Radial glia require pdgfd–pdgfrβ signalling in human but not mouse neocortex
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Evolutionary expansion of the human neocortex underlies many of our unique mental abilities. This expansion has been attributed to the increased proliferative potential1,2 of radial
glia (RG; neural stem cells) and their subventricular dispersion from the periventricular niche3,4,5 during neocortical development. Such adaptations may have evolved through gene
expression changes in RG. However, whether or how RG gene expression varies between humans and other species is unknown. Here we show that the transcriptional profiles of human and mouse
neocortical RG are broadly conserved during neurogenesis, yet diverge for specific signalling pathways. By analysing differential gene co-expression relationships between the species, we
demonstrate that the growth factor _PDGFD_ is specifically expressed by RG in human, but not mouse, corticogenesis. We also show that the expression domain of PDGFRβ, the cognate receptor6,7
for PDGFD, is evolutionarily divergent, with high expression in the germinal region of dorsal human neocortex but not in the mouse. Pharmacological inhibition of PDGFD–PDGFRβ signalling in
slice culture prevents normal cell cycle progression of neocortical RG in human, but not mouse. Conversely, injection of recombinant PDGFD or ectopic expression of constitutively active
PDGFRβ in developing mouse neocortex increases the proportion of RG and their subventricular dispersion. These findings highlight the requirement of PDGFD–PDGFRβ signalling for human
neocortical development and suggest that local production of growth factors by RG supports the expanded germinal region and progenitor heterogeneity of species with large brains. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal
Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may
be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS A HUMAN-SPECIFIC ENHANCER FINE-TUNES RADIAL GLIA POTENCY AND CORTICOGENESIS Article 14 May 2025 A CELL FATE DECISION MAP REVEALS ABUNDANT DIRECT
NEUROGENESIS BYPASSING INTERMEDIATE PROGENITORS IN THE HUMAN DEVELOPING NEOCORTEX Article Open access 28 March 2024 UNRAVELING THE ADULT CELL PROGENY OF EARLY POSTNATAL PROGENITOR CELLS
Article Open access 04 November 2020 ACCESSION CODES PRIMARY ACCESSIONS GENE EXPRESSION OMNIBUS * GSE62064 DATA DEPOSITS Microarray data from the GCASS dataset have been deposited in Gene
Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE62064. REFERENCES * Rakic, P. Evolution of the neocortex: a perspective from developmental biology. _Nature
Rev. Neurosci._ 10, 724–735 (2009) Article CAS Google Scholar * Lui, J. H., Hansen, D. V. & Kriegstein, A. R. Development and evolution of the human neocortex. _Cell_ 146, 18–36
(2011) Article CAS Google Scholar * Smart, I. H. et al. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to
striate and extrastriate cortex in the monkey. _Cereb. Cortex_ 12, 37–53 (2002) Article Google Scholar * Hansen, D. V., Lui, J. H., Parker, P. R. & Kriegstein, A. R. Neurogenic radial
glia in the outer subventricular zone of human neocortex. _Nature_ 464, 554–561 (2010) ADS CAS Google Scholar * Fietz, S. A. et al. OSVZ progenitors of human and ferret neocortex are
epithelial-like and expand by integrin signaling. _Nature Neurosci._ 13, 690–699 (2010) Article CAS Google Scholar * Bergsten, E. et al. PDGF-D is a specific, protease-activated ligand
for the PDGF β-receptor. _Nature Cell Biol._ 3, 512–516 (2001) Article CAS Google Scholar * LaRochelle, W. J. et al. PDGF-D, a new protease-activated growth factor. _Nature Cell Biol._ 3,
517–521 (2001) Article CAS Google Scholar * Rakic, P. Specification of cerebral cortical areas. _Science_ 241, 170–176 (1988) Article ADS CAS Google Scholar * Oldham, M. C. In _The
OMICs: Applications in Neuroscience_ Vol. 1 (ed. Coppola, G. ) 85–113 (Oxford Univ. Press, 2014) Google Scholar * Oldham, M. C. et al. Functional organization of the transcriptome in human
brain. _Nature Neurosci._ 11, 1271–1282 (2008) Article CAS Google Scholar * Workman, A. D. et al. Modeling transformations of neurodevelopmental sequences across mammalian species. _J.
Neurosci._ 33, 7368–7383 (2013) Article CAS Google Scholar * Fietz, S. A. et al. Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix
in progenitor self-renewal. _Proc. Natl Acad. Sci. USA_ 109, 11836–11841 (2012) Article ADS CAS Google Scholar * Miller, J. A. et al. Transcriptional landscape of the prenatal human
brain. _Nature_ 508, 199–206 (2014) Article ADS CAS Google Scholar * Horvath, S. & Dong, J. Geometric interpretation of gene coexpression network analysis. _PLOS Comput. Biol._ 4,
e1000117 (2008) Article ADS MathSciNet Google Scholar * Oldham, M. C., Horvath, S. & Geschwind, D. H. Conservation and evolution of gene coexpression networks in human and chimpanzee
brains. _Proc. Natl Acad. Sci. USA_ 103, 17973–17978 (2006) Article ADS CAS Google Scholar * Zhang, B. & Horvath, S. A general framework for weighted gene co-expression network
analysis. _Stat. Appl. Genet. Mol. Biol._ 4, Article17 (2005) Article MathSciNet Google Scholar * Kawaguchi, A. et al. Single-cell gene profiling defines differential progenitor
subclasses in mammalian neurogenesis. _Development_ 135, 3113–3124 (2008) Article CAS Google Scholar * Pinto, L. et al. Prospective isolation of functionally distinct radial glial
subtypes–lineage and transcriptome analysis. _Mol. Cell. Neurosci._ 38, 15–42 (2008) Article CAS Google Scholar * Wang, Z. et al. Emerging roles of PDGF-D signaling pathway in tumor
development and progression. _Biochim. Biophys. Acta_ 1806, 122–130 (2010) CAS PubMed PubMed Central Google Scholar * Diez-Roux, G. et al. A high-resolution anatomical atlas of the
transcriptome in the mouse embryo. _PLoS Biol._ 9, e1000582 (2011) Article CAS Google Scholar * Roberts, W. G. et al. Antiangiogenic and antitumor activity of a selective PDGFR tyrosine
kinase inhibitor, CP-673,451. _Cancer Res._ 65, 957–966 (2005) CAS PubMed Google Scholar * Magnusson, P. U. et al. Platelet-derived growth factor receptor-β constitutive activity promotes
angiogenesis _in vivo_ and _in vitro_. _Arterioscler. Thromb. Vasc. Biol._ 27, 2142–2149 (2007) Article CAS Google Scholar * Golub, T. R., Barker, G. F., Lovett, M. & Gilliland, D.
G. Fusion of PDGF receptor β to a novel _ets_-like gene, _tel_, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. _Cell_ 77, 307–316 (1994) Article CAS Google
Scholar * McLean, C. Y. et al. Human-specific loss of regulatory DNA and the evolution of human-specific traits. _Nature_ 471, 216–219 (2011) Article ADS CAS Google Scholar * Bae, B. I.
et al. Evolutionarily dynamic alternative splicing of GPR56 regulates regional cerebral cortical patterning. _Science_ 343, 764–768 (2014) Article ADS CAS Google Scholar * Geschwind, D.
H. & Rakic, P. Cortical evolution: judge the brain by its cover. _Neuron_ 80, 633–647 (2013) Article CAS Google Scholar * Johnson, M. B. et al. Functional and evolutionary insights
into human brain development through global transcriptome analysis. _Neuron_ 62, 494–509 (2009) Article CAS Google Scholar * Kang, H. J. et al. Spatio-temporal transcriptome of the human
brain. _Nature_ 478, 483–489 (2011) Article ADS CAS Google Scholar * Hawrylycz, M. J. et al. An anatomically comprehensive atlas of the adult human brain transcriptome. _Nature_ 489,
391–399 (2012) Article ADS CAS Google Scholar * Ajioka, I., Maeda, T. & Nakajima, K. Identification of ventricular-side-enriched molecules regulated in a stage-dependent manner
during cerebral cortical development. _Eur. J. Neurosci._ 23, 296–308 (2006) Article Google Scholar Download references ACKNOWLEDGEMENTS We thank the staff at San Francisco General
Hospital Women’s Options Center for their consideration in allowing access to donated human prenatal tissue. We thank J. DeYoung and the staff at the Southern California Genotyping
Consortium at the University of California Los Angeles for microarray data generation. We are grateful to A. Holloway for her critical reading of the manuscript, and also thank W. Walantus,
S. Wang, Y. Wang and other University of California San Francisco personnel for technical and administrative support. We thank C. Stiles and D. Rowitch for the TEL–PDGFRβ construct. This
work was supported by grants from the NIH, NINDS (A.R.K.), the Bernard Osher Foundation, a California Institute for Regenerative Medicine Predoctoral Fellowship for J.H.L. (TG2-01153), a
Damon Runyon Foundation Postdoctoral Fellowship for A.A.P. (DRG-2013), and the University of California San Francisco Program for Breakthrough Biomedical Research, which is funded in part by
the Sandler Foundation (M.C.O.). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the California
Institute for Regenerative Medicine or any other agency of the State of California. AUTHOR INFORMATION Author notes * Jan H. Lui & Ashkan Javaherian Present address: Present addresses:
Department of Biology and Howard Hughes Medical Institute, Stanford University, Stanford, California 94305, USA (J.H.L.); Gladstone Institute of Neurological Disease, San Francisco,
California 94158, USA (A.J.)., * Jan H. Lui and Tomasz J. Nowakowski: These authors contributed equally to this work. * Arnold R. Kriegstein and Michael C. Oldham: These authors jointly
supervised this work. AUTHORS AND AFFILIATIONS * Department of Neurology and The Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research, University of California, San
Francisco, San Francisco, California 94143, USA, Jan H. Lui, Tomasz J. Nowakowski, Alex A. Pollen, Ashkan Javaherian, Arnold R. Kriegstein & Michael C. Oldham Authors * Jan H. Lui View
author publications You can also search for this author inPubMed Google Scholar * Tomasz J. Nowakowski View author publications You can also search for this author inPubMed Google Scholar *
Alex A. Pollen View author publications You can also search for this author inPubMed Google Scholar * Ashkan Javaherian View author publications You can also search for this author inPubMed
Google Scholar * Arnold R. Kriegstein View author publications You can also search for this author inPubMed Google Scholar * Michael C. Oldham View author publications You can also search
for this author inPubMed Google Scholar CONTRIBUTIONS M.C.O. conceived the GCASS strategy and J.H.L generated the GCASS data set. A.J. generated the FACS mRG data set. M.C.O. conceived,
designed and performed the bioinformatic analyses. J.H.L., T.J.N. and A.A.P. designed and performed the experiments leading up to the prioritization of PDGFD as the focus of this study.
T.J.N. performed the majority of the _in situ_ hybridizations and the _in vivo_ mouse experiments. J.H.L. performed the human and mouse slice culture experiments, as well as all of the
immunostaining, imaging and image analysis in the study. M.C.O. and J.H.L. wrote the manuscript, which was edited by all the authors. M.C.O. and A.R.K provided conceptual guidance at every
stage of the project. CORRESPONDING AUTHORS Correspondence to Arnold R. Kriegstein or Michael C. Oldham. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial
interests. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIGURE 1 HUMAN BRAIN DISSECTION FOR GCASS AND SCHEMATIC FOR GENERATION OF FACS MRG DATA SET. A, Top: to generate the GCASS data set,
an almost-intact prenatal human telencephalic hemisphere (GW14.5) was microdissected to separate the dorsal telencephalon from the ventral telencephalon (including medial and lateral
ganglionic eminences). Bottom: the dorsal fragment was flash-frozen and serially sectioned (150 μm) for transcriptional profiling with Illumina HT-12 v4 Beadchip microarrays. Scale bars, 2.5
mm. B, To generate the FACS mRG data set, dorsal neocortices of Eomes–GFP mouse embryos were microdissected, pooled (_n_ = 3 litters, 5–8 pooled embryos per litter), dissociated and
FACS-sorted according to the gating scheme depicted to isolate RG and intermediate progenitor cells. Transcriptional profiling of the resultant populations was performed using Illumina
mouseRef-8 v1.0 Beadchip microarrays. EXTENDED DATA FIGURE 2 GENES COMPRISING THE SIX RG CO-EXPRESSION MODULES IDENTIFIED BY GCASS ARE EXPRESSED IN A MANNER CONSISTENT WITH THE KNOWN
DISTRIBUTION OF RG IN DEVELOPING HUMAN NEOCORTEX. Six candidate hRG gene co-expression modules (Fig. 1d) were superimposed on three independent gene expression data sets generated from
laser-microdissected samples from prenatal human cortex: ABI.1 (ref. 13) (GW17), ABI.2 (ref. 13) (GW18), and Fietz _et al._ (ref. 12) (GW15–18) (as listed in Extended Data Table 1). The
characteristic expression patterns of the superimposed modules were summarized by singular value decomposition; the first principal component (PC1) for each module in each data set is shown.
In all cases, PC1 revealed substantially higher expression levels for these genes in germinal zones (VZ, ISVZ, and OSVZ, highlighted in red) versus non-germinal zones (IZ, SP, ICP, OCP, CP,
MZ, SG). Permutation analysis indicated that the per cent variance explained (VE) by PC1 of each superimposed module was significantly greater than expected by chance (_n_ = 10,000
permutations). CP, cortical plate; CTX, cortex; GW, gestational week; ICP, inner cortical plate; ISVZ, inner subventricular zone; IZ, intermediate zone; MZ, marginal zone; OCP, outer
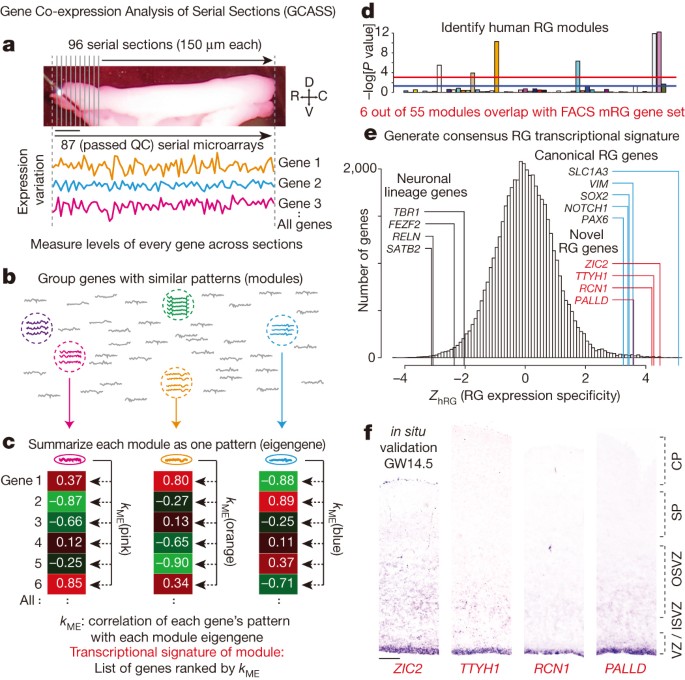
cortical plate; OSVZ, outer subventricular zone; SG, subpial granular layer; SP, subplate; VZ, ventricular zone. EXTENDED DATA FIGURE 3 GCASS SUCCESSFULLY PREDICTS NOVEL MARKERS OF
NEOCORTICAL HRG. A, Genome-wide distribution of predicted GW14.5 neocortical hRG expression specificity (_Z_hRG). Red lines indicate predicted RG genes (validated in B, C). B, C,
Immunostaining and _in situ_ hybridization in GW14.5 human neocortex confirms RG expression specificity for novel candidate markers predicted in A (B, scale bar, 50 μm; C, scale bar, 100
μm). Analysed tissue sections were independent from the sample used for microarray analysis (Fig. 1a). ISVZ, inner subventricular zone; OSVZ, outer subventricular zone; VZ, ventricular zone.
EXTENDED DATA FIGURE 4 WORKFLOW OF BIOINFORMATIC PROCEDURES AND EXPERIMENTAL RATIONALE FOR THE ENTIRE STUDY. The bioinformatic component of this study sought to identify a homologous gene
co-expression signature for human and mouse RG that is robust across multiple sampling strategies/technology platforms and can be normalized to facilitate comparisons within and between
species. This pipeline illustrates the steps that were taken to identify, integrate and compare RG gene co-expression signatures in eight transcriptomic data sets generated from prenatal
human and mouse neocortex. EXTENDED DATA FIGURE 5 GENOME-WIDE PREDICTIONS OF EXPRESSION SPECIFICITY FOR HRG AND MRG ARE ROBUST ACROSS INDEPENDENT DATA SETS. A, B, Heat maps of Spearman
correlation coefficients for predicted RG expression specificity (RG PR) over 10,929 genes present in all five human data sets (A) and 10,649 genes present in all three mouse data sets (B)
(as indicated in columns BE and BI in Supplementary Table 3). Data sets are denoted by the sample ages listed in Extended Data Table 1, although factors besides age also contribute to the
observed correlations (for example, choice of technology platform, sample preparation strategy). E, embryonic; GW, gestational week. EXTENDED DATA FIGURE 6 _IN SITU_ HYBRIDIZATION (ISH)
VALIDATES PREDICTED PRESENCE OR ABSENCE OF GENE EXPRESSION IN HRG AND MRG. A, Pink box: human _in situ_ probes for six genes predicted to be expressed by hRG but not mRG were generated and
hybridized in GW15 human neocortical tissue to validate predicted hRG expression (human scale bar, 200 μm). Blue box (Eurexpress20: http://www.eurexpress.org/ee/): _in situ_ hybridizations
for 13 genes predicted to be expressed by hRG but not mRG reveal no expression by mRG in E14.5 mouse cortex. Green box: mouse _in situ_ probes for three genes predicted to be expressed by
hRG but not mRG were generated and revealed no expression by mRG (E13.5). Positive control expression in cells other than RG are labelled in red. B, Expression patterns of genes predicted to
be expressed by mRG (that is, those with the highest values in Supplementary Table 3) are shown as further validation (blue (E14.5, Eurexpress20: http://www.eurexpress.org/ee/); orange
(E14.5, GenePaint: http://www.genepaint.org); mouse scale bars, ∼500 μm). One other gene in the top 15, _Cks2_, is not shown, but has been validated previously30. EXTENDED DATA FIGURE 7
_PDGFD_ IS EXPRESSED BY NEOCORTICAL RG DURING NEUROGENESIS IN HUMANS BUT NOT MICE. A, _In situ_ hybridization of _PDGFD_ in GW14.5, 16.5, 17.3 and 18.2 human neocortex demonstrates
consistent expression in RG across multiple ages (scale bar, 200 μm). B, _In situ_ hybridization of _Pdgfd_ in E14.5 mouse (Eurexpress20: http://www.eurexpress.org/ee/) demonstrates lack of
expression (scale bar, ∼500 μm). C, To demonstrate the lack of _Pdgfd_ expression in mouse neocortex across multiple ages, a pCAG-_PDGFD_-IG expression plasmid was electroporated into the
mouse VZ at E13.5 as an internal positive control and harvested at E14.5, E15.5 or E17.5. At E14.5 and E15.5, _Pdgfd_ (blue signal) is seen only in the electroporated region in the
ventricular zone (scale bar, 200 μm; inset scale bar, 50 μm). At E17.5, _Pdgfd_ is in the cortical plate and not in the ventricular zone or anywhere else (scale bar, 500 μm, inset scale bar,
50 μm). EXTENDED DATA FIGURE 8 _PDGFRB_ IS STRONGLY EXPRESSED BY VENTRAL RG AND WEAKLY EXPRESSED BY LATERAL RG IN MICE. _In situ_ hybridization of _Pdgfrb_ in sagittal sections through the
mouse forebrain (E14.5) across a medial–lateral axis (Eurexpress20: http://www.eurexpress.org/ee/) demonstrates progenitor expression in the ventral germinal regions. This expression extends
into the dorsal cortex in the lateral aspect of the brain, but is not widespread. In contrast, no progenitor expression is detected in dorsomedial cortex (scale bar, 500 μm; inset scale
bar, 100 μm). Expression is also detected in the pia and vascular pericytes. EXTENDED DATA FIGURE 9 MANIPULATION OF PDGFRΒ SIGNALLING IN HUMAN AND MOUSE NEOCORTEX. A–D, Chemical blockade of
PDGFRβ signalling in cultured slices of GW14.5 human neocortex impairs RG cell cycle progression. Four pharmacological inhibitors of PDGFRβ signalling were screened at different
concentrations to determine their effects on RG proliferation in cultured slices of GW17.5 human neocortex (2 days). Slices were treated with BrdU for the duration of the experiment and RG
proliferation was quantified as the fraction of SOX2+ cells that incorporated BrdU after treatment with inhibitor or vehicle. Statistical significance was assessed with the Wilcoxon rank sum
test using the wilcox.test R function with default settings. Images are derived from ≥3 slices in each condition. Control + DMSO, _n_ = 18; control no DMSO, _n_ = 9; CP673451 all
concentrations, _n_ = 9; Sutent all concentrations, _n_ = 6; imatinib (0.1 μM, 10 μM), _n_ = 9 (100 μM), _n_ = 6; tivozanib (1 μM, 10 μM), _n_ = 9 (0.1 μM), _n_ = 6. Significance indicated
by: n.s. _P_ > 0.05, **_P_ ≤ 0.01, ***_P_ ≤ 0.001, ****_P_ ≤ 0.0001. E, Slice cultures of E13.5 mouse neocortex were treated with BrdU and DMSO (control) or a pharmacological inhibitor of
PDGFRβ signalling (CP673451) for 1 or 2 days (slices from at least three independent litters). RG (SOX2+) or intermediate progenitor (TBR2+) cell proliferation was assessed as the fraction
of each population that incorporated BrdU or was Ki67+ (1d: _n_ = 10 (DMSO) versus _n_ = 9 (CP673451); 2d: _n_ = 11 (DMSO) versus _n_ = 9 (CP673451)). This experiment serves as a negative
control to compare with the human. F, Ectopic PDGFRβ signalling promotes RG identity in E13.5 mouse neocortex. _In utero_ electroporation of constitutively active TEL–PDGFRβ (ref. 23) was
compared with control (mouse E13.5–E15.5) and assessed for the proportion and distribution of SOX2+ RG cells or Ki67+ progenitors (out of GFP+) in the cortical wall (quantified in G: at
least _n_ = 3 slices per embryo from two independent litters, _n_ = 15 (control); _n_ = 18 (TEL–PDGFRβ) or (PDGFRβ(D850V)); scale bar, 50 μm). Ki67+GFP+ cell quantification following
PDGFRβ(D850V)22 electroporation was performed in a similar fashion and is also shown. The spatial distributions of RG (GFP+SOX2+) in the cortical wall were assessed by quantitative image
analysis (spanning ventricle to pia). The grey band delineates a 95% confidence interval for a test of equal univariate densities based on 10,000 permutations. All error bars represent mean
± s.e.m. Statistical significance for the effects of treatment was calculated by ANOVA of multiple linear regression while controlling for individual (E) and litter (F) variability
(significance indicated by: n.s. _P_ > 0.05, *_P_ ≤ 0.05, **_P_ ≤ 0.01, ***_P_ ≤ 0.001, ****_P_ ≤ 0.0001). SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION This file contains
Supplementary Text and Data, Supplementary Figures 1-4, Supplementary Table 1 and additional references (see Contents page for details). (PDF 5714 kb) SUPPLEMENTARY TABLE 1 This file shows
sample characteristics of the GCASS dataset. (XLSX 61 kb) SUPPLEMENTARY TABLE 2 The file shows genes expressed significantly higher in E14 mouse neocortical RG vs. IPC (the FACS-mRG
dataset). (XLSX 53 kb) SUPPLEMENTARY TABLE 3 This file contains a summary of predicted RG expression specificity, mean expression levels, and associated information for all genes and all
datasets. (XLSX 13994 kb) SUPPLEMENTARY TABLE 4 This file contains results of enrichment analyses for conserved and species-specific RG-expressed genes depicted in Fig. 2a, d. (XLSX 40 kb)
POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Lui, J., Nowakowski, T., Pollen, A. _et al._ Radial glia require PDGFD–PDGFRβ signalling in human but not mouse neocortex. _Nature_ 515, 264–268 (2014).
https://doi.org/10.1038/nature13973 Download citation * Received: 15 April 2014 * Accepted: 16 October 2014 * Published: 12 November 2014 * Issue Date: 13 November 2014 * DOI:
https://doi.org/10.1038/nature13973 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative