Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Intensive glucose control increases the all-cause mortality in type 2 diabetes mellitus (T2DM); however, the underlying mechanisms remain unclear. We hypothesized that strict diet
control to achieve euglycemia in diabetes damages major organs, increasing the mortality risk. To evaluate effects on major organs when euglycemia is obtained by diet control, we generated a
model of end-stage T2DM in 13-week-old Sprague-Dawley rats by subtotal pancreatectomy, followed by _ad libitum_ feeding for 5 weeks. We divided these rats into two groups and for the
subsequent 6 weeks provided _ad libitum_ feeding to half (AL, _n_=12) and a calorie-controlled diet to the other half (R, _n_=12). To avoid hypoglycemia, the degree of calorie restriction in
the R group was isocaloric (g per kg body weight per day) compared with a sham-operated control group (C, _n_=12). During the 6-week diet control period, AL rats ate three times more than
rats in the C or R groups, developing hyperglycemia with renal hyperplasia. R group achieved euglycemia but lost overall body weight significantly compared with the C or AL group (49 or 22%,
respectively), heart weight (39 or 23%, respectively) and liver weight (50 or 46%, respectively). Autophagy levels in the heart and liver were the highest in the R group (_P_<0.01),
which also had the lowest pAkt/Akt levels among the groups (_P_<0.05 in the heart; _P_<0.01 in the liver). In conclusion, glycemic control achieved by diet control can prevent
hyperglycemia-induced renal hyperplasia in diabetes but may be deleterious even at isocaloric rate when insulin is deficient because of significant loss of heart and liver mass via increased
autophagy. SIMILAR CONTENT BEING VIEWED BY OTHERS RENAL DENERVATION AMELIORATES CARDIAC METABOLIC REMODELING IN DIABETIC CARDIOMYOPATHY RATS BY SUPPRESSING RENAL SGLT2 EXPRESSION Article 13
November 2021 HIGH-FAT DIET PROMOTES RENAL INJURY BY INDUCING OXIDATIVE STRESS AND MITOCHONDRIAL DYSFUNCTION Article Open access 24 October 2020 COMPARATIVE EVALUATION OF METFORMIN AND
LIRAGLUTIDE CARDIOPROTECTIVE EFFECT IN RATS WITH IMPAIRED GLUCOSE TOLERANCE Article Open access 23 March 2021 INTRODUCTION Correction of hyperglycemia is the ultimate goal in diabetes care,
as uncontrolled long-term hyperglycemia can cause complications in both type 1 and type 2 diabetes mellitus (T2DM).1, 2 According to the Diabetes Control and Complications Trial, the
incidence of diabetic retinopathy, nephropathy and neuropathy increases as hemoglobin A1c (HbA1c, measure of mean blood glucose during the previous 2–3 months) increases, and intensive
therapy targeting near-euglycemia reduces the risk of microvascular complications compared with conventional therapy.2, 3 Similarly, the United Kingdom Prospective Diabetes Study 35 reported
that lower HbA1c level was associated with significant reductions in the risk of death related to diabetes, myocardial infarction and microvascular complications, suggesting that good
glycemic control can prevent diabetic complications.1 Data from subsequent studies have consistently supported that improved glycemic control is associated with lower risks of mortality and
diabetic complications.4, 5, 6, 7 However, recent studies have reported that intensive glucose-lowering treatment targeting euglycemia is not always beneficial to patients with diabetes.8,
9, 10 In fact, the Action to Control Cardiovascular Risk in Diabetes study terminated the intensive-therapy arm (target HbA1c<6.0%) in the middle of the trial because of higher mortality
compared with the standard-therapy arm (target HbA1c 7.0–7.9%).8 Moreover, the all-cause mortality and cardiac events of patients in the lowest HbA1c decile (mean HbA1c 6.4%) and patients in
the highest HbA1c decile (mean HbA1c 10.6%) were higher than those of the patients with the lowest hazard ratio (mean HbA1c 7.5%).11 However, causes of higher mortality and cardiovascular
events in intensive glycemic control have not yet been identified, although iatrogenic hypoglycemia has been suggested.8, 9, 11 Diet control to achieve optimal glycemia is emphasized in
diabetes care,12, 13, 14 but the possibility of harmful effects on major organs when insulin deficiency is not completely corrected has not been well described. Although blood glucose levels
are high in diabetic patients, glucose uptake decreases in cells dependent on insulin for glucose transport but increases in cells that are not dependent on insulin. It is unclear how these
various cell types react to euglycemia obtained by diet control when insulin deficiency exists. In our previous study, we observed that insulin deficiency given physiological needs is
common in patients with T2DM receiving oral antidiabetic agents or insulin injections when they need to restrict calorie to achieve euglycemia and that they do not require calorie
restriction to achieve euglycemia when insulin requirements are corrected for physiological needs by continuous subcutaneous insulin infusion therapy.15 However, few patients with T2DM use
continuous subcutaneous insulin infusion therapy, and therefore they require calorie restriction in addition to medical treatment to achieve euglycemia. Based on these findings, to explain
the increased mortality in intensive glycemic control group we hypothesized that diet control strict enough to achieve euglycemia in diabetic patients with insulin deficiency may result in
loss of functional mass of major organs, such as the heart, liver and kidneys. Thus calorie restriction may lead to life-threatening events such as organ failure due to the additive effects
of decreased insulin signaling and restricted energy supply. We hypothesized that a molecular mechanism underlying the loss of functional mass in major organs is autophagy, which is a
crucial mechanism for cell survival during nutrient and growth factor deprivation but is also an inducer of cell death through apoptotic or non-apoptotic pathways.16, 17, 18 To confirm our
hypotheses, we generated insulin-deficient diabetic rats by subtotal pancreatectomy to mimic end-stage T2DM as β-cell function and mass decrease over time and diet control becomes more
important to control hyperglycemia.19, 20 We used mature 13-week-old rats in this study, because calorie restriction and insulin deficiency early in life are associated with decreased organ
weight/function and disease in later life.21, 22, 23 After pancreatectomy, we determined how much more energy diabetic rats naturally ingest compared with sham-operated control rats when fed
_ad libitum_. We fed one group of diabetic rats a reduced-calorie diet to control hyperglycemia and another group an _ad libitum_ diet. We expected that hyperglycemia could be controlled
without profound energy depletion or hypoglycemia in the calorie-controlled group by feeding them the same number of calories per kilogram body weight as the sham-operated control group,
using a diet designed according to the nutrition recommendations for diabetes management.14 Finally, we compared overall body weight, weights of major organs and insulin-dependent tissues
and autophagy levels of major organs in the three experimental groups to determine whether euglycemia obtained by diet control in insulin deficiency causes loss of functional mass in major
organs through increased autophagy. MATERIALS AND METHODS ETHICS STATEMENT All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of
Laboratory Animals. The study protocol was approved by the Konkuk University Institutional Animal Care and Use Committee. ANIMALS Thirty-six 11-week-old specific pathogen-free male
Sprague-Dawley (SD) rats were purchased from OrientBio (Sungnam, South Korea). The rats were weighed upon arrival and housed individually for 2 weeks before surgery to minimize body weight
variation during the acclimation period. After surgery, the rats were maintained in individual cages for the control and measurement of daily food intake in a temperature- and
humidity-controlled environment with a 12-h light–dark cycle (lights on 0800–2000 hours, temperature 20–23 °C, relative humidity 40–65%). The rats were given a standard chow (GF 2005; Feed
Laboratory, Guri, South Korea), which is a modified AIN-76A diet (4.1 kcal g−1; 62% carbohydrate, 18% protein and 20% fat by calories). The rats had _ad libitum_ access to tap water
throughout the study. At the end of experiment, rats were anesthetized by CO2 after overnight fasting and euthanized. STUDY DESIGN After the 2-week acclimation period, the 13-week-old rats
were randomly divided into two groups: 12 rats underwent a sham operation (C group) and 24 rats subtotal pancreatectomy. Then all rats were fed _ad libitum_ for 5 weeks, which could induce
diabetes in pancreatectomized rats. When overt diabetes was confirmed 5 weeks after the pancreatectomy, the pancreatectomized rats were divided into two groups: an _ad libitum_-fed group (AL
group, _n_=12) and a calorie-controlled diet group (R group, _n_=12). During the subsequent 6 weeks of diet control period, the R group was fed a calorie-restricted diet compared with the
AL group, which, however, was isocaloric (g per kg body weight per day) compared with the C group, determined by using the average daily food intake (g day−1) and body weight (g) of each
animal in the C group during the previous week. Rats in the AL and C groups had continued _ad libitum_ access to food. SUBTOTAL PANCREATECTOMY To generate an insulin-deficient diabetes model
in adult rats, we performed a subtotal pancreatectomy at 13 weeks of age. Briefly, we opened the abdominal wall under anesthesia using 0.7 mg per kg body weight Zoletil 50 (Virbac, Carros,
France) and 0.2 mg per kg body weight Rompun (Bayer Korea, Ansan, South Korea). Pancreatic tissue was carefully removed with cotton swabs, from the attachment to the spleen to 1 mm from the
common bile duct. The pancreatectomized rats were covered with blankets to maintain normal body temperature. The sham operation was performed using the same procedure but without removing
pancreatic tissue. FOOD INTAKE, FASTING BLOOD GLUCOSE (FBG), BODY WEIGHT AND RATE OF DAILY FOOD INTAKE The food intake (g) of each rat was measured daily, and the average daily food intake
of each group (g day−1) was calculated weekly. Every other week FBG (mg dl−1) was measured after an overnight fast at 0900 a.m. from the tail vein blood using a portable glucometer (CareSens
II; Gentrol Co., Incheon, South Korea). Body weight (g) was measured every week and at the end of the study just before euthanasia. The rate of daily food intake (g per kg body weight per
day) was calculated for each rat every week by dividing average daily food intake by body weight. PLASMA INSULIN AND C-PEPTIDE AND SERUM TRIACYLGLYCEROL (TAG), HIGH-DENSITY LIPOPROTEIN
(HDL)-CHOLESTEROL AND ALBUMIN ANALYSIS Immediately before excision of organs, blood samples were taken from the inferior vena cava. Plasma insulin and C-peptide levels were analyzed using
radioimmunoassay kits (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions, and radioactivity was measured by using a γ-counter (Beckman Coulter, Brea, CA, USA).
Serum TAG level was measured using an enzymatic TAG assay kit (Bio Clinical System Co., Ansan, South Korea) and serum albumin level using a bromcresol green-albumin assay kit (Bio Clinical
System Co.) according to the manufacturer’s instructions. HDL-cholesterol level was quantified using a polyethylene glycol precipitation kit (Young Dong, Seoul, South Korea) combined with
the cholesterol oxidase method for cholesterol measurement. TWENTY-FOUR HOUR URINE GLUCOSE AND ALBUMIN ANALYSIS At the fourth week of the diet control period, 24-h urine samples were
collected while the rats were placed in metabolic cages. The total amounts of glucose and albumin in 24-h urine samples were measured with an automated analyzer (TBA-200 FR, Toshiba Medical
Systems Corporation, Tokyo, Japan), using a glucose assay kit (Denka Seiken Co., Tokyo, Japan) and an albumin assay kit (Abbott Laboratries, Abbott Park, IL, USA). ORGANS AND TISSUES At the
end of the 6-week diet control period, the rats were fasted overnight and the liver, heart, both epididymal fat pads, both kidneys and both soleus muscle tissues were quickly excised after
CO2 anesthesia, weighed and immediately frozen in liquid nitrogen. Frozen organs and tissues were stored in a −80 °C freezer until use. WESTERN BLOTTING ANALYSIS Each tissue from the excised
organs was pulverized to a fine powder in liquid nitrogen and homogenized in ice-cold buffer ocntaining 25 mM HEPES, 25 mM benzamidine, 100 mM sodium fluoride, 10 mM sodium pyrophosphate, 2
mM sodium orthovanadate, 1% Triton X-100, 4 mM EDTA, 5 μl ml−1 phosphatase inhibitor cocktail I (Sigma Aldrich, St Louis, MO, USA), 5 μl ml−1 phosphatase inhibitor cocktail II (Sigma
Aldrich) and 5 μl ml−1 protease inhibitor cocktail (Sigma Aldrich). After centrifugation (18 400 _g_ for 30 min at 4 °C), the supernatant was collected, and the protein concentration was
determined using a BCA protein quantification kit (Pierce, Rockford, IL, USA) according to the manufacturer’s instructions. The proteins were separated by sodium dodecyl sulfate
polyacrylamide gel electrophoresis (13.5% gel for LC3 and 8% gel for Akt and p62) and transferred to nitrocellulose membranes (0.45 μm, Bio-Rad Laboratories Inc., Hercules, CA, USA) at 250
mA for 90 min. After blocking with 5% bovine serum albumin in TBS-T buffer for 1 h at room temperature, the membranes were incubated overnight with an anti-LC3 antibody (Cell Signaling
Technology Inc., Danvers, MA, USA, 1:1000), an anti-p62 antibody (BD Biosciences, Franklin Lakes, NJ, USA, 1:1000) or an anti-Akt antibody (Cell Signaling Technology Inc., 1:5000) at 4 °C.
We also detected phospho-Ser473 Akt (Cell Signaling Technology Inc., 1:5000). The membranes were then incubated with a horseradish peroxidase-conjugated anti-rabbit IgG (Cell Signaling
Technology Inc., 1:5000) followed by detection with enhanced chemiluminescence (GE Healthcare, Wauwatosa, WI, USA). The immunoreactive protein bands were quantified using the MultiGauge
software (version 3.1; Fujifilm, Tokyo, Japan). STATISTICAL ANALYSIS Groups were compared by one-way analysis of variance followed by Tukey’s _post hoc_ test. Pearson’s correlation tests
were performed to determine the degree of correlation between organ weight and body weight or insulin level in pancreatectomized rats. Data are presented as mean±s.d.; _P_<0.05 was
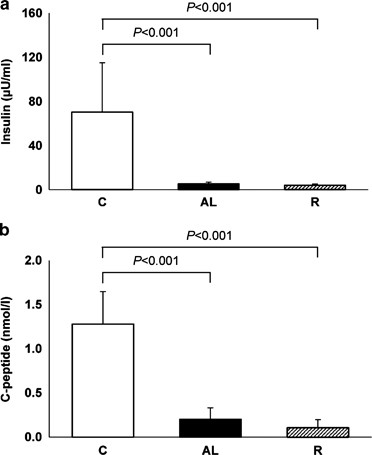
considered significant. Statistical analysis was performed using the SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). RESULTS PLASMA INSULIN AND C-PEPTIDE LEVELS To confirm the generation
of a rat model of insulin-deficient diabetes, we determined plasma insulin and C-peptide levels at the end of the study. The mean plasma insulin levels of pancreatectomized rats were 7.6%
(AL group) and 5.7% (R group) than that of the C group (Figure 1a; _P_<0.001 vs C group for both). The mean plasma C-peptide levels of pancreatectomized rats were 15.6% (AL group) and
8.6% (R group) than that of the C group (Figure 1b; _P_<0.001 vs C group for both). Insulin and C-peptide levels did not differ significantly between the AL and R groups. RATE OF DAILY
FOOD INTAKE As shown in Figure 2a, the rate of daily food intake did not differ significantly among the groups during the 2-week acclimation period (C group 78.2±16.4, AL group 80.0±17.2, R
group 76.9±15.5 g per kg body weight per day; _P_=0.893). However, the rate of daily food intake in the C group decreased over time (58.9±13.5 g per kg body weight per day during the 5-week
diabetes induction period), whereas that of the pancreatectomized rats fed _ad libitum_ for 5 weeks after surgery increased significantly (AL group 95.1±43.0, R group 93.9±39.3 g per kg body
weight per day; _P_<0.001 vs C group for both; Figure 2b). Rats in the AL group ate about three times more than rats in the C and R groups during the diet control period (_P_<0.001;
Figure 2c). FBG LEVELS Figure 2d shows sequential changes in FBG levels of all the groups throughout the experiment. Before surgery, FBG levels were within the normal range for all groups.
However, the pancreatectomized AL and R groups showed hyperglycemia during the 5 weeks after surgery, in which all groups were fed _ad libitum_ (C group 112.2±7.9 vs AL group 445.5±139.3 or
R group 454.5±152.7 mg dl−1; _P_<0.001 for both; Figure 2e), confirming that all pancreatectomized rats developed diabetes. However, the hyperglycemia in rats of the R group improved over
time to near-normoglycemic levels during the period of diet control (Figure 2d). Figure 2f shows that at the last week of the diet control period, FBG levels were within normal range for
both the C and R groups (113.0±15.9 and 125.2±14.9 mg dl−1, respectively; _P_=0.906); however, the mean FBG level of the AL group was 448.3±119.6 mg dl−1 (_P_<0.001 vs C and R groups).
BODY WEIGHTS Figure 2g shows sequential changes in body weights of all the groups throughout the experiment. Body weight did not differ significantly among the groups during the 2-week
acclimation period. Although the mean body weight of the C group steadily increased throughout the experimental period, body weight in the AL and R groups significantly decreased during the
5 weeks after pancreatectomy, even though they were fed _ad libitum_ (C group 469.3±37.4 g vs AL group 399.9±32.8 g and R group 404.0±29.6 g; _P_<0.001 for both; Figure 2h). As shown in
Figure 2g, the AL group maintained body weight during the rest of the study, whereas the R group lost body weight. Figure 2i shows that during the diet control period, the mean body weight
of the R group (336.5±62.5 g; 60 and 87% that of the C and AL groups, respectively) was significantly lower than that of the C group (563.5±38.5 g, _P_<0.001) and the AL group (389.0±50.7
g, _P_=0.045). SERUM TAG, HDL-CHOLESTEROL AND ALBUMIN LEVELS AND 24-H URINE GLUCOSE AND ALBUMIN LEVELS As shown in Table 1, serum TAG, HDL-cholesterol and albumin levels were significantly
lower in the AL and R groups than the C group, but the AL and R groups did not differ significantly. Twenty-four hour urine glucose level increased in both the AL and R groups compared with
the C group, whereas the level of the AL group was five times greater than that of the R group (_P_<0.001). Moreover, the AL group showed albuminuria above microalbuminuria range (30–300
mg 24 h−1), and the level was 10.5 times greater than that of the R group (_P_<0.01; Table 1). However, the C and R groups did not differ significantly. WEIGHTS OF ORGANS AND TISSUES To
determine whether reduced body weight due to insulin deficiency and/or diet control was accompanied by decreased weight of organs and tissues, we weighed the heart, liver, both epididymal
fat pads, both kidneys and both soleus muscle tissues of all rats at the end of the study. Figure 3a shows that the mean heart weights of the AL group (1.33±0.20 g) and R group (1.02±0.20 g)
were 80 and 61%, respectively, that of the C group (1.66±0.15 g; _P_<0.001 for both). It is noteworthy that the mean heart weight of the R group was only 77% that of the AL group
(_P_<0.001). Figure 3b shows that the mean liver weight of the R group (8.3±2.2 g) was 50% that of the C group (16.7±3.8 g) and 54% that of the AL group (15.4±1.9 g; _P_<0.001 for
both); the C and AL groups did not differ significantly. Figure 3c shows that the mean weight of both kidneys of the AL group (5.43±0.61 g) was 163% that of the C group (3.33±0.30 g) and
189% that of the R group (2.86±0.38 g; _P_<0.001 for both); the C and R groups did not differ significantly. Figure 3d shows that the mean weights of both soleus muscle tissues of the AL
group (0.364±0.049 g) and R group (0.318±0.063 g) were 77 and 67%, respectively, that of C group (0.471±0.047 g; _P_<0.001 for both); the AL and R groups did not differ significantly.
Figure 3e shows that the mean weights of both epididymal fat pads of the AL group (3.4±1.5 g) and R group (1.4±1.1 g) were 20 and 8%, respectively, that of C group (16.9±4.3 g; _P_<0.001
for both); the AL and R groups did not differ significantly. In pancreatectomized rats, the weights of all organs and tissues examined were significantly correlated with body weights and
plasma insulin levels (Table 2). PAKT/AKT RATIO AND AUTOPHAGY LEVEL To investigate potential mechanisms underlying differential changes in organ weights of insulin-deficient diabetic rats
fed _ad libitum_ or a calorie controlled diet, we investigated the ratio of phosphorylated Akt to total Akt, which is a crucial marker for insulin signaling, and the ratio of LC3 II to LC3 I
(the conversion of LC3 I to LC3 II) and p62 level, which are markers for autophagy activity (the ratio increases as autophagy is active)24 and autophagy flux (p62 level decreases as
autophagosomes are cleared from cytoplasm by lysosomal degradation),25 respectively. We found that Akt activation in heart tissue decreased by 40% in the R group compared with the C and AL
groups (_P_<0.05 for both; Figures 4a and b). In addition, the ratio of LC3 II to LC3 I in heart tissue increased by 5.6-fold in the R group compared with the C and AL groups (_P_<0.01
for both; Figures 5a and b), while p62 level of the R group was the lowest among all groups (Figure 5c). However, Akt activation, LC3 conversion and p62 level in heart tissue did not differ
significantly between the C and AL groups. Akt activation in liver tissue decreased by 31% in the R group compared with the C and AL groups (_P_<0.01 for both; Figures 4c and d). And the
ratio of LC3 II to LC3 I in liver tissue increased in the R group compared with the C group (1.6-fold, _P_=0.017) and AL group (2.2-fold, _P_=0.002; Figures 5d and e), while p62 level of
the R group was the lowest among all groups (Figure 5f). However, Akt activation, LC3 conversion and p62 level in liver tissue did not differ significantly between the C and AL groups. Akt
activity in kidney tissue increased by 50% in the AL group compared with the C group (_P_<0.05; Figures 4e and f) and increased by 72% compared with the R group (_P_<0.01; Figures 4e
and f ). The ratio of LC3 II to LC3 I in kidney tissue decreased by 40% in the AL group compared with the C and R groups (_P_<0.01 for both; Figures 5g and h), while p62 level did not
differ between the C and AL groups and was the highest in the R group (Figure 5i). However, Akt activation and LC3 conversion in kidney tissue did not differ significantly between the C and
R groups. DISCUSSION In the present study, we demonstrated that pancreatectomized diabetic SD rats achieved euglycemia and were protected against diabetic renal hyperplasia and excessive
albuminuria through diet control (R group) but displayed significant loss of overall body weight and weight of the heart and liver compared with both rats that underwent a sham operation (C
group) and diabetic rats with _ad libitum_ access to food (AL group). Although hyperglycemia induced renal hyperplasia and excessive albuminuria in the AL group, the loss of heart weight was
less than that of the R group, and liver weight was maintained similar to that of the C group (Figures 3a and b). Euglycemia has been known to prevent hyperglycemia-induced microvascular
complications, including diabetic nephropathy;2, 3 however, our results suggest that a strict diet control to achieve euglycemia may be deleterious in insulin deficiency, resulting in the
loss of functional mass in heart and liver tissues (Figures 3a and b). To understand the mechanism underlying the loss of organ weight in the R group, we investigated autophagy level,
because excessive autophagy has been known to induce cell death, leading to a reduction in organ weight.17, 26, 27, 28, 29, 30 We found that autophagy activity as well as autophagy flux
significantly increased in heart and liver tissues of the R group compared with the C group (Figures 5a–f), even though their daily food intake per body weight was the same (Figures 2a and
c). Insulin inhibits autophagy via the class I PI3K-Akt pathway.18, 31, 32 The mean plasma insulin level of the R group was <10% that of the C group, and Akt activity in those tissues was
also significantly lower in the R group (Figures 4a–d). Although the mean plasma insulin level of the AL group was similar to that of the R group, neither Akt activity nor autophagy
