Lessons and revelations from biomimetic syntheses
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

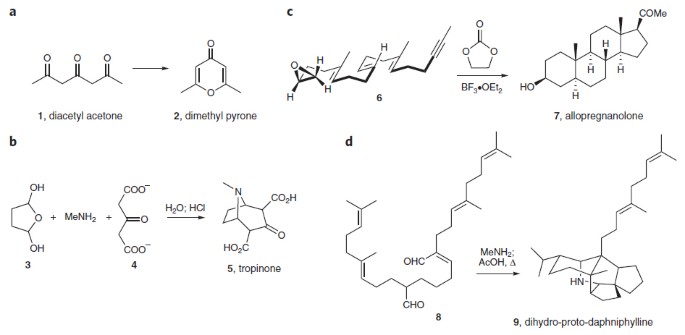
Biomimetic synthesis describes the field of organic chemistry that aims to emulate the natural, biosynthetic processes toward natural products. As well as providing insight into how
molecules are formed in nature, the benefits of this approach to total synthesis are numerous and extend beyond the gains typical of traditional synthesis. For example, using biosynthetic
proposals to design a synthetic route can highlight alternative methods to the desired target. The pursuit of biomimetic syntheses also promotes the development of new reactions to prove or
disprove a biosynthetic proposal or to unravel mechanistic implications of a proposed biosynthesis and can lead to the identification of new natural products. Here we look at some recent
compelling examples and examine how biomimetic synthesis has led to the discovery of new procedures and principles that would not have been found by other approaches.
We thank J.M. Ready and U.K.Tambar (University of Texas Southwestern) for critical reading of this manuscript. We gratefully acknowledge financial support by the National Institutes of
Health (CA 90349) and the Robert A. Welch Foundation (I-1422).
Department of Biochemistry, The University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA
Anyone you share the following link with will be able to read this content: