Musashi expression in β-cells coordinates insulin expression, apoptosis and proliferation in response to endoplasmic reticulum stress in diabetes
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Diabetes is associated with the death and dysfunction of insulin-producing pancreatic β-cells. In other systems, Musashi genes regulate cell fate via Notch signaling, which we recently
showed regulates β-cell survival. Here we show for the first time that human and mouse adult islet cells express mRNA and protein of both Musashi isoforms, as well
Numb/Notch/Hes/neurogenin-3 pathway components. Musashi expression was observed in insulin/glucagon double-positive cells during human fetal development and increased during directed
differentiation of human embryonic stem cells (hESCs) to the pancreatic lineage. De-differentiation of β-cells with activin A increased Msi1 expression. Endoplasmic reticulum (ER) stress
increased Msi2 and Hes1, while it decreased Ins1 and Ins2 expression, revealing a molecular link between ER stress and β-cell dedifferentiation in type 2 diabetes. These effects were
independent of changes in Numb protein levels and Notch activation. Overexpression of MSI1 was sufficient to increase Hes1, stimulate proliferation, inhibit apoptosis and reduce insulin
expression, whereas Msi1 knockdown had the converse effects on proliferation and insulin expression. Overexpression of MSI2 resulted in a decrease in MSI1 expression. Taken together, these
results demonstrate overlapping, but distinct roles for Musashi-1 and Musashi-2 in the control of insulin expression and β-cell proliferation. Our data also suggest that Musashi is a novel
link between ER stress and the compensatory β-cell proliferation and the loss of β-cell gene expression seen in specific phases of the progression to type 2 diabetes.
The destruction and dysfunction of pancreatic insulin-producing β-cells are hallmarks of diabetes, but the molecular links between the stresses that cause β-cell death and pathways that
control β-cell function and differentiation are unclear. Although once thought to be quiescent and terminally differentiated, we and others have shown that individual adult pancreatic cells
exhibit a dynamic range in differentiation status.1, 2, 3 Indeed, accumulating evidence reveals striking plasticity between pancreatic cell types and the islet endocrine lineages,4, 5 but
the effects of cellular stress on plasticity pathways remain unknown.
Notch and neurogenin-3 (Ngn3) have established roles in pancreas development.6 Ngn3 is essential for the development of all pancreatic endocrine lineages.6 During embryonic development,
Notch negatively regulates Ngn3 via its target gene Hes1.7 Thus, the Notch signaling pathway is critical at maintaining a proliferating pool of endocrine progenitor cells and influences the
timely cell lineage specification of exocrine and endocrine cell differentiation.8 Accumulating evidence suggests that these developmental gene networks also have roles in the adult. We have
demonstrated that Ngn3 mRNA and protein are present in adult human and mouse islets.9 Inhibiting Notch signaling with
N-[(3,5-difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester (DAPT) increased Ngn3 levels and induced apoptosis, suggesting an important role for this pathway in adult
β-cell survival.9 Subsequently, it was shown that conditional knockout of Ngn3 in adult islets impaired β-cell function.10 Unfortunately, little is known about the factors that regulate
Notch and Ngn3 signaling in the adult pancreatic β-cell.
In other tissues, the RNA-binding protein Musashi, a translational repressor of Numb,11 regulates cell fate via Notch signaling by maintaining a pool of self-renewing stem cells.12, 13
Targeted disruption of Musashi-1 (Msi1) and Musashi-2 (Msi2) revealed their roles in maintaining neural stem cell populations in the adult central nervous system.14 Expression of Msi1 and
Hes1 was also reported in the stem cell niche of highly proliferative intestinal crypt cells.15 The Musashi target Numb plays a role in determining cell fate during embryonic development in
some tissues16 and can also be found in the developing mouse pancreas.17 To date, there are no reports regarding Musashi expression in developing or mature β-cells or its possible
mechanistic roles. We hypothesized that Musashi expression remains in a subset of adult β-cells and that it regulates cell fate decisions in response to cellular stress.
