DRAM1 regulates apoptosis through increasing protein levels and lysosomal localization of BAX
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

DRAM1 (DNA damage-regulated autophagy modulator 1) is a TP53 target gene that modulates autophagy and apoptosis. We previously found that DRAM1 increased autophagy flux by promoting
lysosomal acidification and protease activation. However, the molecular mechanisms by which DRAM1 regulates apoptosis are not clearly defined. Here we report a novel pathway by which DRAM1
regulates apoptosis involving BAX and lysosomes. A549 or HeLa cells were treated with the mitochondrial complex II inhibitor, 3-nitropropionic acid (3NP), or an anticancer drug, doxorubicin.
Changes in the protein and mRNA levels of BAX and DRAM1 and the role of DRAM1 in BAX induction were determined. The interaction between DRAM1 and BAX and its effect on BAX degradation, BAX
lysosomal localization, the release of cathepsin B and cytochrome c by BAX and the role of BAX in 3NP- or doxorubicin-induced cell death were studied. The results showed that BAX, a
proapoptotic protein, was induced by DRAM1 in a transcription-independent manner. BAX was degraded by autophagy under basal conditions; however, its degradation was inhibited when DRAM1
expression was induced. There was a protein interaction between DRAM1 and BAX and this interaction prolonged the half-life of BAX. Furthermore, upregulated DRAM1 recruited BAX to lysosomes,
leading to the release of lysosomal cathepsin B and cleavage of BID (BH3-interacting domain death agonist). BAX mediated the release of mitochondrial cytochrome c, activation of caspase-3
and cell death partially through the lysosome-cathepsin B-tBid pathway. These results indicate that DRAM1 regulates apoptosis by inhibiting BAX degradation. In addition to mitochondria,
lysosomes may also be involved in BAX-initiated apoptosis.
Autophagy and apoptosis have important roles in many biological functions of cells and are involved in pathogenesis of many diseases. The mitochondria have an important role in the
initiation of apoptosis by releasing proapoptotic factors and activation of caspases, whereas the lysosomes are essential for autophagy as they provide digestive enzymes for the completion
of autophagic process. Autophagy promotes cell survival or cell death under basal and various stressed conditions through its autophagic pathway or crosstalk to apoptosis.1, 2 The
interaction between the autophagic protein beclin1 and the antiapoptotic protein Bcl-2 has defined the first molecular pathway of crosstalk between autophagy and apoptosis.3 DRAM1 (DNA
damage-regulated autophagy modulator 1) is a TP53 target gene coding a lysosomal membrane protein and has an essential role in TP53-mediated autophagy activation and apoptosis.4, 5, 6 We
have previously reported that DRAM1 was involved in the mitochondrial complex II inhibitor (3-nitropropionic acid (3NP))-induced autophagy activation and cell death.7 We have also
characterized a mechanism by which DRAM1 stimulates autophagy flux by enhancing vacuole ATPase activity and lysosomal acidification.8 These studies suggest that DRAM1 regulates autophagy
partially through lysosomes. However, the regulation of apoptosis by DRAM1 remains to be elucidated.
The classical apoptosis is triggered by the translocation of proapoptotic protein BAX from the cytosol to the mitochondria and induced by the release of cytochrome c,9, 10 which leads to the
activation of procaspase-9 and -3. The induction of BAX has been found in the apoptotic process in response to physiological and pathological stimuli.11, 12 The upregulation of BAX protein
was achieved by increasing its transcription or decreasing its degradation. Although ubiquitin-proteasome system (UPS) was reportedly involved in the degradation of BAX,13 other mechanisms
were also apparently involved in the degradation of BAX.14, 15 Whether an autophagy-lysosomal pathway (ALP) has a role in the degradation of BAX remains to be clarified.
On the other hand, the release of cytochrome c from the mitochondria is also triggered by truncated BH3-interacting domain death agonist (tBID). BID is normally present in the cytosol and
cleaved by activated caspase-8 or the lysosomal cathepsin B to form tBID (carboxy-terminal region of BID).16, 17, 18 tBID relocalizes to the mitochondria from the cytosol to release
cytochrome c.19 Some investigators suggest that members of the Bcl-2 family proteins that mediate permeabilization of mitochondrial membranes may also be involved in the permeabilization of
lysosomal membranes. For example, BAX was reported to increase the permeability of lysosomes,20, 21 but some essential evidence is still missing. We speculated that DRAM1 might regulate
apoptosis through BAX and lysosomes.
In the present study, we sought to investigate if DRAM1 regulated BAX levels and if DRAM1 would recruit BAX to lysosomes through a protein–protein interaction. The recruitment of BAX to
lysosomes would release lysosomal cathepsin B to cleave BID. We found that DRAM1 inhibited BAX autophagic degradation and increased BAX lysosomal localization under stress conditions,
leading to lysosomal permeabilization, cathepsin B release, BID cleavage and activation of mitochondrial apoptotic pathway.
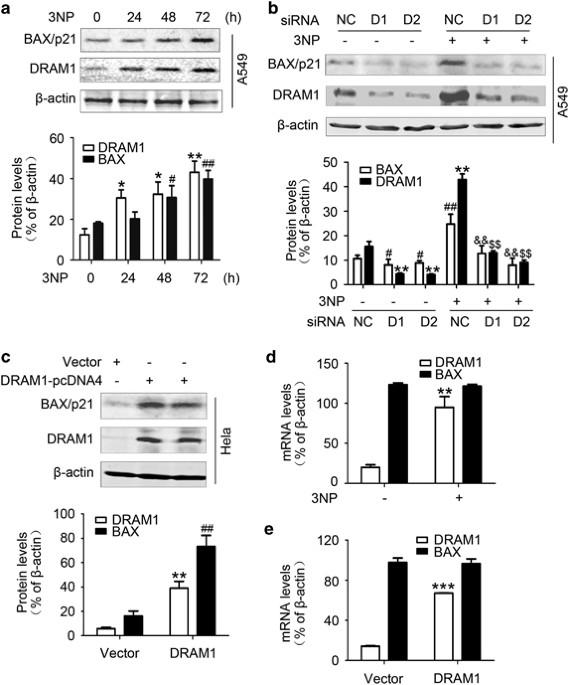
To determine if DRAM1 expression was involved in the regulation of apoptosis, the induction of proapoptotic protein BAX was investigated in A549 and HeLa cells. The results demonstrated that
3NP induced a significant increase in the protein levels of DRAM1 and BAX in A549 cells (Figure 1a) and HeLa cells (Supplementary Figure 1a). The upregulation of DRAM1 and BAX was also
found in A549 and HeLa cells after treatment with doxorubicin (Supplementary Figures 1b and c). To evaluate whether elevation of BAX was regulated by DRAM1, the present study examined the
effects of DRAM1 siRNA on 3NP- and doxorubicin-induced changes in BAX protein levels. It was shown that silencing of DRAM1 expression markedly reduced basal levels and 3NP- (Figure 1b) and
doxorubicin-induced (Supplementary Figure 1d) upregulation of BAX in A549 cells. In addition, BAX induction was also observed in HeLa cells transfected with DRAM1-pcDNA4 (Figure 1c),
suggesting that DRAM1 could regulate BAX protein levels. On the other hand, overexpression of BAX had no effect on DRAM1 protein levels (Supplementary Figure 1e).
DRAM1 increases BAX protein levels independent of transcription. (a) A549 cells were treated with 3NP (500 μM) and harvested 24, 48 and 72 h later. Bars represent mean±S.E.; n=4; **P