Linking environmental heterogeneity and reproductive success at single-cell resolution
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Individual-based microbial ecology (IBME) is a developing field of study in need of experimental tools to quantify the individual experience and performance of microorganisms in
their natural habitats. We describe here the conception and application of a single-cell bioreporter approach with broad utility in IBME. It is based on the dilution of stable green
fluorescent protein (GFP) in dividing bacteria. In the absence of _de novo_ synthesis, GFP fluorescence of a daughter cell approximates half of that of its mother, from which follows that
the fluorescence of a progeny cell is a quantitative measure for the reproductive success of its ancestor. To test this concept, we exposed GFP-filled bacteria to different degrees of
environmental heterogeneity and assessed how this affected individual cells by the analysis of GFP content in their progeny. Reporter bacteria growing in rich medium in a shaking flask
showed no variation in reproductive success, confirming that life in a broth is experienced much the same from one bacterium to the next. In contrast, when reporter bacteria were released
onto plant leaf surfaces, representing a microscopically heterogeneous environment, clear intrapopulation differences in reproductive success were observed. Such variation suggests that
individual cells in the founding population experienced different growth-permitting conditions, resulting in unequal contributions of individual bacteria to future offspring and population
sizes. Being able to assess population changes bottom-up rather than top-down, the bioreporter offers opportunities to quantify single-cell competitive and facilitative interactions, assess
the role of chance events in individual survivorship and reveal causes that underlie individual-based environmental heterogeneity. SIMILAR CONTENT BEING VIEWED BY OTHERS ECO-EVOLUTIONARY
INTERACTION BETWEEN MICROBIOME PRESENCE AND RAPID BIOFILM EVOLUTION DETERMINES PLANT HOST FITNESS Article 11 March 2021 COMMUNITY AND SINGLE CELL ANALYSES REVEAL COMPLEX PREDATORY
INTERACTIONS BETWEEN BACTERIA IN HIGH DIVERSITY SYSTEMS Article Open access 16 September 2021 GRADIENTS AND CONSEQUENCES OF HETEROGENEITY IN BIOFILMS Article 11 February 2022 INTRODUCTION
Environmental heterogeneity, defined as spatial and temporal variation in the physical, chemical and biological environment, is a fundamental property of ecosystems (Scheiner and Willig,
2008). At the scale of individual organisms, it affects the ability to survive, reproduce, co-exist and interact with other organisms. For plants and animals, environmental impact can be
assessed and quantified relatively simply at the level of individual organisms (Melbourne et al., 2007). In microbial ecology, however, the effect of environmental variability on microbial
activity and diversity is commonly assessed at a scale that is several orders of magnitude greater than the dimensions of the microorganisms under study (Hellweger and Bucci, 2009). This
‘coarse-grained’ (Templeton and Rothman, 1978) approach to environmental heterogeneity suffers from the averaging effect that is typical of many population-based approaches (Brehm-Stecher
and Johnson, 2004). It is increasingly being recognized that ‘fine-grained’ environmental heterogeneity, that is the one experienced by microscopic individuals at the micrometer-scale is a
key factor in explaining microbial activity, diversity, distribution and evolution (Davey and Winson, 2003; Green and Bohannan, 2006; Prosser et al., 2007; Davidson and Surette, 2008).