Here, we show that both Musashi isoforms are expressed in developing and adult human and mouse islets and β-cells at the transcript and protein levels. Musashi was more abundant in
dedifferentiated and immature β-cells. Conditions associated with type 2 diabetes, such as endoplasmic reticulum (ER) stress induced by sarco-endoplasmic reticulum Ca2+ ATPase inhibition (ER
Ca2+ depletion) or palmitate treatment, increased the expression of the Musashi genes. Overexpression of MSI1 in mouse insulinoma cell line 6 (MIN6) β-cells increased Hes1 gene expression,
increased proliferation and downregulated insulin gene expression, whereas knockdown had the opposite effects. Our data suggest that Musashi genes regulate insulin expression, apoptosis and
proliferation in response to ER stress via Hes1, but independently of the Numb/Notch pathway.
The expression and function of Notch pathway components in adult islets was recently demonstrated,9, 10 but the upstream regulators of Notch are unclear in these cells. Our identification of
MSI1 via mass spectroscopy in a proteomics study of human islets provided the impetus to investigate this translational repressor protein. Published gene expression databases, Tag-Seq
libraries and microarray data sets of fluorescence-activated cell sorting (FACS)-purified β-cells18, 19 pointed to the presence of both Musashi homologs in adult islets and purified β-cells
from humans, mice and rats, as well as Numb/Numb-like, Notch1-4, Hes1 and Ngn3 (Figure 1a). However, the functional relationships between the components of this classical Notch pathway
remain understudied in adult β-cells.
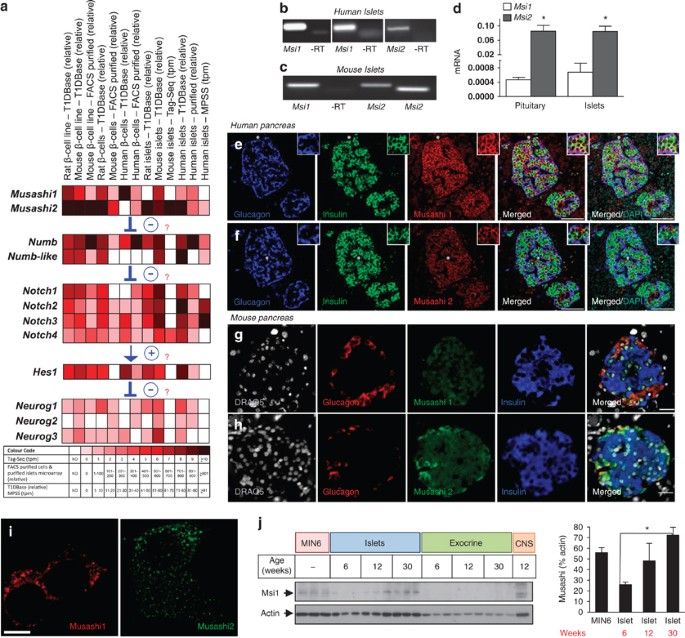
Expression of Musashi genes in pancreatic islets and β-cells. (a) mRNA expression data were extracted from four independent sources for Musashi-1 (Msi1) and Musashi-2 (Msi2), as well as
other Notch pathway genes for human, mouse and/or rat islets and β-cells . Expression levels for T1DBase (relative expression), FACS-purified β-cells and purified islets microarray (relative
expression), MPSS (tpm=transcripts per million) and Tag-Seq library (tpm=tags per million) are displayed by color (see look-up table; nd, data not determined). (b) RT-PCR analysis of human
Msi1 (using two different primer sets spanning different exons) and Msi2 in human islets (representative of >3 experiments). (c) RT-PCR analysis of Msi1 and Msi2 (using two different primer
sets spanning different exons) in mouse islets. (d) RT-qPCR expression analysis of Msi1 and Msi2 in mouse islets (n=4) and pituitary gland (n=3). Glucagon and insulin staining in adult human
pancreas along with Msi1 (e) and Msi2 (f). Scale bar=100 μm. Glucagon and insulin staining in adult mouse pancreas along with Msi1 (g) and Msi2 (h). Scale bar=20 μm. (i) Deconvolution
microscopy of Msi1 and Msi2 subcellular location in MIN6 cells. Scale bar=5 μm. Similar results were observed with overexpression of Msi1 and Msi2 fluorescent fusion proteins. (j) Western
blot for Msi1 protein in MIN6 cells, islets and exocrine tissue from mice aged 6, 12 and 30 weeks (n=3). Mouse brain tissue was used as a positive control. *P