XW, Schneider PA, Pang YP, Kaufmann SH . Transient binding of
an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. _J Cell Biol_ 2011; 194: 39–48. CAS PubMed PubMed Central Google Scholar * Czabotar PE, Westphal D,
Dewson G, Ma S, Hockings C, Fairlie WD _et al_. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. _Cell_ 2013; 152: 519–531.
CAS PubMed Google Scholar * O'Neill JW, Manion MK, Maguire B, Hockenbery DM . BCL-XL dimerization by three-dimensional domain swapping. _J Mol Biol_ 2006; 356: 367–381. CAS PubMed
Google Scholar * Denisov AY, Sprules T, Fraser J, Kozlov G, Gehring K . Heat-induced dimerization of BCL-xL through alpha-helix swapping. _Biochemistry_ 2007; 46: 734–740. CAS PubMed
Google Scholar * Walensky LD . Direct BAKtivation. _Nat Struct Mol Biol_ 2013; 20: 536–538. CAS PubMed Google Scholar * Zhou HX, Cross TA . Influences of membrane mimetic environments on
membrane protein structures. _Annu Rev Biophys_ 2013; 42: 361–392. CAS PubMed PubMed Central Google Scholar * Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM _et al_. To
trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. _Mol Cell_ 2008; 30: 369–380. CAS PubMed Google Scholar * Bleicken S, Classen M, Padmavathi
PV, Ishikawa T, Zeth K, Steinhoff HJ _et al_. Molecular details of Bax activation, oligomerization, and membrane insertion. _J Biol Chem_ 2010; 285: 6636–6647. CAS PubMed Google Scholar *
Zhang Z, Zhu W, Lapolla SM, Miao Y, Shao Y, Falcone M _et al_. Bax forms an oligomer via separate, yet interdependent, surfaces. _J Biol Chem_ 2010; 285: 17614–17627. CAS PubMed PubMed
Central Google Scholar * Dewson G, Ma S, Frederick P, Hockings C, Tan I, Kratina T _et al_. Bax dimerizes via a symmetric BH3:groove interface during apoptosis. _Cell Death Differ_ 2012;
19: 661–670. CAS PubMed Google Scholar * Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O _et al_. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell
death program. _Science_ 2010; 330: 1390–1393. CAS PubMed PubMed Central Google Scholar * Gavathiotis E, Walensky LD . Tracking BAX once its trigger is pulled. _Cell Cycle_ 2011; 10:
868–870. CAS PubMed PubMed Central Google Scholar * Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA _et al_. An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. _Nature_ 2005; 435: 677–681. CAS PubMed Google Scholar * Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S _et al_. ABT-263: a potent and orally
bioavailable Bcl-2 family inhibitor. _Cancer Res_ 2008; 68: 3421–3428. CAS PubMed Google Scholar * Losonczi JA, Olejniczak ET, Betz SF, Harlan JE, Mack J, Fesik SW . NMR studies of the
anti-apoptotic protein Bcl-xL in micelles. _Biochemistry_ 2000; 39: 11024–11033. CAS PubMed Google Scholar * Franzin CM, Choi J, Zhai D, Reed JC, Marassi FM . Structural studies of
apoptosis and ion transport regulatory proteins in membranes. _Magn Reson Chem_ 2004; 42: 172–179. CAS PubMed PubMed Central Google Scholar * Gong XM, Choi J, Franzin CM, Zhai D, Reed
JC, Marassi FM . Conformation of membrane-associated proapoptotic tBid. _J Biol Chem_ 2004; 279: 28954–28960. CAS PubMed Google Scholar * Malia TJ, Wagner G . NMR structural investigation
of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. _Biochemistry_ 2007; 46: 514–525. CAS PubMed Google Scholar * Denisov AY, Chen G, Sprules
T, Moldoveanu T, Beauparlant P, Gehring K . Structural model of the BCL-w-BID peptide complex and its interactions with phospholipid micelles. _Biochemistry_ 2006; 45: 2250–2256. CAS PubMed
Google Scholar * Xu XP, Zhai D, Kim E, Swift M, Reed JC, Volkmann N _et al_. Three-dimensional Structure of Bax-mediated Pores in Membrane Bilayers. _Cell Death Dis_ 2013; 4: e683. CAS
PubMed PubMed Central Google Scholar * Oh KJ, Barbuto S, Meyer N, Kim RS, Collier RJ, Korsmeyer SJ . Conformational changes in BID, a pro-apoptotic BCL-2 family member, upon membrane
binding. A site-directed spin labeling study. _J Biol Chem_ 2005; 280: 753–767. CAS PubMed Google Scholar * Epand RF, Martinou JC, Montessuit S, Epand RM . Transbilayer lipid diffusion
promoted by Bax: implications for apoptosis. _Biochemistry_ 2003; 42: 14576–14582. CAS PubMed Google Scholar * Garcia-Saez AJ, Coraiola M, Serra MD, Mingarro I, Muller P, Salgado J .
Peptides corresponding to helices 5 and 6 of Bax can independently form large lipid pores. _FEBS J_ 2006; 273: 971–981. CAS PubMed Google Scholar * Qian S, Wang W, Yang L, Huang HW .
Structure of transmembrane pore induced by Bax-derived peptide: evidence for lipidic pores. _Proc Natl Acad Sci USA_ 2008; 105: 17379–17383. CAS PubMed PubMed Central Google Scholar *
Fuertes G, Garcia-Saez AJ, Esteban-Martin S, Gimenez D, Sanchez-Munoz OL, Schwille P _et al_. Pores formed by Baxalpha5 relax to a smaller size and keep at equilibrium. _Biophys J_ 2010; 99:
2917–2925. CAS PubMed PubMed Central Google Scholar * Bogner C, Leber B, Andrews DW . Apoptosis: embedded in membranes. _Curr Opin Cell Biol_ 2010; 22: 845–851. CAS PubMed Google
Scholar * Cross TA, Sharma M, Yi M, Zhou HX . Influence of solubilizing environments on membrane protein structures. _Trends Biochem Sci_ 2011; 36: 117–125. CAS PubMed Google Scholar *
Katayama H, Wang J, Tama F, Chollet L, Gogol EP, Collier RJ _et al_. Three-dimensional structure of the anthrax toxin pore inserted into lipid nanodiscs and lipid vesicles. _Proc Natl Acad
Sci USA_ 2010; 107: 3453–3457. CAS PubMed PubMed Central Google Scholar * Ye F, Hu G, Taylor D, Ratnikov B, Bobkov AA, McLean MA _et al_. Recreation of the terminal events in
physiological integrin activation. _J Cell Biol_ 2010; 188: 157–173. CAS PubMed PubMed Central Google Scholar * Shi L, Shen QT, Kiel A, Wang J, Wang HW, Melia TJ _et al_. SNARE proteins:
one to fuse and three to keep the nascent fusion pore open. _Science_ 2012; 335: 1355–1359. CAS PubMed PubMed Central Google Scholar * Parker MW, Tucker AD, Tsernoglou D, Pattus F .
Insights into membrane insertion based on studies of colicins. _Trends Biochem Sci_ 1990; 15: 126–129. CAS PubMed Google Scholar * Song HY, Cohen FS, Cramer WA . Membrane topography of
ColE1 gene products: the hydrophobic anchor of the colicin E1 channel is a helical hairpin. _J Bacteriol_ 1991; 173: 2927–2934. CAS PubMed PubMed Central Google Scholar * Shin YK,
Levinthal C, Levinthal F, Hubbell WL . Colicin E1 binding to membranes: time-resolved studies of spin-labeled mutants. _Science_ 1993; 259: 960–963. CAS PubMed Google Scholar * Schafer B,
Quispe J, Choudhary V, Chipuk JE, Ajero TG, Du H _et al_. Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation. _Mol Biol Cell_ 2009; 20: 2276–2285. CAS
PubMed PubMed Central Google Scholar * Pang X, Moussa SH, Targy NM, Bose JL, George NM, Gries C _et al_. Active Bax and Bak are functional holins. _Genes Dev_ 2011; 25: 2278–2290. CAS
PubMed PubMed Central Google Scholar * Terrones O, Antonsson B, Yamaguchi H, Wang HG, Liu J, Lee RM _et al_. Lipidic pore formation by the concerted action of proapoptotic BAX and tBID.
_J Biol Chem_ 2004; 279: 30081–30091. CAS PubMed Google Scholar * Kushnareva Y, Andreyev AY, Kuwana T, Newmeyer DD . Bax activation initiates the assembly of a multimeric catalyst that
facilitates Bax pore formation in mitochondrial outer membranes. _PLoS Biol_ 2012; 10: e1001394. CAS PubMed PubMed Central Google Scholar * Campelo F, Fabrikant G, McMahon HT, Kozlov MM
. Modeling membrane shaping by proteins: focus on EHD2 and N-BAR domains. _FEBS Lett_ 2010; 584: 1830–1839. CAS PubMed Google Scholar * Kozlov MM, McMahon HT, Chernomordik LV .
Protein-driven membrane stresses in fusion and fission. _Trends Biochem Sci_ 2010; 35: 699–706. CAS PubMed PubMed Central Google Scholar * McMahon HT, Kozlov MM, Martens S . Membrane
curvature in synaptic vesicle fusion and beyond. _Cell_ 2010; 140: 601–605. CAS PubMed Google Scholar * Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S,
Schwarzenbacher R _et al_. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. _Cell_ 2010; 142: 889–901. CAS PubMed PubMed Central Google
Scholar * Landeta O, Landajuela A, Gil D, Taneva S, Di Primo C, Sot B _et al_. Reconstitution of proapoptotic BAK function in liposomes reveals a dual role for mitochondrial lipids in the
BAK-driven membrane permeabilization process. _J Biol Chem_ 2011; 286: 8213–8230. CAS PubMed PubMed Central Google Scholar * Martinez-Caballero S, Dejean LM, Kinnally MS, Oh KJ, Mannella
CA, Kinnally KW . Assembly of the mitochondrial apoptosis-induced channel, MAC. _J Biol Chem_ 2009; 284: 12235–12245. CAS PubMed PubMed Central Google Scholar * Lee YJ, Jeong SY,
Karbowski M, Smith CL, Youle RJ . Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. _Mol Biol Cell_ 2004; 15: 5001–5011. CAS PubMed
PubMed Central Google Scholar * Takahashi Y, Karbowski M, Yamaguchi H, Kazi A, Wu J, Sebti SM _et al_. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis.
_Mol Cell Biol_ 2005; 25: 9369–9382. CAS PubMed PubMed Central Google Scholar * Etxebarria A, Terrones O, Yamaguchi H, Landajuela A, Landeta O, Antonsson B _et al_. Endophilin B1/Bif-1
stimulates BAX activation independently from its capacity to produce large scale membrane morphological rearrangements. _J Biol Chem_ 2009; 284: 4200–4212. CAS PubMed Google Scholar *
Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T _et al_. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer
membrane permeabilization. _Dev Cell_ 2008; 14: 193–204. CAS PubMed PubMed Central Google Scholar * Garcia-Saez AJ, Ries J, Orzaez M, Perez-Paya E, Schwille P . Membrane promotes tBID
interaction with BCL(XL). _Nat Struct Mol Biol_ 2009; 16: 1178–1185. CAS PubMed Google Scholar * Denisov AY, Madiraju MS, Chen G, Khadir A, Beauparlant P, Attardo G _et al_. Solution
structure of human BCL-w: modulation of ligand binding by the C-terminal helix. _J Biol Chem_ 2003; 278: 21124–21128. CAS PubMed Google Scholar * Hinds MG, Lackmann M, Skea GL, Harrison
PJ, Huang DC, Day CL . The structure of Bcl-w reveals a role for the C-terminal residues in modulating biological activity. _EMBO J_ 2003; 22: 1497–1507. CAS PubMed PubMed Central Google
Scholar * Anfinsen CB . Principles that govern the folding of protein chains. _Science_ 1973; 181: 223–230. CAS PubMed Google Scholar * Murphy KM, Streips UN, Lock RB . Bax membrane
insertion during Fas(CD95)-induced apoptosis precedes cytochrome c release and is inhibited by Bcl-2. _Oncogene_ 1999; 18: 5991–5999. CAS PubMed Google Scholar * Murphy KM, Ranganathan V,
Farnsworth ML, Kavallaris M, Lock RB . Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. _Cell Death Differ_ 2000; 7:
102–111. CAS PubMed Google Scholar * Murphy KM, Streips UN, Lock RB . Bcl-2 inhibits a Fas-induced conformational change in the Bax N terminus and Bax mitochondrial translocation. _J Biol
Chem_ 2000; 275: 17225–17228. CAS PubMed Google Scholar * Billen LP, Kokoski CL, Lovell JF, Leber B, Andrews DW . Bcl-XL inhibits membrane permeabilization by competing with Bax. _PLoS
Biol_ 2008; 6: e147. PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by National Institutes of Health (NIH) grants P01-GM098412 (DH, NV),
P01-AI074805 and R01-GM100265 (FMM) and R01-GM62289 (DDN). AUTHOR INFORMATION Author notes * N Volkmann, F M Marassi, D D Newmeyer and D Hanein: All authors contributed equally to this
review. AUTHORS AND AFFILIATIONS * Bioinformatics and Systems Biology Program, Sanford Burnham Medical Research Institute, La Jolla, 92037, CA, USA N Volkmann & D Hanein * Apoptosis and
Cell Death Research Program, Sanford Burnham Medical Research Institute, La Jolla, 92037, CA, USA F M Marassi * Division of Immune Regulation, La Jolla Institute for Allergy and Immunology,
La Jolla, CA, USA D D Newmeyer Authors * N Volkmann View author publications You can also search for this author inPubMed Google Scholar * F M Marassi View author publications You can also
search for this author inPubMed Google Scholar * D D Newmeyer View author publications You can also search for this author inPubMed Google Scholar * D Hanein View author publications You can
also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to D Hanein. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest.
ADDITIONAL INFORMATION Edited by C Borner RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Volkmann, N., Marassi, F., Newmeyer, D. _et al._ The rheostat
in the membrane: BCL-2 family proteins and apoptosis. _Cell Death Differ_ 21, 206–215 (2014). https://doi.org/10.1038/cdd.2013.153 Download citation * Received: 03 July 2013 * Revised: 22
August 2013 * Accepted: 17 September 2013 * Published: 25 October 2013 * Issue Date: February 2014 * DOI: https://doi.org/10.1038/cdd.2013.153 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * BCL-XL * BAX * BID * mitochondria * structure * membrane