Haplotype architecture of the norepinephrine transporter gene slc6a2 in four populations
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The norepinephrine transporter (NET) regulates levels of monoamine neurotransmitters integral to a variety of behaviors and autonomic functions. Two _SLC6A2_ polymorphisms have been
used in genetic association studies, generating intriguing but nondefinitive results on traits such as hypertension and mood. One of these _SLC6A2_ variants is functional but rare. The
other is common but not informative over the entire 48 kb _SLC6A2_ region and is insufficient to capture the functional diversity potentially contained within any _SLC6A2_ region. To
elucidate _SLC6A2_ haplotype structure and define markers sufficient to capture haplotype diversity within detected haplotype blocks, 26 single-nucleotide polymorphisms (SNPs) were genotyped
in 384 individuals evenly divided across Finnish Caucasian, US Caucasian, Plains American Indian, and African American populations. Three conserved blocks, 13.6, 12.5, and 25 kb in size and
showing little evidence for historical recombination were observed in all populations. Haplotype diversity in block 1 and numbers of common haplotypes were highest in African Americans,
among whom 5–6 optimal markers were sufficient to maximize diversity of each block. For other populations, 2–3 markers/block sufficed, but the optimal markers differed across populations.
The _SLC6A2_ haplotype map and 25-marker panel (excluding the monomorphic one) is a comprehensive tool for genetic linkage studies on phenotypes related to NET function. SIMILAR CONTENT
BEING VIEWED BY OTHERS IDENTIFICATION AND FUNCTIONAL CHARACTERIZATION OF THE EXTREMELY LONG ALLELE OF THE SEROTONIN TRANSPORTER-LINKED POLYMORPHIC REGION Article Open access 11 February 2021
DEVELOPMENT OF A METHOD FOR THE IMPUTATION OF THE MULTI-ALLELIC SEROTONIN-TRANSPORTER-LINKED POLYMORPHIC REGION (5-HTTLPR) IN THE JAPANESE POPULATION Article 25 September 2024 SEROTONIN
TRANSPORTER FUNCTIONAL POLYMORPHISMS POTENTIALLY INCREASE RISK OF SCHIZOPHRENIA SEPARATELY AND AS A HAPLOTYPE Article Open access 25 January 2022 INTRODUCTION In humans, norepinephrine (NE)
is essential to fundamental cognitive and emotional processes including attention, learning and memory, perception of emotions, and perception of pain (Foote et al. 1983; Jasmin et al.
2002). NE is also involved in autonomic control via its actions in the brainstem and as the primary neurotransmitter at postganglionic sympathetic nerve terminals (Hahn et al. 2003). The
majority of brain noradrenergic neurons are concentrated in the locus coeruleus, a phylogenetically ancient and developmentally precocious structure. These NE neurons project to limbic
regions critical to cognition and affect. NE released at central and peripheral synapses is inactivated through active transport into terminals by the presynaptically localized
norepinephrine transporter (NET) (Iversen 1974). NET recaptures as much as 90% of released NE making it a critical mediator of NE inactivation and presynaptic catecholamine homeostasis
(Schomig et al. 1989). Thus, NET plays a role in controlling the intensity and duration of signal transduction (Zahniser et al. 2001). NE interacts with many other neurotransmitters both in
normal cortical regulation and in the therapeutic response to psychoactive compounds, and one critical interacting neurotransmitter is dopamine (Jordan et al. 1994). Dopamine is the NE
precursor so that levels of both neurotransmitters are regulated by common factors, for example, tyrosine hydroxylase activity. The NET has the ability to transport dopamine, and drugs that
block the NET increase extracellular levels of both NE and DA (Tanda et al. 1997; Bymaster et al. 2002; Gu et al. 1996). Monoamine transporters are initial sites of action for several
antidepressant drugs (including several which are relatively NET selective) as well as psychostimulants including cocaine and the amphetamines (Pacholczyk et al. 1991; Ritz et al. 1990;
Tatsumi et al. 1997; Sacchetti et al. 1999). Decreases in NE uptake sites and activity have been observed in hypertension, diabetes, cardiomyopathy, and heart failure (Esler et al. 1981;
Merlet et al. 1992; Bohm et al. 1995; Schnell et al. 1996; Backs et al. 2001), and insufficient NE clearance may contribute to the progression of these diseases (Bohm et al. 1998). The human
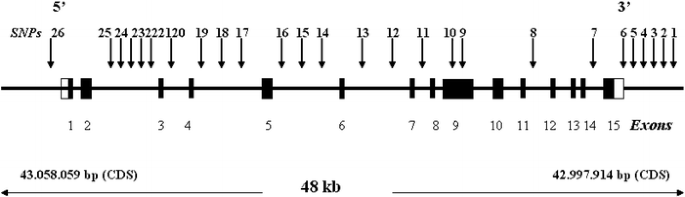
NET gene (_SLC6A2_, hCG2025341) is located on chromosome 16q12.2 (Brüss et al. 1993) and has 15 exons spanning ∼48 kb (Pörzgen et al. 1995, 1998). The cDNA sequence encodes a 617-amino acid
protein with 12 highly hydrophobic membrane domains and a high level of amino acid identity to other members of the Na+/Cl−-dependent monoamine transporter family, e.g., _HTT_ (serotonin
transporter) and _DAT_ (dopamine transporter) (Nelson 1998; Hahn and Blakely 2002). _SLC6A2_ has five alternative splice transcripts. Resequencing of _SLC6A2_ identified 13 DNA sequence
variants, among them five low-frequency missense substitutions (Stober et al. 1996). The reported missense substitutions Val69Ile, Thr99Ile, Val245Ile, Val449Ile, and Gly478Ser are located
in putative transmembrane domains 1, 2, 4, 9, and 10, respectively. The Thr99Ile substitution is at the 5th position of a putative leucine zipper in transmembrane domain 2. A rare Ala457Pro
substitution in exon 9 resulting in more than 98% loss of function has recently been detected (Ivancsits et al. 2003). A synonymous substitution also located in exon 9, A1287G, has been used
in a series of association studies to NE-related phenotypes including hypertension and mood disorders (Stober et al. 1996; Leszczynska-Rodziewicz et al. 2002; Samochowiec et al. 2002).
However, no common functional NET polymorphisms are known. The NET markers used so far in linkage studies do not capture the potential information on NET functional variation, and the
results obtained so far have been nondefinitive. A haplotype approach combining abundant missense polymorphisms with a series of loci chosen for haplotype informativeness offers the
potential for detection of effects of any allele of moderate abundance and effect size, regardless of whether the allele is presently known or unknown (Gabriel et al. 2002). Concerning
linkage disequilibrium (LD), many regions of the genome have a block-like structure such that all loci within the block region tend to be in strong LD. However, haplotype block boundaries,
strength of LD, haplotype diversity, and optimal marker panels to fully capture haplotype diversity vary across populations. In this study, we report the haplotype structure of _SLC6A2_
obtained using 26 single-nucleotide polymorphisms (SNPs) genotyped in four populations: Finnish and American Caucasians, American Indians, and African Americans. We also describe marker
panels for each block, which maximize haplotype information content. MATERIALS AND METHODS PARTICIPANTS A total of 384 participants were genotyped, including 96 individuals from each of four
populations: Finns, US Caucasians, African Americans, and Plains American Indians. Informed consent was obtained according to human research protocols approved by the human research
committees of the recruiting institutes, including the National Institute on Alcohol Abuse and Alcoholism, National Institute of Mental Health, Rutgers University, and University of
Helsinki. All participants had been psychiatrically interviewed, and none had been diagnosed with a psychiatric disorder. SNP MARKERS The physical position and frequency of minor alleles
(>0.05) from a commercial database (Celera Discovery System, CDS, July, 2003) were used to select SNPs (including A1287G and Ala457Pro). A total of 50 _SLC6A2_ SNPs were identified in the
database. 5′ nuclease assays (_vide infra_) could be designed for 35, and of these, 26 SNP assays detected sequence polymorphisms and could be genotyped in highly accurate fashion. This
panel of 26 equally spaced markers covered the 48-kb gene plus 4 kb upstream and 4 kb downstream. GENOMIC DNA Genomic DNA was extracted from lymphoblastoid cell lines and diluted to a
concentration of 10 ng/ul; 1-ul aliquots were dried in 384-well plates. POLYMERASE CHAIN REACTION (PCR) AMPLIFICATION Genotyping was performed by the 5′ nuclease method (Shi et al. 1999)
using fluorogenic allele-specific probes. Oligonucleotide primer and probe sets were designed based on gene sequence from the CDS, July 2003. Primers and detection probes for each locus are
listed in Table 1. In each reaction well, 2.5 μl of PCR Master Mix (Applied Biosystems, CA, USA) containing AmpliTaq Gold DNA Polymerase, dNTPs, gold buffer, and MgCl2 was mixed with 900
nmol of each forward and reverse primer and 100 nmol of each reporter and quencher probe. DNA was allowed to stand at 50°C for 2 min and at 95°C for 10 min, amplified by 40 cycles at 95°C
for 15 s and 60°C for 1 min, and then held at 4°C. PCR was carried out with a GeneAmp PCR system 9700 (Applied Biosystems). Allele-specific signals were distinguished by measuring endpoint
6-FAM or VIC fluorescence intensities at 508 and 560 nm, respectively, and genotypes were generated using Sequence Detection System Software Version 1.7 (Applied Biosystems). Genotyping
error rate was directly determined by regenotyping 25% of the samples, randomly chosen, for each locus. The overall error rate was <0.005. Genotype completion rate was 0.98. HAPLOTYPE
ANALYSIS Haplotype frequencies were estimated using a Bayesian approach implemented with PHASE (Stephens et al. 2001). These frequencies closely agreed with results from a maximum likelihood
method implemented via an expectation–maximization (EM) algorithm (Long et al. 1995). RESULTS AND DISCUSSION Of a total of 26 _SLC6A2_ SNPs, 25 were polymorphic in all four populations.
Dramatic interpopulation differences in allele frequencies were observed for most of the SNPs. Ala457Pro, the previously reported in vitro functional variant, was monomorphic in the 384
individuals representing the four populations. Allele frequencies of _SLC6A2_ SNPs and their locations in the gene are shown in Table 2. The majority of the markers are located in the
intronic sequence of the gene, one synonymous substitution (A1287G) is located in exon 9, one marker is located in the 3′ UTR region, five are in the 3′ region, and one is in the 5′ region
(Fig. 1). All genotype frequencies conformed to Hardy–Weinberg equilibrium. Within _SLC6A2_, three conserved LD blocks (1, 13.6 kb; 2, 12.5 kb; 3, 25 kb) were observed in all four
populations. Definition of haplotype blocks and block boundaries is an inexact science. Some disruptions of _D_′ (a measure of LD) occurring within blocks are clearly attributable to low
allele frequencies that lead to increased variance in estimation of _D_′. We discounted low _D_′ values, which appeared to originate from this cause. In the _SLC6A2_ haplotype block regions,
_D_′ was generally >0.85 from one end of the region to the other. _D_′ averaged was 0.83, 0.94 and 0.94 in blocks 1, 2, and 3, and perhaps more importantly, the median _D_′ value within
haplotype blocks was 0.97, 0.97, and 1.00 for blocks 1, 2, and 3, meaning that most of the SNP loci were in very high LD. We note that in the situation that haplotype block boundaries are
drawn too widely, an increased number of haplotypes will be observed for the block and an increased number of markers will be required to capture this diversity. _SLC6A2_ haplotype block
boundaries could be drawn somewhat differently than we have done, and it can also be observed that there is some variation from population to population. For example, there was some
disruption of LD within block 1 in both Finns and Plains Indians. However, the marker panels we genotyped were sufficient to capture diversity in the blocks in the four populations we
studied, as described below. Pairwise LD values within each haplotype block are summarized in Tables 3, 4, and 5; all pairwise LD values among 25 SNPs across four populations are represented
in Tables 6, 7, 8, and 9. Haplotype frequencies for the three blocks in four populations are shown in Table 10. For each population and haplotype block, 3–6 common (≥0.05) haplotypes
accounted for most of the total: 85–96% of Caucasian and Plains Indian haplotypes and 75–89% of African American haplotypes. The number of common (≥0.05) haplotypes were block 1: 4, 5, 3, 6;
block 2: 5, 5, 4, 5, and block 3: 4, 5, 5, 6 for U.S. and Finnish Caucasians, Plains Indians, and African Americans, respectively. For each haplotype block, a panel of markers sufficient to
maximize genetic information content was available. Excluding Ala457Pro, the number of SNPs available for the three haplotype blocks were 8, 8, and 9 for blocks 1, 2, and 3, respectively.
Knowing this, the value of additional SNP markers for haplotype diversity (informativeness) can then be evaluated. We began with the haplotypes derived from all available markers in the
block and successively subtracted markers, first subtracting markers that resulted in no change whatever in haplotype diversity and then subtracting markers which changed diversity in the
most minimal fashion and so on until we were left with a single, independent, highest heterozygosity SNP marker. The figures graphically depict a reversal of this process, showing that
diversity can be maximized using a smaller group of tag SNPs selected from a larger panel. After each marker addition using the pathway determined by the subtraction analysis, haplotype
frequencies, and diplotype heterozygosity, the chosen measure of diversity were recalculated. At some point for each haplotype block and each population, the addition of a new SNP marker did
not appreciably increase diversity, as shown in Fig. 2. The required number of tag SNPs varies according to the haplotype diversity of the region (and population) and the information
content of the markers available. Haplotype diversity in block 1 was greatest in African Americans, and to maximize it, more markers were needed (5–6 markers). For the other populations, 2–3
markers sufficed for block 1, but the optimal markers differed across populations. Thus, each SNP had different information content in different populations. For association/linkage
studies, different tag SNPs could be used in different target populations. Alternatively, the entire panel of 25 SNPs could be applied to reliably capture haplotype diversity across
populations. As illustrated in Fig. 2, genotyping larger panels of markers yields a steadily diminishing return, but another purpose of this approach is to capture more information on
certain rarer haplotypes. The focus of haplotype-based genetic association studies has been the detection of effects of moderately abundant loci, because haplotypes and functional alleles of
low frequency are not well represented in small datasets. However, power increases in larger datasets that may be available for certain noradrenergic related phenotypes, for example,
diseases such as hypertension and mood disorders, which are readily diagnosed and which afflict very large segments of populations. For _SLC6A2_, the 25-locus SNP panel defines a three-block
LD structure across the entire gene region and would be sufficient to capture the signal of any moderately abundant SNP. For example, A1287G is the marker most extensively used in NET
linkage studies, and this SNP is located in block 2, for which the _SLC6A2_ SNP panel includes another seven markers in addition to A1287G. When A1287G is excluded, 99% of the information
content of block 2 is still captured. In conclusion, the _SLC6A2_ haplotype map and marker panel are a comprehensive tool for genetic linkage studies on phenotypes related to noradrenergic
function. This map is a surrogate for moderately abundant effective alleles, which may be unknown or unrecognized as functional. REFERENCES * Backs J, Haunstetter A, Gerber SH, Metz J, Borst
MM, Strasser RH, Kubler W, Haass M (2001) The neuronal norepinephrine transporter in experimental heart failure: evidence for a posttranscriptional downregulation. J Mol Cell Cardiol
33:461–472 Article CAS Google Scholar * Bohm M, La Rosee K, Schwinger RH, Erdmann E (1995) Evidence for reduction of norepinephrine uptake sites in the failing human heart. J Am Coll
Cardiol 25:146–153 Article CAS Google Scholar * Bohm M, Castellano M, Flesch M, Maack C, Moll M, Paul M, Schiffer F, Zolk O (1998) Chamber-specific alterations of norepinephrine uptake
sites in cardiac hypertrophy. Hypertension 32:831–837 Article CAS Google Scholar * Brüss M, Kunz J, Lingen B, Bönisch H (1993) Chromosomal mapping of the human gene for the tricyclic
antidepressant-sensitive noradrenaline transporter. Hum Genet 91:278–280 Article Google Scholar * Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH,
Morin SM, Gehlert DR, Perry KW (2002) Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention
deficit/hyperactivity disorder. Neuropsychopharmacology 27:699–711 Article CAS Google Scholar * Esler M, Jackman G, Bobik A, Leonard P, Kelleher D, Skews H, Jennings G, Korner P (1981)
Norepinephrine kinetics in essential hypertension. Defective neuronal uptake of norepinephrine in some patients. Hypertension 3:149–156 Article CAS Google Scholar * Foote SL, Bloom FE,
Aston-Jones G (1983) Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63:844–914 Article CAS Google Scholar * Gabriel SB, Schaffner SF, Nguyen
H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D (2002) The structure
of haplotype blocks in the human genome. Science 296:2225–2229 Article CAS Google Scholar * Gu HH, Wall S, Rudnick G (1996) Ion coupling stoichiometry for the norepinephrine transporter
in membrane vesicles from stably transfected cells. J Biol Chem 271:6911–6919 Article CAS Google Scholar * Hahn MK, Blakely RD (2002) Gene organization and polymorphisms of monoamine
transporters. Relationship to psychiatric and other complex diseases. In: Reith MEA (ed) Neurotransmitter transporters. Structure, function, and regulation, Humana, Totowa, NJ, pp 111–169 *
Hahn MK, Robertson D, Blakely RD (2003) A mutation in the human norepinephrine transporter gene (SLC6A2) associated with orthostatic intolerance disrupts surface expression of mutant and
wild-type transporters. J Neurosci 23:4470–4478 Article CAS Google Scholar * Ivancsits S, Heider A, Rudiger HW, Winker R (2003) Orthostatic intolerance is not necessarily related to a
specific mutation (Ala457Pro) in the human norepinephrine transporter gene. Am J Med Sci 325:63–65 Article Google Scholar * Iversen LL (1974) Uptake mechanisms for neurotransmitter amines.
Biochem Pharmacol 23:1927–1935 Article CAS Google Scholar * Jasmin L, Tien D, Weinshenker D, Palmiter RD, Green PG, Janni G, Ohara PT (2002) The NK1 receptor mediates both the
hyperalgesia and the resistance to morphine in mice lacking noradrenaline. Proc Natl Acad Sci 99:1029–1034 Article CAS Google Scholar * Jordan S, Kramer GL, Zukar PK, Moeller M, Petty F
(1994) In vivo biogenic amine efflux in medial prefrontal cortex with imipramine, fluoxetine, and fluvoxamine. Synapse 18:294–297 Article CAS Google Scholar * Leszczynska-Rodziewicz A,
Czerski PM, Kapelski P, Godlewski S, Dmitrzak-Weglarz M, Rybakowski J, Hauser J (2002) A polymorphism of the norepinephrine transporter gene in bipolar disorder and schizophrenia: lack of
association. Neuropsychobiology 45:182–185 Article CAS Google Scholar * Long JC, Williams RC, Urbanek M (1995) An E–M algorithm and testing strategy for multiple locus haplotypes. Am J
Hum Genet 56:799–810 CAS PubMed PubMed Central Google Scholar * Merlet P, Dubois-Rande JL, Adnot S, Bourguignon MH, Benvenuti C, Loisance D, Valette H, Castaigne A, Syrota A (1992)
Myocardial beta-adrenergic desensitization and neuronal norepinephrine uptake function in idiopathic dilated cardiomyopathy. J Cardiovasc Pharmacol 19:10–16 Article CAS Google Scholar *
Nelson N (1998) The family of Na+/Cl− neurotransmitter transporters. J Neurochem 71:1785–1803 Article CAS Google Scholar * Pacholczyk T, Blakely RD, Amara SG (1991) Expression cloning of
a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature 350:350–354 Article CAS Google Scholar * Pörzgen P, Bönisch H, Brüss M (1995) Molecular cloning and
organization of the coding region of the human norepinephrine transporter gene. Biochem Biophys Res Commun 215:1145–1150 Article Google Scholar * Pörzgen P, Bönisch H, Hammermann R, Brüss
M (1998) The human noradrenaline transporter gene contains multiple polyadenylation sites and two alternatively spliced C-terminal exons. Biochim Biophys Acta 1398:365–370 Article Google
Scholar * Ritz MC, Cone EJ, Kuhar MJ (1990) Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci 46:635–645
Article CAS Google Scholar * Sacchetti G, Bernini M, Bianchetti A, Parini S, Invernizzi RW, Samanin R (1999) Studies on the acute and chronic effects of reboxetine on extracellular
noradrenaline and other monoamines in the rat brain. Br J Pharmacol 128:1332–1338 Article CAS Google Scholar * Samochowiec J, Kucharska-Mazur J, Kaminski R, Smolka M, Rommelschpacher H,
Wernicke C, Tymicz A, Schmidt LG (2002) Norepinephrine transporter gene polymorphism is not associated with susceptibility to alcohol dependence. Psychiatry Res 111:229–233 Article CAS
Google Scholar * Schnell O, Muhr D, Weiss M, Dresel S, Haslbeck M, Standl E (1996) Reduced myocardial 123I-metaiodobenzylguanidine uptake in newly diagnosed IDDM patients. Diabetes
45:801–805 Article CAS Google Scholar * Schomig E, Fischer P, Schonfeld CL, Trendelenburg U (1989) The extent of neuronal re-uptake of 3H-noradrenaline in isolated vasa deferentia and
atria of the rat. Arch Pharmacol 340:502–508 CAS Google Scholar * Shi MM, Myrand SP, Bleavins MR, de la Iglesia FA (1999) High throughput genotyping for the detection of a single
nucleotide polymorphism in NAD(P)H quinone oxidoreductase (DT diaphorase) using TaqMan probes. Mol Pathol 52:295–299 Article CAS Google Scholar * Stephens M, Smith NJ, Donnelly P (2001) A
new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989 Article CAS Google Scholar * Stober G, Nothen MM, Porzgen P, Bruss M, Bonisch H, Knapp
M, Beckmann H, Propping P (1996) Systematic search for variation in the human norepinephrine transporter gene: identification of five naturally occurring missense mutations and study of
association with major psychiatric disorders. Am J Med Genet 67:523–532 Article CAS Google Scholar * Tanda G, Pontier FE, Frau R, DiChiara G (1997) Contribution of blockade of the
noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci 9:2077–2085 Article CAS Google Scholar * Tatsumi M,
Groshan K, Blakely RD, Richelson E (1997) Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol 340:249–258 Article CAS Google
Scholar * Zahniser NR, Doolen S (2001) Chronic and acute regulation of Na+/Cl−-dependent neurotransmitter transporters: drugs, substrates, presynaptic receptors, and signaling systems.
Pharmacol Ther 92:21–55 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to Dr. Alec Roy and Dr. Matti Virkkunen for subsets of their population datasets,
to Longina Akhtar for assistance with cell culture, and Ilona Lorincz for technical assistance. Supported by NIDCR Intramural Grant Z01, NIAAA Intramural Grant Z01 AA000301 (National
Institutes of Health, Bethesda, MD, USA), and the Comprehensive Neuroscience Program Grant USUHS G192BR-C4 (Henry Jackson Foundation, Rockville, MD, USA). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Pain and Neurosensory Mechanisms Branch, National Institute of Dental and Craniofacial Research, Bethesda, MD, USA Inna Belfer, Gabriel Phillips, Heather Hipp & Mitchell
B. Max * Laboratory of Neurogenetics, Department of Health and Human Services, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD, 20892-1258,
USA Inna Belfer, Gabriel Phillips, Julie Taubman, Heather Hipp, Robert H. Lipsky, Mary-Anne Enoch & David Goldman Authors * Inna Belfer View author publications You can also search for
this author inPubMed Google Scholar * Gabriel Phillips View author publications You can also search for this author inPubMed Google Scholar * Julie Taubman View author publications You can
also search for this author inPubMed Google Scholar * Heather Hipp View author publications You can also search for this author inPubMed Google Scholar * Robert H. Lipsky View author
publications You can also search for this author inPubMed Google Scholar * Mary-Anne Enoch View author publications You can also search for this author inPubMed Google Scholar * Mitchell B.
Max View author publications You can also search for this author inPubMed Google Scholar * David Goldman View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to Inna Belfer. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Belfer, I., Phillips, G., Taubman, J. _et al._
Haplotype architecture of the norepinephrine transporter gene _SLC6A2_ in four populations. _J Hum Genet_ 49, 232–245 (2004). https://doi.org/10.1007/s10038-004-0140-9 Download citation *
Received: 13 January 2004 * Accepted: 13 February 2004 * Published: 01 May 2004 * Issue Date: May 2004 * DOI: https://doi.org/10.1007/s10038-004-0140-9 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * Single-nucleotide polymorphism * Linkage disequilibrium * Haplotype * Norepinephrine transporter * _SLC6A2_