activity and flux differed from those of the C group (Figures 4a–d and Figures 5a–f). These findings are consistent with organ weight in the AL group, in which a considerable loss of heart
weight appeared to develop during the 5 weeks of induction of diabetes, but subsequently, the weight appeared to be maintained until the end of the study with an _ad libitum_ diet (Figure
2g), as estimated by the relationship between organ and body weight (Table 2). Several studies support these observations in the AL group: hyperglycemia can activate Akt33, 34 and sufficient
nutrient levels within cells inhibit autophagy, independent of insulin level.18 Taken together, our findings suggest that although an _ad libitum_ diet after subtotal pancreatectomy can
result in renal hyperplasia and excessive albuminuria, indicating hyperglycemic damage to endothelial cells as explained elsewhere,35 this more natural eating behavior may compensate for
decreased insulin signaling in heart and liver tissues. By contrast, the amount of food consumed by the healthy control rats may not be sufficient to protect the R group against autophagic
destruction of heart and liver tissues in insulin deficiency. These findings may explain the reason that the T2DM patients with moderate degree of hyperglycemia showed the lowest mortality
compared with those with high degree of hyperglycemia or near-euglycemia,11 for, though even moderate hyperglycemia is toxic to vascular endothelial cells compared with euglycemia, the
glycemic level seems to be high enough to suppress autophagic response by increasing insulin signaling in heart and liver tissues of diabetic patients but low enough not to induce severe
damage on endothelial cells as shown in high degree of hyperglycemia. Our results are reminiscent of studies on the autophagic response during starvation. One study reported that autophagy
in the heart and liver was increased in mice after a 24-h starvation period.36 Another study reported that heart weight decreased by 21% and liver weight decreased by 55% in 16-week-old rats
after 10 days of starvation; these rats died approximately 2 days later, when starved persistently.37 Because of the comparable loss of heart and liver weight, we speculate that diet
control to achieve euglycemia has a starvation-like effect in insulin-deficient rats. Although calorie restriction can extend lifespan and prevent age-associated diseases such as diabetes,
cardiovascular disease and cancer,38, 39 our study demonstrates that in insulin deficiency, calorie-restricted diet control to achieve euglycemia can increase autophagy above the basal level
in the heart and liver. These findings may explain the reason that the intensive glycemic control group showed higher mortality from cardiovascular causes compared with the standard-therapy
group, whose HbA1c levels were 6.4 and 7.5%, respectively.8 Finally, we found that loss of soleus muscle and epididymal fat pad weight was similar in both the groups of diabetic rats
compared with the sham-operated control group, indicating that insulin-dependent tissues, including skeletal muscle and adipose tissues, may not be protected against weight loss by the _ad
libitum_ diet when insulin is deficient. In conclusion, although diet control becomes more important for optimal glycemic control especially in end-stage T2DM because of decreased β-cell
function and drug failure over time,40, 41, 42 strict diet control to achieve euglycemia should be avoided to prevent loss of functional mass of the heart and liver via increased autophagy.
The effects of calorie restriction (beneficial or deleterious) are dependent on insulin status. In addition, polyphagia may be a compensatory mechanism for insulin deficiency in diabetic
patients, by which resulting high glucose level may activate insulin signaling and maintain the heart and liver weights at the cost of renal hyperplasia. Further studies are needed to better
understand factors that increase the all-cause mortality and cardiovascular death in individuals treated with intensive glycemic control. REFERENCES * Stratton IM, Adler AI, Neil HA,
Matthews DR, Manley SE, Cull CA _et al_. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. _BMJ_
2000; 321: 405–412. Article CAS PubMed PubMed Central Google Scholar * Shamoon H, Duffy H, Fleischer N, Engel S, Saenger P, Strelzyn M _et al_. The effect of intensive treatment of
diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. _New Engl J Med_ 1993; 329: 977–986. Article Google Scholar * Skyler JS .
Diabetic complications. The importance of glucose control. _Endocrinol Metab Clin North Am_ 1996; 25: 243–254. Article CAS PubMed Google Scholar * Klein R, Klein BE, Moss SE, Davis MD,
DeMets DL . Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. _JAMA_ 1988; 260: 2864–2871. Article CAS PubMed Google Scholar * Reichard P, Nilsson
BY, Rosenqvist U . The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. _New Engl J Med_ 1993; 329: 304–309. Article
CAS PubMed Google Scholar * Gerstein HC, Pogue J, Mann JF, Lonn E, Dagenais GR, McQueen M _et al_. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic
and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. _Diabetologia_ 2005; 48: 1749–1755. Article CAS PubMed Google Scholar * Holman RR, Paul SK,
Bethel MA, Matthews DR, Neil HA . 10-year follow-up of intensive glucose control in type 2 diabetes. _New Engl J Med_ 2008; 359: 1577–1589. Article CAS PubMed Google Scholar * Gerstein
HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB _et al_. Effects of intensive glucose lowering in type 2 diabetes. _New Engl J Med_ 2008; 358: 2545–2559. Article CAS PubMed
Google Scholar * Brown A, Reynolds LR, Bruemmer D . Intensive glycemic control and cardiovascular disease: an update. _Nat Rev Cardiol_ 2010; 7: 369–375. Article CAS PubMed Google
Scholar * Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B _et al_. Effect of intensive glucose lowering treatment on all cause mortality,
cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. _BMJ_ 2011; 343: d4169. Article PubMed PubMed Central Google Scholar *
Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL _et al_. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. _Lancet_ 2010; 375:
481–489. Article CAS PubMed Google Scholar * Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M _et al_. Management of hyperglycemia in type 2 diabetes: a
patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). _Diabetes Care_ 2012; 35: 1364–1379.
Article CAS PubMed PubMed Central Google Scholar * American Diabetes Association. Standards of medical care in diabetes—2011. _Diabetes Care_ 2011; 34 (Suppl 1): S11–S61. * Bantle JP,
Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ _et al_. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association.
_Diabetes Care_ 2008; 31 (Suppl 1): S61–S78. CAS PubMed Google Scholar * Noh YH, Lee WJ, Kim KA, Lim I, Lee JH, Kim S _et al_. Insulin requirement profiles of patients with type 2
diabetes after achieving stabilized glycemic control with short-term continuous subcutaneous insulin infusion. _Diabetes Technol Ther_ 2010; 12: 271–281. Article CAS PubMed Google Scholar
* Shintani T, Klionsky DJ . Autophagy in health and disease: a double-edged sword. _Science_ 2004; 306: 990–995. Article CAS PubMed PubMed Central Google Scholar * Kourtis N,
Tavernarakis N . Autophagy and cell death in model organisms. _Cell Death Differ_ 2009; 16: 21–30. Article CAS PubMed Google Scholar * Wang RC, Levine B . Autophagy in cellular growth
control. _FEBS Lett_ 2010; 584: 1417–1426. Article CAS PubMed PubMed Central Google Scholar * Turner RC, Holman RR, Cull CA, Stratton IM, Matthews DR, Frighi V _et al_. Intensive
blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study
(UKPDS) Group. _Lancet_ 1998; 352: 837–853. Article Google Scholar * Turner RC, Holman RR, Stratton IM, Cull CA, Matthews DR, Manley SE _et al_. Effect of intensive blood-glucose control
with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. _Lancet_ 1998; 352: 854–865. Article Google Scholar *
Cella SG, Locatelli V, Mennini T, Zanini A, Bendotti C, Forloni GL _et al_. Deprivation of growth hormone-releasing hormone early in the rat's neonatal life permanently affects
somatotropic function. _Endocrinology_ 1990; 127: 1625–1634. Article CAS PubMed Google Scholar * Harding JE . The nutritional basis of the fetal origins of adult disease. _Int J
Epidemiol_ 2001; 30: 15–23. Article CAS PubMed Google Scholar * Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS . Fetal nutrition and cardiovascular disease in
adult life. _Lancet_ 1993; 341: 938–941. Article CAS PubMed Google Scholar * Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T . The Atg16L complex specifies the site of LC3
lipidation for membrane biogenesis in autophagy. _Mol Biol Cell_ 2008; 19: 2092–2100. Article CAS PubMed PubMed Central Google Scholar * Komatsu M, Kageyama S, Ichimura Y .
p62/SQSTM1/A170: physiology and pathology. _Pharmacol Res_ 2012; 66: 457–462. Article CAS PubMed Google Scholar * Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M _et
al_. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. _J Clin Invest_ 2006; 116: 2161–2172. Article CAS PubMed PubMed Central Google Scholar *
Kunchithapautham K, Rohrer B . Apoptosis and autophagy in photoreceptors exposed to oxidative stress. _Autophagy_ 2007; 3: 433–441. Article CAS PubMed Google Scholar * Yu L, Wan F, Dutta
S, Welsh S, Liu Z, Freundt E _et al_. Autophagic programmed cell death by selective catalase degradation. _Proc Natl Acad Sci USA_ 2006; 103: 4952–4957. Article CAS PubMed PubMed Central
Google Scholar * Takemura G, Miyata S, Kawase Y, Okada H, Maruyama R, Fujiwara H . Autophagic degeneration and death of cardiomyocytes in heart failure. _Autophagy_ 2006; 2: 212–214.
Article CAS PubMed Google Scholar * Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H . Autophagic degeneration as a possible mechanism of myocardial cell death in dilated
cardiomyopathy. _Jpn Circ J_ 2001; 65: 965–968. Article CAS PubMed Google Scholar * Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P . Distinct classes of
phosphatidylinositol 3'-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. _J Biol Chem_ 2000; 275: 992–998. Article CAS PubMed Google Scholar *
Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P _et al_. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein
kinase B pathway. _J Biol Chem_ 2001; 276: 35243–35246. Article CAS PubMed Google Scholar * Li SY, Fang CX, Aberle NS 2nd, Ren BH, Ceylan-Isik AF, Ren J . Inhibition of PI-3
kinase/Akt/mTOR, but not calcineurin signaling, reverses insulin-like growth factor I-induced protection against glucose toxicity in cardiomyocyte contractile function. _J Endocrinol_ 2005;
186: 491–503. Article CAS PubMed Google Scholar * Mariappan MM, Shetty M, Sataranatarajan K, Choudhury GG, Kasinath BS . Glycogen synthase kinase 3beta is a novel regulator of high
glucose- and high insulin-induced extracellular matrix protein synthesis in renal proximal tubular epithelial cells. _J Biol Chem_ 2008; 283: 30566–30575. Article CAS PubMed PubMed
Central Google Scholar * Brownlee M . The pathobiology of diabetic complications: a unifying mechanism. _Diabetes_ 2005; 54: 1615–1625. Article CAS PubMed Google Scholar * Mizushima N,
Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y . _In vivo_ analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. _Mol
Biol Cell_ 2004; 15: 1101–1111. Article CAS PubMed PubMed Central Google Scholar * Goodman MN, Ruderman NB . Starvation in the rat. I. Effect of age and obesity on organ weights, RNA,
DNA, and protein. _Am J Physiol_ 1980; 239: E269–E276. CAS PubMed Google Scholar * Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM _et al_. Caloric restriction
delays disease onset and mortality in rhesus monkeys. _Science_ 2009; 325: 201–204. Article CAS PubMed PubMed Central Google Scholar * Hansen BC, Bodkin NL . Primary prevention of
diabetes mellitus by prevention of obesity in monkeys. _Diabetes_ 1993; 42: 1809–1814. Article CAS PubMed Google Scholar * U.K. prospective diabetes study 16. Overview of 6 years'
therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. _Diabetes_ 1995; 44: 1249–1258. Article Google Scholar * Kahn SE, Haffner SM, Heise MA, Herman
WH, Holman RR, Jones NP _et al_. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. _New Engl J Med_ 2006; 355: 2427–2443. Article CAS PubMed Google Scholar *
Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S _et al_. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. _New Engl J Med_ 2007; 357:
1716–1730. Article CAS PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biochemistry, Konkuk University School of Medicine, Seoul,
Republic of Korea Jun-Ho Lee, Ju-Han Lee, Mingli Jin, Seonguk Kim, Sung-Young Kim & Yun-Hee Noh * Rmedica-Stem Cell, Seoul, Republic of Korea Ju-Han Lee * Department of Neurology, Konkuk
University School of Medicine, Chungju Hospital, Chungju, Republic of Korea Sang-Don Han * Department of Pulmonary and Critical Care Medicine, Konkuk University School of Medicine, Chungju
Hospital, Chungju, Republic of Korea Gyu-Rak Chon * Department of Surgery, Konkuk University School of Medicine, Chungju Hospital, Chungju, Republic of Korea Ick-Hee Kim * Department of
Internal Medicine, Konkuk University School of Medicine, Chungju Hospital, Chungju, Republic of Korea Soo-Bong Choi Authors * Jun-Ho Lee View author publications You can also search for this
author inPubMed Google Scholar * Ju-Han Lee View author publications You can also search for this author inPubMed Google Scholar * Mingli Jin View author publications You can also search
for this author inPubMed Google Scholar * Sang-Don Han View author publications You can also search for this author inPubMed Google Scholar * Gyu-Rak Chon View author publications You can
also search for this author inPubMed Google Scholar * Ick-Hee Kim View author publications You can also search for this author inPubMed Google Scholar * Seonguk Kim View author publications
You can also search for this author inPubMed Google Scholar * Sung-Young Kim View author publications You can also search for this author inPubMed Google Scholar * Soo-Bong Choi View author
publications You can also search for this author inPubMed Google Scholar * Yun-Hee Noh View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING
AUTHORS Correspondence to Soo-Bong Choi or Yun-Hee Noh. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. RIGHTS AND PERMISSIONS This work is licensed
under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to
reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lee, JH., Lee,
JH., Jin, M. _et al._ Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with _ad libitum_ diet in diabetic rats. _Exp Mol
Med_ 46, e111 (2014). https://doi.org/10.1038/emm.2014.52 Download citation * Received: 25 April 2014 * Accepted: 30 June 2014 * Published: 29 August 2014 * Issue Date: August 2014 * DOI:
https://doi.org/10.1038/emm.2014.52 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative