Clinical implications of proliferation activity in T1 or T2 male gastric cancer patients
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Proliferation activity has already been established as a prognostic marker or as a marker for anticancer drug sensitivity. In gastric cancer, however, the prognostic significance of
proliferation activity is still being debated. Several studies evaluating proliferation activity using Ki-67 have shown controversial results in terms of the relationship between
proliferation activity and overall survival (OS) or drug sensitivity in gastric cancer patients. Because cytoskeleton-associated protein 2 (CKAP2) staining has recently been introduced as a
marker of proliferation activity, we analyzed 437 gastric cancer tissues through CKAP2 immunohistochemistry, and we evaluated the chromatin CKAP2-positive cell count (CPCC) for proliferation
activity. Although the CPCC did not show any significant correlation with OS in the male, female or total number of cases, it did show a significant correlation in the T1 or T2 male patient
subgroup, according to log-rank tests (P=0.001) and univariate analysis (P=0.045). Additionally, multivariate analysis with the Cox proportional hazard regression model showed a significant
correlation between the CPCC and OS (P=0.039) for the co-variables of age, gender, T stage, N stage, histology, tumor location, tumor size and adjuvant chemotherapy. In male gastric cancer
cell lines, faster-growing cancer cells showed higher sensitivity to cisplatin than slow-growing cells. Thus our study indicates that CPCC-measured proliferation activity demonstrates a
significantly worse prognosis in T1 or T2 male gastric cancer patients. The CPCC will help to more precisely classify gastric cancer patients and to select excellent candidates for adjuvant
chemotherapy, which in turn will facilitate further clinical chemotherapeutic trials.
Because cell proliferation is one of the most vital biological mechanisms in oncogenesis,1 cell proliferation activity has been posited as a promising prognostic marker. Whereas the
prognostic significance of cell proliferation activity has been well established in various cancers, including breast cancers, meningiomas, gastrointestinal stromal tumors and head and neck
cancers,2, 3, 4, 5 its utility remains in doubt in other cancers. Particularly in cases of gastric cancer, while some studies have reported a positive correlation between higher
proliferation and worse survival,6 others have demonstrated no such relationship.7, 8, 9 Recently, an inverse correlation was reported in a relatively large (245 cases) cohort.10 Therefore,
additional studies are required to clarify the prognostic significance of proliferation activity in gastric cancer.
High proliferation activity has been related to greater sensitivity to anticancer drugs.11 In breast cancer, for example, proliferation has been recognized as a reliable predictor of the
response to adjuvant12, 13 and neoadjuvant chemotherapy.14, 15, 16 In gastric cancer, however, the correlation between proliferation activity and anticancer drug sensitivity remains unclear.
If such a correlation is also observed in gastric cancer, the measurement of proliferation activity for that disease could have therapeutic implications.
Ki-67 and mitotic counts have been the most widely employed tools used to evaluate proliferation activity in various cancers, including gastric cancer. Cytoskeleton-associated protein 2
(CKAP2) has been recently established as a new mitotic marker, with chromatin CKAP2-positive cells being identified as mitotic cells17; indeed, there is a strong correlation between the
CKAP2-positive cell count (CPCC) and the mitotic figure count.18 The prognostic significance of the CPCC has also been demonstrated in breast cancer, for which it was equivalent to or better
than the significance of the mitotic activity index,19 one of the most widely accepted proliferation activity measurements in breast cancer.20 In the present study, to evaluate the
prognostic significance of proliferation activity in gastric cancer, CKAP2 immunohistochemical staining was performed on 437 gastric cancer tissues, and the correlation between the CPCC and
overall survival (OS) was evaluated.
Cases of gastric cancer patients who underwent curative resection at the National Cancer Center between 2002 and 2003 were accrued, and microarrays were created from paraffin-embedded
tissues from 521 gastric cancer patients. Access to and usage of the patients’ clinical information and the relevant archival tissues were approved by the Institutional Review Board of the
National Cancer Center, which waived the need for informed consent. The human male gastric cancer cell lines Kato III, SNU-484, SNU-601 and SNU-668 were obtained from the Korean Cell Line
Bank (http://cellbank.snu.ac.kr) and cultured in RPMI-1640 culture media (Thermo Fisher Scientific Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (Thermo Fisher Scientific
Hyclone) at 37 °C under 5% CO2.
Immunohistochemical staining was performed using the Ultravision LP Detection kit (Thermo Fisher Scientific Inc., Fremont, CA, USA), as previously reported, for the same CKAP2 antibody.12
Briefly, after deparaffinization of the formalin-fixed, paraffin-embedded tissues, antigen was retrieved in 10 mM citrate buffer, pH 6.0, containing 0.05% Tween 20. The tissues were
sequentially treated with 3% hydrogen peroxide and Ultra V block solution for 15 min each. After being incubated for 1 h at room temperature with anti-CKAP2 antibody, the slides were washed
in Tris-buffered saline with Tween 20 (TBST), incubated with primary antibody enhancer for 10 min and exposed to horseradish peroxidase-conjugated secondary antibody for 15 min. After
re-washing in TBST, the tissue slides were incubated with diaminobenzidine chromogen (Scytek Laboratories Inc., Logan, UT, USA) and counterstained with Mayer’s hematoxylin (Dako Cytomation,
Glostrup, Denmark).
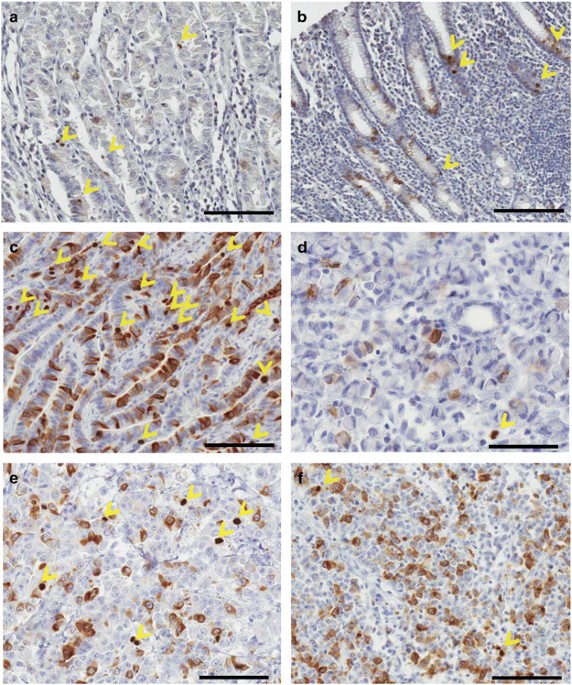
In the CPCC determination, the number of chromosomal CKAP2-positive cells under one × 200 power field (instead of 10 × 400 power fields) was counted because of the limited number of
microscopic fields in the tissue microarrays. Strongly to moderately stained chromatin-positive cells were included in the count. Cores containing