However, due to the relative lack of tools to probe environments for micrometer-scale differences in physical, chemical or biological variables, little is known about the heterogeneity that
individual microorganisms are exposed to and, more importantly, how this affects their activity and reproductive success. Bioreporter technology (Leveau and Lindow, 2002; Harms et al., 2006;
Tecon and van der Meer, 2006) relies on microorganisms themselves to report on local environmental conditions. Many of these bioreporters involve the conditional expression of green
fluorescent protein (GFP), a reporter that can be quantified with relative ease in individual cells by fluorescence image microscopy (Jaspers et al., 2001; Leveau and Lindow, 2001a) or flow
cytometry (Axtell and Beattie, 2002; Maksimow et al., 2002; Harms et al., 2006; Roostalu et al., 2008). When properly calibrated, the GFP signal becomes a measure for exposure to a
particular environmental stimulus. For example, Leveau and Lindow (2001a) used a fructose-responsive promoter fused to the gene for GFP to probe the availability of this sugar to bacteria on
plant leaf surfaces, also known as the phyllosphere (Leveau, 2006). Temporal and spatial variation in single-cell green fluorescence indicated substantial heterogeneity in the availability
of fructose to individual leaf colonizers (Leveau and Lindow, 2001a). Such heterogeneity has also been reported for other nutrients or stimuli that leaf bacteria are exposed to, including
iron (Joyner and Lindow, 2000), water (Axtell and Beattie, 2002), UV light (Gunasekera and Sundin, 2006) and phenolic compounds (Sandhu et al., 2007). While bacterial bioreporters, such as
the ones described above, are useful in micrometer mapping of differences in the bacterial experience of single environmental variables, they cannot communicate how each of those variables,
individually or jointly, impact the fate of bacteria in the environment under study. We therefore designed a bioreporter tool that describes micrometer-scale environmental heterogeneity in
general terms, that is as a sum of all variables expressed into a single, quantifiable effect on the bacterium. The bioreporter we introduce here records environmental heterogeneity in terms
of past reproductive success. In concept, it is based on the observation that upon cell division, GFP in a bacterial cell is distributed in a predictable manner between its two daughter
cells (Rosenfeld et al., 2006; Roostalu et al., 2008): one division leaves cells approximately half as green fluorescent as their parent, two divisions one-fourth as fluorescent, and so on.
Thus, the GFP content of an individual offspring cell becomes a quantifiable measure of reproductive success. This approach resembles the method that was used (Mailloux and Fuller, 2003) to
estimate _in situ_ doubling times for bacteria released into an aquifer after staining them with carboxyfluorescein diacetate succinimidyl ester, a fluorescent protein stain that dilutes
from the bacteria with every cell division. However, whereas these authors were interested solely in population averages of _in situ_ growth, we tested our GFP-based bioreporter by exposure
to microscopic conditions of low (that is, LB broth) and high (that is, the phyllosphere) environmental heterogeneity to reveal sub-population differences in the reproduction of single
bacteria. The implications of our findings extend broadly to studies on other microbial habitats dealing with the question of how individual bacteria in founder populations differ in their
contribution to future population sizes. MATERIALS AND METHODS BACTERIAL STRAINS AND CULTURE CONDITIONS _Erwinia herbicola_ 299R∷JBA28 (pCPP39) (_Eh_299R∷JBA28 (pCPP39)) (Leveau and Lindow,
2001b) carries a chromosomal mini-Tn_5_-Km transposon insertion that expresses stable GFP from a LacIq-repressible P_A1/O4/O3_ promoter fusion to _gfp_mut3. The transposon confers resistance
to kanamycin. The strain also harbors plasmid pCPP39, which confers tetracycline resistance and harbors a _lacI_q gene for control of P_A1/O4/O3_ activity, and thus GFP production by
isopropyl-β-D-thiogalactopyranoside (IPTG). Bacteria were cultivated at 28 °C on LB agar or in LB broth at 300 r.p.m. Where appropriate, IPTG, kanamycin or tetracycline were added to final
concentrations of 1 mM, 50 or 15 μg ml−1, respectively. Optical densities of bacterial cultures were measured at 600 nm (OD600) in a Unico 1100 spectrophotometer (Unico, Dayton, NJ, USA).
GFP-LOADING, RELEASE AND RECOVERY OF BIOREPORTER _EH_299R∷JBA28 (PCPP39) Exponentially growing cells of _Eh_299R∷JBA28 (pCPP39) were diluted 300-fold into fresh LB broth containing 1 mM IPTG
and grown to mid-exponential phase. These GFP-loaded cells were used to inoculate plant leaves (see below) or LB broth. In the latter case, 25 ml of LB was inoculated with 200 μl of
GFP-loaded bacteria and incubated at 28 °C and 300 r.p.m. Samples were taken every 30 min to measure OD600 and to collect bacteria for fixation (see below). For plant inoculations,
GFP-loaded bacteria were diluted in Milli-Q water to a final concentration of 5 × 104 colony-forming units ml−1. Leaves of 12–14-day old _Phaseolus vulgaris_ plants (green snap bean, variety
Blue Lake Bush 274) were inoculated by brief submersion into this bacterial suspension, shaken to dispose of excessive liquid and transferred to a closed translucent box for high-humidity
incubation at 21 °C. At different time intervals, two leaves were transferred to a 50-ml Falcon tube with 20 ml 1 × PBS buffer, vortexed briefly and sonicated for 7 min. Part of the
bacterial cells in the leaf washing was plated on agar for counting colony forming units, whereas the rest was collected on 0.2-μm Durapore filters (Millipore, Amsterdam, The Netherlands),
recovered by vortexing for 15 s in 1 ml 1 × PBS, and fixed (see below). FLUORESCENCE _IN SITU_ HYBRIDIZATION, FLUORESCENCE MICROSCOPY AND IMAGE CYTOMETRY Bacterial cells collected from LB
broth or plant leaves were fixed as described previously (Leveau and Lindow, 2001a) and stored at −20 °C in 50% 1 × PBS/50% ethanol for no longer than 2 weeks. To distinguish cells of
_Eh_299R∷JBA28 (pCPP39) from indigenous bacteria on the bean leaves, fixed leaf washings were subjected to fluorescence _in situ_ hybridization using an _Eh_299R-specific, TAMRA-labeled
probe (Brandl et al., 2001) at a final concentration of 5.5 ng μl−1. LB- or leaf-exposed cells were examined with an Axio Imager.M1 (Zeiss, Oberkochen, Germany) using 470/20 nm excitation
for the visualization of GFP and 546/6 nm for TAMRA. Digital images were captured at 1000-fold magnification with an AxioCam MRm camera (Zeiss) in phase contrast and through a 525/25 nm
(GFP) or 575–640 nm (TAMRA) filter set. Using AxioVision 2.6 Software (Zeiss), single-cell GFP fluorescence was quantified as the mean-pixel intensity (Leveau and Lindow, 2001a), and
expressed in units of Sfere (Standardized fluorescence reference), where 1 milliSfere equals one-thousandth of the average mean-pixel intensity of 1-μm Tetraspeck Fluorescent Microsphere
Standards (Molecular probes, Eugene, OR, USA). Data analyses and simulations were performed in Microsoft Excel 2003 (Microsoft Corporation, Redmond, WA, USA). For computer simulations
presented in Figure 3a, reproductive success was calculated for 100 cells with a green fluorescence (GF) equal to X/2_t_, in which X equals the Excel formula ‘=norminv(rand(),1000,250)’.
Figure 3b shows the temporal changes in reproductive success of individual bacteria as a function of _t_ from a population of 90 cells with GF=X and 10·2_t_ cells with GF=X/2_t_. Figure 3c
shows the reproductive success of 20 bacteria with GF=X, 20·20.125·_t_ bacteria with GF=X/20.125·_t_, 20·20.25·_t_ bacteria with GF=X/20.25·_t_, 20·20.5·_t_ bacteria with GF=X/20.5·_t_ and
20·2_t_ bacteria with GF=X/2_t_. Figure 3d shows the reproductive success in a population of 20 bacteria with GF=X, 20·2_t_ bacteria with GF=X/2_t_ (_t_⩽1) or 20·21 bacteria with GF=X/21
(_t_>1), 20·2_t_ bacteria with GF=X/2_t_ (_t_⩽2) or 20·22 bacteria with GF=X/22 (_t_>2), 20·2_t_ bacteria with GF=X/2_t_ (_t_⩽3) or 20·23 bacteria with GF=X/23 (_t_>3) and 20·2_t_
bacteria with GF=X/2_t_. RESULTS GFP DILUTION IS A QUANTITATIVE MEASURE FOR REPRODUCTIVE SUCCESS A previously formulated mathematical model of GFP expression in bacteria (Leveau and Lindow,
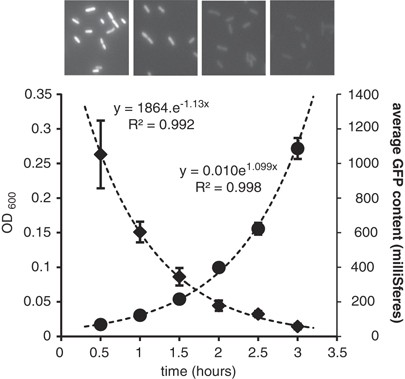
2001b) predicts that in the absence of _de novo_ synthesis, GFP dilutes from dividing cells at a rate equal to growth rate μ. We verified this prediction here using _Eh_299R∷JBA28 (pCPP39),
which accumulates stable GFP in the presence of the synthetic inducer IPTG. Upon transfer of IPTG-induced GFP-loaded cells to broth that lacked IPTG, GFP fluorescence declined exponentially
and at a rate that was not significantly different from μ (Figure 1). No such decrease in fluorescence was observed when GFP-loaded cells were transferred to sterile Milli-Q water (data not
shown), confirming that GFP is extremely stable in this strain and that its dilution from cells depends on division. From Figure 1, it follows that the average number of cell divisions since
_t_=0 can be calculated from the GFP fluorescence at times _t_ (GFP_t_) and _t_=0 (GFP_0_) as 2log(GFP_0_/GFP_t_). This value is essentially a measure of reproductive success; in simple
terms, it means that the dimmer a cell is, the more successful its ancestor was in producing offspring. Figure 2a shows that in an exponentially growing bacterial population in LB broth, the
reproductive success of individual cells (which for any individual cell was calculated as 2log[average cell's GFP fluorescence at _t_=0 divided by single cell's fluorescence at
time _t_]) was normally distributed. The parallel lines signify that over time, all cells contributed with equal success to the population increase (Figure 2a). This observation is
consistent with the assumption that a shaking LB culture represents a homogeneous environment. A very different result was obtained when GFP-loaded cells of _Eh_299R∷JBA28 (pCPP39) were
released onto leaf surfaces of bean plants. In this environment (which is devoid of IPTG, see below), progeny bacteria showed considerably more variation in reproductive success compared
with those in LB (Figure 2b), with clear deviation from the parallel lines observed in Figure 2a. This suggests that some immigrant cells to the leaves contributed more progeny to the
population than others. In a control experiment, we introduced an uninduced culture of _Eh_299R∷JBA28 (pCPP39) onto leaf surfaces. Analysis of bacteria recovered from leaves after 24 h
revealed that they had remained non-fluorescent, confirming that (1) the leaf surface was devoid of IPTG or other compounds that might induce _de novo_ synthesis of GFP and (2) loss of
plasmid pCPP39 (which would lead to constitutive expression of GFP) did not occur during the course of the experiment. MODELING OF GFP DILUTION IN INDIVIDUAL CELLS To facilitate
interpretation of the experimental data from the leaf surface, we ran four simple simulations (see Materials and methods) to examine the effects of different scenarios of environmental
heterogeneity on the shape of reproductive success distributions. In the first scenario, all cells experienced the same conditions for maximal growth, much like the experiment in LB broth.
This resulted in normal distributions of reproductive success (Figure 3a), as expected and as observed experimentally (Figure 2a). In a second scenario, the starter population was split into
two sub-populations, one of which, representing an arbitrary 10% of the cells, had a maximum reproductive success rate, whereas 90% were unsuccessful at producing offspring (Figure 3b). In
the other two simulations, the starter population was divided into five equal sub-populations, each of which produced progeny at different rates (Figure 3c) or produced progeny at the same
maximum rate but ceased doing so at different times during the course of the simulation (Figure 3d). Comparison of the simulated distribution curves to the experimental ones suggests that it
is unlikely that the leaf surface consists of only two types of locales: one that fully supports bacterial growth and another that does not. Based on Figure 3b, this would have resulted in
the clear separation of two sub-populations in the distribution curves. Instead, it seems more likely that leaf locales represent a sliding scale in their ability to support growth of
initial colonizers. Figures 3c and d show that patterns of increased heterogeneity can be simulated by assuming sub-populations that differ in their ability to reproduce, either through
being offered less than favorable growth conditions or by being offered less time or resources to reproduce. Which one of these scenarios applies to the leaf surface, or whether it is a
combination of the two, cannot be easily resolved by comparison of experimental to simulated data. However, both simulations prove the point that heterogeneity in reproductive success is
indicative of a starter population in which cells are exposed to different growth-permitting conditions, resulting in unequal contributions to future offspring and population sizes.
BACTERIAL IMMIGRANTS TO THE PHYLLOSPHERE CONTRIBUTE DIFFERENTIALLY TO LEAF POPULATION SIZES Based on these simulations, we interpreted the experimental leaf data (Figure 2b) as follows.
During the first 3 h, GFP content did not differ significantly from _t_=0 across the population, suggesting that the bacteria did not reproduce during that time. Six hours after inoculation,
>90% cells appeared in a straight line more or less parallel to the _t_=0 distribution, but with an average reproductive success of 2.9 divisions. This suggests that during the early
period of colonization, most cells encountered similar conditions, allowing them to contribute equally to an approximately 22.9, that is 7.5-fold population increase. About 5% of the cells
appeared brighter than expected. These might represent cells with ancestors that settled in spots unfavorable for growth. With time, the shape of the distribution curve changed (Figure 2b).
At _t_=9 h, approximately 3% of the cells had divided 0 times, 2% 1 time, 8% 2 times, 36% 3 times and 51% 4 times or more. Extrapolated to _t_=0, this means that 22%, 7%, 15%, 33% and 23% of
the starter population contributed 3%, 2%, 8%, 36% and 51%, respectively, of the population at _t_=9 (Figure 4). In other words, while nearly one-third of the starter cells contributed only
5% to the population size at _t_=9, less than one-fourth eventually contributed more than half. These data confirm that bacterial immigrants to the leaf surface contributed differently to
population sizes, which is consistent with the hypothesis that the fate of individual immigrants is determined in large part by the environmental heterogeneity at the microscopic leaf level.
We were unable to make estimates of relative contribution for the _t_=24 population, as 79% of the cells had divided beyond the limit of GFP detection. ESTIMATING POPULATION CHANGES FROM
SINGLE-CELL DATA Figure 5a shows the changes in bacterial population sizes on the leaves, as determined by plate counting. The growth curve follows a pattern that is typical for this type of
plant inoculation experiment, including a short lag, a phase of rapid growth and a level-off to apparent carrying capacity. It is interesting to note that this pattern can be reproduced
quite well using only the single-cell data. From _N_=_N_0·e_μt_ (in which _N_ is the number of cells at time _t_, and _N_0 is the number of cells at _t_=0) and GFP=GFP0·e−_μt_ (in which GFP
is the average single-cell GFP content at time _t_, and GFP0 is the average single-cell GFP content at _t_=0), μ and _t_ can be eliminated to reveal that N/N0 equals GFP0/GFP. In other
words, a plot of GFP0/GFP as a function of time produces in essence a growth curve, which is indeed confirmed for our data in Figure 5b. The underestimation of growth by the single-cell data
at _t_=9 and _t_=24 is likely because of the fact that the reproductive success of a portion of the cells was undervalued because their GFP content was below the limit of fluorescence
detection. Combined, Figures 4 and 5 demonstrate that our GFP bioreporter allows the assessment of population growth at the individual as well as population level. DISCUSSION The
experimental data we present here demonstrate the utility of the ‘reproductive success’ concept by offering new insight into bacterial phyllosphere colonization. The heterogeneity that we
observed in the ability of individual immigrants to produce offspring on the leaf surface is a novel observation. It corroborates findings of others who have documented leaf-based
heterogeneity in environmental stimuli (Joyner and Lindow, 2000; Leveau and Lindow, 2001a; Axtell and Beattie, 2002; Gunasekera and Sundin, 2006), each of which is likely to affect
reproduction. Together, these data support the hypothesis that local conditions are the key determinants of the abundance and dynamics of microbes on plant leaf surfaces (Woody et al.,
2007). We noted striking similarities in the interpretation of leaf colonization patterns based on our bioreporter data and those from a previously described bioreporter that is also _E.
herbicola_-based but measures fructose availability (Leveau and Lindow, 2001a). In both cases, bacterial cells appeared to experience a period of adaptation immediately after immigration to
the leaf surface. In the experiment presented here, this period of adaptation was accompanied by a reduction in cell size (data not shown), which concentrated the GFP fluorescence signal in
the bacteria, resulting in brighter green fluorescent cells and lower apparent values for reproductive success (Figure 2b). This initial period of adaptation was followed by a period of
reproduction for nearly all immigrants. The fructose bioreporter revealed that bacteria differ substantially in their subsequent access to fructose, causing them to deplete their resources
and cease dividing at different times during colonization. This parallels our observation here of unequal contributions to the population size (Figure 2b). These lines of evidence suggest
that heterogeneity in fructose availability at the micrometer scale has an important role in the reproductive success of individual bacterial immigrants to the leaf surface. Our observations
of intra-population variability in the reproduction of bacteria on leaf surfaces are compatible with current theories of aggregative behavior of bacteria in the phyllosphere. Various
studies (Morris et al., 1998; Monier and Lindow, 2004) have shown that many or most bacteria on naturally or experimentally inoculated leaves occur not as isolated cells but in aggregates.
Aggregation has been explained to result from the differential survival and growth of solitary and aggregated cells (Monier and Lindow, 2003). In the case of _Pseudomonas syringae_ (Monier
and Lindow, 2004), the frequency distribution of the number of cells per aggregate was found to be right-hand skewed, representing a sliding scale from many aggregates with few bacteria to
few aggregates with many bacteria. Assuming that each aggregate arose from a single founder cell, a right-hand skewed distribution of aggregate sizes would indeed translate into a curved
distribution of reproductive success, much like we observed for _E. herbicola_ cells on leaves after prolonged exposure to the leaf surface (Figures 2b and 5b). A limitation of our
bioreporter is the inability to interpret reproductive success for cells in which GFP is diluted beyond the limit of detection. In our most optimal setup, this corresponded to six doublings,
or 64 progeny cells from a single ancestor. This is sufficient for studies that are relatively short-term, involve bacteria with low rates of reproduction, or habitats with low or
intermediate degrees of environmental heterogeneity. The need for fluorescence _in situ_ hybridization to distinguish bioreporters from indigenous cells made it impossible to follow
bacterial reproduction beyond four divisions. In future versions of the bioreporter, this may be solved by complementation of the bioreporter with a GFP-compatible, constitutively expressed
fluorescent protein, for example red fluorescent mCherry (Shaner et al., 2004). An additional advantage is the prospect of _in situ_ observation of the bioreporter independent of its GFP
fluorescence. Thus, one can start to interpret ancestral success of individual bacteria in the context of their location in the micrometer landscape. Despite this room for improvement, the
bioreporter in its current form offers several unique opportunities and advantages. One of its strengths is that reproductive success is recorded in the GFP content of each cell, which is a
major advantage for studies that allow only intermittent observation or that necessitate destructive sampling of the environment, as most experiments in microbial ecology do. Another plus of
the reproductive success bioreporter is that it offers microbial ecologists low-ambiguity output. Most GFP bioreporters are promoter-based, and although promoters can be quite specific in
response to the environmental variable under investigation, their activity can be modulated in unpredictable ways by other input from the environment (Leveau and Lindow, 2001b). Such
ambiguity makes promoter-based bioreporters susceptible to misinterpretation, particularly in the absence of proper controls (Leveau and Lindow, 2001b). The reproductive-success bioreporter
is promoter-independent in that it is solely based on dilution of previously synthesized GFP from the cell by division, hence with the minimal likelihood of misinterpretation of GFP output.
Such types of bioreporters are expected to have the broadest and most reliable utility in microbial ecology. Another major advantage of our bioreporter is its compatibility with many other
single-cell interrogation techniques (Davey and Kell, 1996; Brehm-Stecher and Johnson, 2004) with the ultimate goal to link reproductive success at single-cell resolution to specific
bacterial behaviors or environmental experiences, and to identify the sources of heterogeneity and their impacts on bacterial individuals and on population structure and activity. REFERENCES
* Axtell CA, Beattie GA . (2002). Construction and characterization of a _proU-gfp_ transcriptional fusion that measures water availability in a microbial habitat. _Appl Environ Microbiol_
68: 4604–4612. Article CAS Google Scholar * Brandl MT, Quinones B, Lindow SE . (2001). Heterogeneous transcription of an indoleacetic acid biosynthetic gene in _Erwinia herbicola_ on
plant surfaces. _Proc Nat Acad Sci USA_ 98: 3454–3459. Article CAS Google Scholar * Brehm-Stecher BF, Johnson EA . (2004). Single-cell microbiology: tools, technologies, and applications.
_Microbiol Mol Biol Rev_ 68: 538–559. Article CAS Google Scholar * Davey HM, Kell DB . (1996). Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of
single-cell analyses. _Microbiol Rev_ 60: 641–696. CAS PubMed PubMed Central Google Scholar * Davey HM, Winson MK . (2003). Using flow cytometry to quantify microbial heterogeneity.
_Curr Issues Mol Biol_ 5: 9–15. PubMed Google Scholar * Davidson CJ, Surette MG . (2008). Individuality in bacteria. _Annu Rev Genet_ 42: 253–268. Article CAS Google Scholar * Green J,
Bohannan BJM . (2006). Spatial scaling of microbial biodiversity. _Trends Ecol Evol_ 21: 501–507. Article Google Scholar * Gunasekera TS, Sundin GW . (2006). Role of nucleotide excision
repair and photoreactivation in the solar UVB radiation survival of _Pseudomonas syringae_ pv. _syringae_ B728a. _J Appl Microbiol_ 100: 1073–1083. Article CAS Google Scholar * Harms H,
Wells MC, van der Meer JR . (2006). Whole-cell living biosensors—are they ready for environmental application? _Appl Microbiol Biotechnol_ 70: 273–280. Article CAS Google Scholar *
Hellweger FL, Bucci V . (2009). A bunch of tiny individuals—individual-based modeling for microbes. _Ecol Modell_ 220: 8–22. Article Google Scholar * Jaspers MCM, Meier C, Zehnder AJB,
Harms H, van der Meer JR . (2001). Measuring mass transfer processes of octane with the help of an _alkS-alkB_∷_gfp_-tagged _Escherichia coli_. _Environ Microbiol_ 3: 512–524. Article CAS
Google Scholar * Joyner DC, Lindow SE . (2000). Heterogeneity of iron bioavailability on plants assessed with a whole-cell GFP-based bacterial biosensor. _Microbiology_ 146: 2435–2445.
Article CAS Google Scholar * Leveau JHJ . (2006). Microbial communities in the phyllosphere. In: Riederer M and Mueller C (eds). _Biology of the Plant Cuticle_. Blackwell Publishing Ltd:
Oxford, UK, pp 334–367. Chapter Google Scholar * Leveau JHJ, Lindow SE . (2001a). Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. _Proc
Nat Acad Sci USA_ 98: 3446–3453. Article CAS Google Scholar * Leveau JHJ, Lindow SE . (2001b). Predictive and interpretive simulation of green fluorescent protein expression in reporter
bacteria. _J Bacteriol_ 183: 6752–6762. Article CAS Google Scholar * Leveau JHJ, Lindow SE . (2002). Bioreporters in microbial ecology. _Curr Opin Microbiol_ 5: 259–265. Article Google
Scholar * Mailloux BJ, Fuller ME . (2003). Determination of _in situ_ bacterial growth rates in aquifers and aquifer sediments. _Appl Environ Microbiol_ 69: 3798–3808. Article CAS Google
Scholar * Maksimow M, Hakkila K, Karp M, Virta M . (2002). Simultaneous detection of bacteria expressing _gfp_ and _dsred_ genes with a flow cytometer. _Cytometry_ 47: 243–247. Article CAS
Google Scholar * Melbourne BA, Cornell HV, Davies KF, Dugaw CJ, Elmendorf S, Freestone AL _et al_. (2007). Invasion in a heterogeneous world: resistance, coexistence or hostile takeover?
_Ecol Lett_ 10: 77–94. Article Google Scholar * Monier JM, Lindow SE . (2003). Differential survival of solitary and aggregated bacterial cells promotes aggregate formation on leaf
surfaces. _Proc Nat Acad Sci USA_ 100: 15977–15982. Article CAS Google Scholar * Monier JM, Lindow SE . (2004). Frequency, size, and localization of bacterial aggregates on bean leaf
surfaces. _Appl Environ Microbiol_ 70: 346–355. Article CAS Google Scholar * Morris CE, Monier JM, Jacques MA . (1998). A technique to quantify the population size and composition of the
biofilm component in communities of bacteria in the phyllosphere. _Appl Environ Microbiol_ 64: 4789–4795. CAS PubMed PubMed Central Google Scholar * Prosser JI, Bohannan BJM, Curtis TP,
Ellis RJ, Firestone MK, Freckleton RP _et al_. (2007). The role of ecological theory in microbial ecology. _Nature Rev Microbiol_ 5: 384–392. Article CAS Google Scholar * Roostalu J,
Joers A, Luidalepp H, Kaldalu N, Tenson T . (2008). Cell division in _Escherichia coli_ cultures monitored at single cell resolution. _BMC Microbiol_ 8: 68. Article Google Scholar *
Rosenfeld N, Perkins TJ, Alon U, Elowitz MB, Swain PS . (2006). A fluctuation method to quantify _in vivo_ fluorescence data. _Biophys J_ 91: 759–766. Article CAS Google Scholar * Sandhu
A, Halverson LJ, Beattie GA . (2007). Bacterial degradation of airborne phenol in the phyllosphere. _Environ Microbiol_ 9: 383–392. Article CAS Google Scholar * Scheiner S, Willig M .
(2008). A general theory of ecology. _Theor Ecol_ 1: 21–28. Article Google Scholar * Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY . (2004). Improved monomeric
red, orange and yellow fluorescent proteins derived from _Discosoma_ _sp._ red fluorescent protein. _Nat Biotechnol_ 22: 1567–1572. Article CAS Google Scholar * Tecon R, van der Meer JR .
(2006). Information from single-cell bacterial biosensors: what is it good for? _Curr Opin Biotechnol_ 17: 4–10. Article CAS Google Scholar * Templeton AR, Rothman ED . (1978). Evolution
in fine-grained environments: 1. environmental runs and evolution of homeostasis. _Theor Popul Biol_ 13: 340–355. Article CAS Google Scholar * Woody ST, Ives AR, Nordheim EV, Andrews JH
. (2007). Dispersal, density dependence, and population dynamics of a fungal microbe on leaf surfaces. _Ecology_ 88: 1513–1524. Article Google Scholar Download references ACKNOWLEDGEMENTS
We thank Maria Marco for useful comments on the paper and Steve Lindow for his support in the conception phase of the project. Funding was provided by the Netherlands Organisation of
Scientific Research (NWO) in the form of a personal VIDI grant to JHJL. This is NIOO-KNAW publication 4626. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Microbial Ecology,
Netherlands Institute of Ecology (NIOO-KNAW), Heteren, The Netherlands Mitja NP Remus-Emsermann & Johan HJ Leveau * Department of Plant Pathology, University of California, Davis, CA,
USA Johan HJ Leveau Authors * Mitja NP Remus-Emsermann View author publications You can also search for this author inPubMed Google Scholar * Johan HJ Leveau View author publications You can
also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Johan HJ Leveau. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Remus-Emsermann, M., Leveau, J. Linking environmental heterogeneity and reproductive success at single-cell resolution. _ISME J_ 4, 215–222 (2010).
https://doi.org/10.1038/ismej.2009.110 Download citation * Received: 23 July 2009 * Revised: 21 September 2009 * Accepted: 21 September 2009 * Published: 29 October 2009 * Issue Date:
February 2010 * DOI: https://doi.org/10.1038/ismej.2009.110 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * individual-based ecology *
environmental heterogeneity * bioreporter * phyllosphere * single-cell microbiology