Discovering novel ligands for macromolecules using x-ray crystallographic screening
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

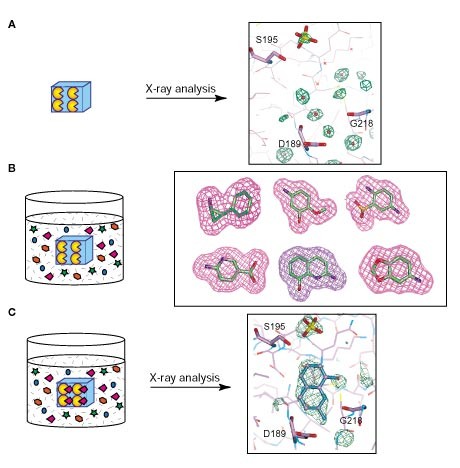
ABSTRACT The need to decrease the time scale for clinical compound discovery has led to innovations at several stages in the process, including genomics/proteomics for target identification,
ultrahigh-throughput screening1 for lead identification, and structure-based drug design2 and combinatorial chemistry3 for lead optimization. A critical juncture in the process is the
identification of a proper lead compound, because a poor choice may generate costly difficulties at later stages. Lead compounds are commonly identified from high-throughput screens of large
compound libraries, derived from known substrates/inhibitors, or identified in computational prescreeusing X-ray crystal structures2,4,5,6. Structural information is often consulted to
efficiently optimize leads, but under the current paradigm, such data require preidentification and confirmation of compound binding. Here, we describe a new X-ray crystallography–driven
screening technique that combines the steps of lead identification, structural assessment, and optimization. The method is rapid, efficient, and high-throughput, and it results in detailed
crystallographic structure information. The utility of the method is demonstrated in the discovery and optimization of a new orally available class of urokinase inhibitors for the treatment
of cancer. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to
this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy
now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact
customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A ROBUST CRYSTAL STRUCTURE PREDICTION METHOD TO SUPPORT SMALL MOLECULE DRUG DEVELOPMENT WITH LARGE SCALE VALIDATION AND BLIND STUDY
Article Open access 05 March 2025 CHEMICAL SPACE DOCKING ENABLES LARGE-SCALE STRUCTURE-BASED VIRTUAL SCREENING TO DISCOVER ROCK1 KINASE INHIBITORS Article Open access 28 October 2022 A
PRACTICAL GUIDE TO LARGE-SCALE DOCKING Article 24 September 2021 REFERENCES * Sundberg, S. High-throughput and ultra-high-throughput screening: solution- and cell-based approaches. _Curr.
Opin. Biotechnol._ 11, 47–53 (2000). Article CAS PubMed Google Scholar * Verlinde, C.L. & Hol, W.G. Structure-based drug design: progress, results and challenges. _Structure_ 2,
577–87 (1994). Article CAS PubMed Google Scholar * Salemme, F., Spurlino, J. & Bone, R. Serendipity meets precision: the integration of structure-based drug design and combinatorial
chemistry for efficient drug discovery. _Structure_ 15, 319–324 (1997). Article Google Scholar * Kuntz, I. Structure-based strategies for drug design and discovery. _Science_ 257,
1078–1082 (1992). Article CAS PubMed Google Scholar * Shuker, S.B., Hajduk, P.J., Meadows, R.P. & Fesik, S.W. Discovering high affinity ligands for proteins: SAR by NMR. _Science_
274, 1531–1534 (1996). Article CAS PubMed Google Scholar * Fejzo, J. et al. The SHAPES strategy: an NMR-based approach for lead generation in drug discovery. _Chem. Biol._ 6, 755–769
(1999). Article CAS PubMed Google Scholar * Allen, K.N. et al. An experimental approach to mapping the binding surfaces of crystalline proteins. _J. Phys. Chem._ 100, 2605–2611 (1996).
Article CAS Google Scholar * Goodford, P.J. A computational procedure for determining energetically favorable binding sites on biologically important molecules. _J. Med. Chem._ 28,
849–857 (1985). Article CAS PubMed Google Scholar * Caflish, A., Miranker, A. & Karplus, M. Multiple copy simultaneous search and construction of ligands in binding sites:
application to inhibitors of HIV-1 aspartic proteinase. _J. Med. Chem._ 36, 2142–2167 (1993). Article Google Scholar * Kuntz, I.D., Meng, E.C. & Shoichet, B.K. Structure-based drug
design. _Accounts Chem. Res._ 27, 117–123 (1994). Article CAS Google Scholar * Verlinde, C., Rudenko, G. & Hol, W. In search of new lead compounds for trypanosomiasis drug desgin: a
protein structure-based linked-fragment approach. _J. Comput. Aided Mol. Des._ 6, 131–147 (1992). Article CAS PubMed Google Scholar * Cheng, X. et al. Using electrospray ionization FTICR
mass spectrometry to study competitive binding of inhibitors to carbonic anhydrase. _J. Am. Chem. Soc._ 117, 8859–8860 (1995). Article CAS Google Scholar * Lin, M., Shapiro, M.J. &
Wareing, J.R. Screening mixtures by affinity NMR. _J. Org. Chem._ 62, 8930–8931 (1997). Article CAS Google Scholar * Muchmore, S. et al. Automated crystal mounting and data collection for
protein crystallography. _Structure_, in press. * Verlinde, C., Kim, H., Berstein, B., Mande, S. & Hol, W. Antitrypanosomiasis drug development based on structures of glycolytic
enzymes. In _Structure-based drug design_ (ed. Veerapandian, P.) 365–394 (Marcel Dekker, Inc, New York, NY; 1997). Google Scholar * Reuning, U. et al. Multifunctional potential of the
plasminogen activation system in tumor invasion and metastasis. _Int. J. Oncol._ 13, 896–906 (1998). Google Scholar * Carmeliet, P. et al. Physiological consequences of loss of plasminogen
activator gene function in mice. _Nature_ 368, 419–24 (1994). Article CAS PubMed Google Scholar * Nienaber, V., Wang, J., Davidson, D. & Henkin, J. Re-engineering of human urokinase
provides a system for structure-based drug design at high resolution and reveals a novel bindng sub-site. _J. Biol. Chem._ 275, 7239–7248 (2000). Article CAS PubMed Google Scholar *
Nienaber, V. et al. Structure-directed discovery of potent non-peptidic inhibitors of human urokinase which access a novel binding sub-site. _Structure_ 8, 553–563 (2000). Article CAS
PubMed Google Scholar * Menear, K. Progress towards the discovery of orally active thrombin inhibitors. _Curr. Med. Chem._ 5, 457–468 (1998). CAS PubMed Google Scholar * _Daylight
chemical information systems_, I. (Mission Viejo, CA,). * Price, S. & Nagai, K. Protein engineering as a tool for crystallography. _Cur. Opin. Biotechnol._ 6, 425–430 (1995). Article
CAS Google Scholar * Berman, H.M. et al. The Protein Data Bank. _Nucleic Acids Res._ 28, 235–242 (2000). Article CAS PubMed PubMed Central Google Scholar * Otwinowski, Z. & Minor,
W. Processing of X-ray dIffraction data collected in oscillation mode. _Methods Enzymol._ 276, 307–326 (1997). Article CAS PubMed Google Scholar * Brunger, A.T. _X-PLOR version 3.1
manual_. (Yale University Press, New Haven, CT; 1993). Google Scholar Download references ACKNOWLEDGEMENTS We thank R. Meadows, S. Fesik, and S. Betz for helpful discussions and comments on
the manuscript; J. Wang for preparation of the urokinase protein; and S. Muchmore and T. Rockway for discussions on the technique. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department
of Structural Biology, Abbott Laboratories, Abbott Park, 60064-6098, IL Vicki L. Nienaber, Paul L. Richardson & Jonathan Greer * Department of Cancer Research, Abbott Laboratories,
Abbott Park, 60064-6098, IL Vered Klighofer, Jennifer J. Bouska & Vincent L. Giranda Authors * Vicki L. Nienaber View author publications You can also search for this author inPubMed
Google Scholar * Paul L. Richardson View author publications You can also search for this author inPubMed Google Scholar * Vered Klighofer View author publications You can also search for
this author inPubMed Google Scholar * Jennifer J. Bouska View author publications You can also search for this author inPubMed Google Scholar * Vincent L. Giranda View author publications
You can also search for this author inPubMed Google Scholar * Jonathan Greer View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to Vicki L. Nienaber. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Nienaber, V., Richardson, P., Klighofer, V. _et al._ Discovering
novel ligands for macromolecules using X-ray crystallographic screening. _Nat Biotechnol_ 18, 1105–1108 (2000). https://doi.org/10.1038/80319 Download citation * Received: 31 May 2000 *
Accepted: 27 July 2000 * Issue Date: October 2000 * DOI: https://doi.org/10.1038/80319 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative