Three mechanisms control e-cadherin localization to the zonula adherens
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT E-cadherin localization to the zonula adherens is fundamental for epithelial differentiation but the mechanisms controlling localization are unclear. Using the _Drosophila_
follicular epithelium we genetically dissect E-cadherin transport in an _in vivo_ model. We distinguish three mechanisms mediating E-cadherin accumulation at the zonula adherens. Two
membrane trafficking pathways deliver newly synthesized E-cadherin to the plasma membrane. One is Rab11 dependent and targets E-cadherin directly to the zonula adherens, while the other
transports E-cadherin to the lateral membrane. Lateral E-cadherin reaches the zonula adherens by endocytosis and targeted recycling. We show that this pathway is dependent on RabX1, which
provides a functional link between early and recycling endosomes. Moreover, we show that lateral E-cadherin is transported to the zonula adherens by an apically directed flow within the
plasma membrane. Differential activation of these pathways could facilitate cell shape changes during morphogenesis, while their misregulation compromises cell adhesion and tissue
architecture in differentiated epithelia. SIMILAR CONTENT BEING VIEWED BY OTHERS IDENTIFICATION OF A NEW REGULATION PATHWAY OF EGFR AND E-CADHERIN DYNAMICS Article Open access 22 November
2021 THE _DROSOPHILA_ EPIDERMAL GROWTH FACTOR RECEPTOR PATHWAY REGULATES HEDGEHOG SIGNALLING AND CYTONEME BEHAVIOUR Article Open access 26 February 2025 NON-JUNCTIONAL ROLE OF CADHERIN3 IN
CELL MIGRATION AND CONTACT INHIBITION OF LOCOMOTION VIA DOMAIN-DEPENDENT, OPPOSING REGULATION OF RAC1 Article Open access 15 October 2020 INTRODUCTION Adherens junctions (AJs) are cell–cell
contacts that mediate intercellular adhesion of epithelial cells. They control tissue architecture by regulating cell shape and adhesion. AJs are formed by the homophilic adhesion receptor
E-cadherin (E-cad), whose cytoplasmic domain is associated with several proteins. Among them are β- and α-catenin, which anchor the actin cytoskeleton at the plasma membrane (PM)1,2,3.
Changes in E-cad distribution at the PM lead to severe tissue disorders that underlie many disease processes. For instance, loss of E-cad in tumour cells releases AJ-mediated cell–cell
contacts, which is a critical first step during metastasis4,5,6. AJs form the zonula adherens (ZA), an adhesive belt that links epithelial cells into a continuous sheet. In _Drosophila_ the
ZA is located in the most apical region of the lateral membrane, where it defines the border between the lateral and the apical membrane domain. AJ components also localize along the lateral
PM, where they have a more punctate pattern compared with the continuous ZA. It has been shown in mammalian cells that punctate E-cad clusters establish the lateral actin cortex7. AJ
components are constantly internalized from and recycled back to the PM8, a trafficking process that allows tissue remodelling during development. Many morphogenetic processes including
gastrulation or epithelial mesenchymal transitions rely on controlled AJ disassembly, whereas the formation of new epithelia during mesenchymal epithelial transitions (MET) requires AJ
assembly at defined cell–cell contact sites2,6,9,10. Furthermore, AJ trafficking is required for epithelial maintenance, and disrupting AJ trafficking affects ZA maintenance and may
eventually lead to loss of epithelial adhesion11,12,13,14. E-cad trafficking occurs in membrane vesicles whose formation, transport and targeting is controlled by Rab GTPases15,16,17. Rab5
is central for the formation of the early endosome to which endocytosed vesicles are delivered. Within the early-endosome internalized proteins are sorted either for degradation in the
endolysosomal pathway or for recycling. Recycling to the PM can occur either in a direct and rapid pathway controlled by Rab4 or via the recycling endosome, which involves Rab11 (ref. 18).
E-cad protein is translated at the ER and membrane vesicles with newly synthesized protein are sorted at the Golgi for transport to the lateral PM. Sorting in vertebrate cells requires a
dileucine motif in the cytoplasmic tail of E-cad19, which is, however, not present in the _Drosophila_ orthologue. Transport of E-cad vesicles to the lateral PM involves the passage through
a Rab11-positive compartment20. A recycling mechanism redistributes E-cad from the lateral PM to the apicolateral PM, which leads to E-cad accumulation at the ZA. Recycling involves
molecular interactions between Rab11, the exocyst complex and β-catenin11. The exocyst complex is thought to provide a landmark on the PM, which targets the fusion of vesicles21,22. Targeted
E-cad recycling is so far the only identified mechanism explaining the accumulation of AJ components at the ZA. Here we identify two additional mechanisms that localize E-cad to the ZA:
direct transport of E-cad vesicles from the Golgi to the ZA and an actin driven and apically directed cadherin flow within the lateral PM. Moreover, we identify with RabX1 a critical new
component of the recycling pathway, which links the early and the recycling endosome. RESULTS _RAB5_ AND _RAB11_ DIFFERENTIALLY AFFECT DE-CAD DISTRIBUTION The _Drosophila_ ovary consists of
egg chambers (or follicles) which form when epithelial precursors migrate towards a cyst of germline cells (pink cells Supplementary Fig. 1a). When they reach the cyst they undergo a MET
including ZA formation and PM polarization23,24. Approximately 30 cuboidal epithelial cells assemble an epithelial monolayer around the cyst when a new egg chamber is formed25. During early
oogenesis stages epithelial cells proliferate, but at mid-oogenesis cell division ceases and morphogenesis starts26. In a genome scale _in vivo_ RNA interference (RNAi) screen for genes
involved in the formation of the follicular epithelium we identified strong epithelial defects after _Rab5_ and _Rab11_ knockdown27. To examine whether the two Rab GTPases contribute to
_Drosophila_ E-cadherin (DE-cad) trafficking we analysed the localization of Armadillo (Arm, _Drosophila_ β-catenin), which binds DE-cad not only at the PM but also during vesicular
transport11,20,28. In wild-type epithelia Arm is detectable along the lateral PM and concentrates apicolaterally at the ZA (Supplementary Fig. 1b,b′). After _Rab5_ depletion by RNAi this
apicolateral concentration is severely reduced and Arm accumulates uniformly along the lateral PM (Supplementary Fig. 1c,c′; Supplementary Table 1, which summarizes the reproducibility of
all RNAi experiments). This is in striking contrast to _Rab11_ RNAi, which leads to strong Arm accumulation within the cell (Supplementary Fig. 1e,e′, see also ref. 29). We validated the
RNAi phenotypes by generating genetic mosaics, which allowed us to directly compare Arm distribution in mutant and neighbouring wild-type cells. Optical confocal sections through the lateral
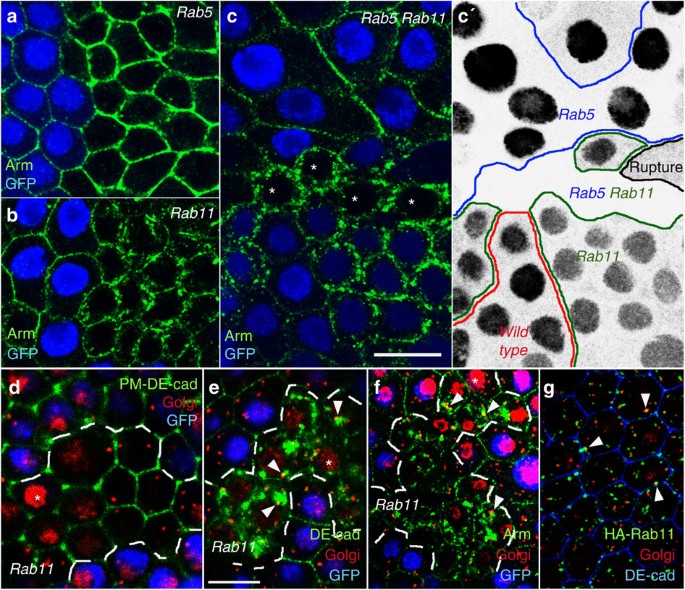
PM reveal a strong Arm and DE-cad accumulation at the periphery of _Rab5_ mutant cells (Fig. 1a, Supplementary Fig. 1d and Supplementary Table 2, which summarizes the reproducibility of all
experiments with genetic mosaics). Ultrastructural analysis in _Drosophila_ ooyctes revealed that loss of _Rab5_ leads to the accumulation of endocytic vesicles close to the PM30, and it
appears therefore likely that the enlarged Arm domain reflects not only the PM but also endocytosed protein near the PM. In _Rab11_ cells Arm and DE-cad are only weakly detectable at the
lateral membrane and accumulate in numerous aggregates within the cell (Fig. 1b,e). Bigger _Rab11_ mutant cell clones show no clear ZA and flatten (yellow arrows in Supplementary Fig. 1f).
This suggests that the intracellular accumulation of DE-cad in _Rab11_ mutant cells results in decreased DE-cad levels at the PM, which lead to cell shape and cell–cell adhesion defects.
RAB11 REGULATED EXOCYTOSIS TRANSPORTS DE-CAD TO THE PM DE-cad is endocytosed at the lateral PM, and Rab11 is involved in a targeted recycling process that leads to DE-cad accumulation at the
ZA11. To test if the intracellular DE-cad aggregation in _Rab11_ cells is caused by a recycling failure we prevented DE-cad influx from the PM by generating _Rab5 Rab11_ double-mutant
cells. If the intracellular DE-cad aggregates in _Rab11_ are formed by endocytosed DE-cad, the block of early-endosome formation in the double mutants would hold internalized DE-cad at the
periphery and intracellular DE-cad aggregation would be suppressed. However, _Rab5 Rab11_ double-mutant cells did not affect intracellular DE-cad accumulation arguing against a recycling
defect (asterisks in Fig. 1c). We also knocked down _Rab5_ and _Rab11_ simultaneously (Supplementary Fig. 1g,g′) using two RNAi lines that phenocopy the null phenotypes (Supplementary Fig.
1c,e). The double knockdown shows the same intracellular accumulation like the _Rab11_ single RNAi confirming that a block of early-endosome formation in _Rab11_ does not prevent
intracellular DE-cad aggregation. These data indicate that a large part of the intracellular DE-cad protein in _Rab11_ cells does not come from the PM, and hence its aggregation is not
primarily caused by a recycling defect. To examine a possible contribution of endocytosed DE-cad to the intracellular aggregates we performed endocytosis experiments. We incubated living
ovaries harbouring _Rab11_ clones with an antibody that binds to the extracellular part of DE-cad31,32. As the PM was not permeabilized during the incubation, the antibody bound exclusively
to DE-cad at the PM (PM-labelled DE-cad) but not to intracellular protein. Studies in cell-free systems suggest that antibody binding inhibits trans interactions between E-cad molecules and
promotes their endocytosis33. After 3-h incubation we fixed the ovaries, permeabilized the PM and detected DE-cad. We could not identify intracellular DE-cad aggregates in these assays
supporting the idea that endocytosed DE-cad does not contribute to the intracellular accumulation (Fig. 1d). We therefore conclude that the intracellular aggregates consist mainly of _de
novo_-synthesized DE-cad. Considering the well-established function of Rab11 in recycling, it is unexpected that endocytosed DE-cad does not accumulate at all. A possible explanation is that
in the absence of Rab11 all endocytosed DE-cad is directly recycled back to the PM. Rab4, the regulator of direct recycling, might mediate DE-cad recycling partially in wild-type cells and
completely if Rab11 is absent. Consistent with this hypothesis we detected cytoplasmic Arm puncta that co-localize with Rab4 in wild-type and _Rab11_ cells (Supplementary Fig. 2a). Our data
suggest that the intracellular aggregates in _Rab11_ cells do not consist of recycled but rather of newly synthesized DE-cad protein. New DE-cad is translated at the ER and its exocytosis to
the PM involves passage through the Golgi. This organelle consists of several transitional ER-Golgi subunits in _Drosophila_34. Notably, the aggregates within _Rab11_ cells directly abut
the Golgi (Fig. 1e,f) supporting the idea that after leaving the Golgi, vesicles with newly synthesized DE-cad protein accumulate within the cell. To examine whether Rab11 localizes to the
Golgi in wild-type cells we expressed HA-tagged Rab11 protein in the follicular epithelium. Quantification of the overlap of Rab11 with the Golgi marker GM130 revealed that 63% of the
detected Golgi puncta (_n_=1211) overlapped with HA-Rab11 puncta (_n_=4811). This concentration of Rab11 at the Golgi is consistent with a role of Rab11 in regulating exocytosis.
Collectively, these data suggest that Rab11 controls the transport of newly synthesized DE-cad from the Golgi to the PM. _RAB11_ CONTROLS DE-CAD EXOCYTOSIS TO THE APICOLATERAL PM DE-cad
localizes to the ZA and to the lateral PM. _De novo_-synthesized DE-cad could therefore be transported by apicolaterally and laterally directed pathways. To examine the lateral pathway we
incubated living ovaries only briefly with the DE-cad antibody to avoid DE-cad endocytosis and redistribution. After short incubation of wild-type ovaries, DE-cad was exclusively detectable
in the basal region of the lateral PM, but not at the ZA (Fig. 2a). Septate junctions, the equivalent of tight junctions in insect cells, could prevent access of the antibody to the ZA.
However, ultrastructural studies35 and analysis of the dynamics of septate junction components36 argue against the existence of functional septate junctions at early and mid-oogenesis
stages. We therefore speculate that the incubation time in this experiment is too short to allow the antibody to reach the ZA. To test whether Rab 11 is required for DE-cad exocytosis to the
lateral PM we knocked down _Rab11_ in all epithelial cells including their precursors. We achieved this by using _traffic jam_-Gal4, which strongly induces RNAi in all somatic cells37.
After brief incubation of these ovaries with the DE-cad antibody, DE-cad was clearly detectable at the basolateral PM (Fig. 2b). This indicates that DE-cad is exocytosed to the lateral PM in
the absence of Rab11. Thus, _Rab11_ depleted cells accumulate newly synthesized DE-cad within the cell but show no defects in basolateral exocytosis. We therefore conclude that the
accumulating DE-cad protein is destined for apicolateral exocytosis to the ZA. Exocytosis to the lateral PM might be mediated by a pathway that is common for all lateral membrane proteins.
To further investigate a possible role of Rab11 in this pathway we examined the localization of the lateral adhesion proteins Fasciclin2 (Fas2) and Fasciclin3 (Fas3). Both proteins localize
normally and reveal no intracellular aggregation in _Rab11_ mutant cells (Fig. 2c,d). Thus, Rab11 is not essential for exocytosis of adhesion proteins to the lateral PM. In summary, our data
indicate the existence of two exocytosis pathways to the lateral PM: a Rab11-independent pathway for exocytosis to the basal region of the lateral PM and a Rab11-dependent pathway that
targets exocytosis to the ZA. It has been shown that Rab11 recruits the exocyst complex to endocytosed DE-cad vesicles to promote their fusion with the ZA11. To examine whether Rab11 also
cooperates with the exocyst complex in targeting newly synthesized DE-cad to the ZA we analysed Sec6, a critical exocyst component. The aggregating AJ components in _Rab11_ mutant cells show
only a very weak overlap with Sec6 protein (Supplementary Fig. 2b). This is consistent with the idea that Rab11 is required to recruit the exocyst complex to vesicles with newly synthesized
DE-cad. _Sec6_ mutant cells form intracellular Arm aggregates resembling the aggregates in _Rab11_ mutant cells. These Arm aggregates also accumulate Rab11 protein. This suggests that AJ
components are trapped in a Rab11 compartment when exocyst function is absent (Supplementary Fig. 2c). Thus, our data support a model in which the Rab11-exocyst interaction regulates the
targeting of vesicles with endocytosed as well as newly synthesized DE-cad. We therefore propose a new exocytosis pathway, in which Rab11 and the exocyst complex guide _de novo_ synthesized
DE-cad to the apicolateral PM. LOSS OF _RABX1_ LEADS TO INTRACELLULAR DE-CAD AGGREGATION To identify new Rab GTPases controlling DE-cad trafficking we focused on Rab proteins that
co-localize with Rab11 in _Drosophila_ neuronal cells38. We found that 31% of the Rab11 vesicles (_n_=4,206) in the follicular epithelium overlap with vesicles of the so far uncharacterised
GTPase RabX1 (_n_=1,586, Fig. 3a). To analyse _RabX1_ function we used a P-element insertion into the 5UTR (_RabX1__KG06805_). After removal of second site mutations homozygous flies were
viable (see Methods). Mutant epithelia polarize their membrane domains normally but show cell-shape defects (Fig. 3c–f). Quantitative analysis of confocal pictures revealed a strong
reduction of hexagonal cells, which is the predominant shape in the wild-type (Table 1). Moreover, measurements of cell areas show an increased average cell size, which is, however,
accompanied by a greater variability in size (compare s.d. in Table 1). Although Arm and DE-cad localize to the lateral PM and accumulate apicolaterally in _RabX1_ mutants, the formation of
a continuous ZA is affected (arrows in Fig. 3f). We observed these ZA discontinuities in 46% of mutant cells (_n_=114), while they were detectable only in 19% of wild-type cells (_n_=118).
Remarkably, _RabX1_ mutant epithelia also show big intracellular Arm aggregates indicating a defect in DE-cad trafficking (arrowheads in Fig. 3d,f). Interestingly, intracellular aggregation
is not restricted to AJ components since DE-cad accumulates together with Fas2 (Fig. 3b, 84% of the analysed DE-cad aggregates (_n_=74) also accumulated Fas2). This suggests that DE-cad and
Fas2 accumulate in the same compartment. _RabX1__KG06805_ is viable over a deficiency (Df(2R)BSC661), and hemizygous egg chambers show the same morphological defects and intracellular
aggregates like homozygous mutants (Supplementary Fig. 3a,b). This indicates that _RabX1__KG06805_ is either a null or a strong allele. Importantly, expression of a UAS-_YFP-RabX1_ transgene
completely rescues all morphological defects as well as the intracellular aggregation of Arm and Fas2 (Supplementary Fig. 3c–f and Supplementary Table 2) confirming that the epithelial
defects are caused by the mutation in _RabX1_. _RABX1_ LINKS EARLY AND RECYCLING ENDOSOMES The intracellular aggregation of membrane proteins in _RabX1_ mutants could reflect defects in
endolysosomal degradation, exocytosis or recycling. Our data argue against the first two possibilities. Defects in the endolysosomal pathway caused by mutations in _Rab5_ or ESCRT components
result in overproliferation. This leads to the formation of elongated cellular connections between egg chambers (see Supplementary Fig. 1c for _Rab5_, arrowheads) and multi-layered
epithelia27,39,40. _RabX1_ mutants do not show these overproliferation phenotypes suggesting that the gene does not affect the endolysosomal pathway. To examine whether exocytosis to the
lateral PM is _RabX1_ dependent we briefly incubated living ovaries of mutants with the DE-cad antibody, and detected the protein at the basolateral PM (Supplementary Fig. 4a). This
indicates that RabX1 is not essential for lateral DE-cad exocytosis. To access whether RabX1 is, like Rab11, involved in DE-cad exocytosis to the ZA we compared the pattern of Arm
aggregation in _RabX1_ and _Rab11_ mutant cells by quantifying confocal images depicting Arm and Golgi localization (see Fig. 1f and Supplementary Fig. 4b for examples). This revealed a
seven times higher frequency of Arm–Golgi overlaps in _Rab11_ cells (Supplementary Table 3). Moreover, in _Rab11_ cells the average size of Arm aggregates is smaller and their total number
higher. Thus, _Rab11_ cells generate numerous small aggregates, whereas _RabX1_ cells form few aggregates which are larger. These differences in Arm aggregation suggest that Rab11 and RabX1
affect different DE-cad localization pathways, and thus argue against a role of RabX1 in apicolateral exocytosis. To test whether _RabX1_ acts in recycling we prevented the DE-cad influx
from the PM in homozygous _RabX1_ mutants by inducing _Rab5_ clones. This led to an accumulation of Arm at the periphery of double-mutant cells, which was accompanied by a strong reduction
or disappearance of the intracellular Arm aggregates (Fig. 4a). We also induced _RabX1_ clones in epithelia with _Rab5_ knockdown, which again suppressed aggregation (Fig. 4b). Thus, the
formation of intracellular DE-cad aggregates in _RabX1_ is _Rab5_ dependent and occurs downstream of early-endosome formation. To further characterize the intracellular DE-cad aggregates in
_RabX1_ cells we analysed whether they co-localize with Rab5 and Rab11. Remarkably, both Rab GTPases concentrated within the aggregates (Fig. 4c,d; 94% of Arm aggregates (_n_=402)
accumulated Rab5 and 95% DE-cad aggregates (_n_=180) accumulated Rab11). Localization of Rab5 and Rab11 was also examined by expressing HA-tagged Rab5 and Rab11 in _RabX1_ mutants, and
confirmed that the two Rab proteins co-accumulate with Arm (Supplementary Fig. 4c,d). In summary, these data suggest that in _RabX1_ cells endocytosed DE-cad is trapped in a large
intracellular compartment, which accumulates regulators of the early and the recycling endosome. Notably, in wild-type cells RabX1 protein strongly overlaps with Rab11 (Fig. 3a, 83% of the
analysed RabX1 puncta (_n_=1,586) overlapped with Rab11) and Rab5 (Supplementary Fig. 4e, 83% RabX1 puncta (_n_=829) overlapped with Rab5). This supports the idea that RabX1 provides a
direct link between the early and the recycling endosome. A possible explanation for the concentration of Rab5 and Rab11 within the DE-cad aggregates in _RabX1_ cells is a disruption of the
recycling process at the level of early to recycling endosome conversion. To test directly whether DE-cad recycling is blocked in _RabX1_ mutants we performed endocytosis assays with ovaries
harbouring _RabX1_ mutant cells. After 3-h incubation with the DE-cad antibody, we detected the protein. We also counterstained for Arm to visualize the intracellular aggregates. Notably,
PM-labelled DE-cad was clearly detectable in the existing aggregates confirming that they accumulate endocytosed protein (Fig. 5c,c′ arrows; DE-cad Arm overlay was detectable in 31 of 32
clones). We next tested the genetic hierarchy between _RabX1_ and _Rab11_ by generating _Rab11_ clones in homozygous _RabX1_ mutant epithelia. _RabX1 Rab11_ double-mutant cells resemble
_Rab11_ single mutants as they form numerous small intracellular aggregates, which show a strong overlap with the Golgi (Fig. 5a,b; Supplementary Table 3). This indicates that DE-cad
exocytosis to the ZA is disturbed like in _Rab11_ single mutants. To assess epistasis during DE-cad recycling we performed endocytosis assays in _RabX1_ mutant ovaries with _Rab11_ clones.
Notably, double-mutant cells accumulated endocytosed DE-cad similar to _RabX1_ single mutants (Fig. 5e,e′; DE-cad Arm overlay was detectable in 12 of 13 clones). Thus, _RabX1_ acts upstream
of _Rab11_ in DE-cad recycling. This raises the possibility that RabX1 activates Rab11. We propose that in the absence of RabX1, inactive Rab11 and DE-cad are trapped together in a blocked
recycling endosome. This provides an explanation for our observation that Rab11 and DE-cad co-accumulate in _RabX1_ mutant cells (Fig. 4d). A CADHERIN FLOW LOCALIZES DE-CAD TO THE ZA Our
DE-cad endocytosis experiments revealed that only a part of PM-labelled DE-cad accumulated within _RabX1_ cells, whereas a significant amount was transported to the ZA (Fig. 5f; arrow).
PM-labelled DE-cad in _RabX1 Rab11_ double and in _Rab11_ single-mutant cells even localized to the apical PM (Fig. 5g,h; arrows). This apical and apicolateral localization of PM-labelled
DE-cad could be mediated by a _RabX1_ and _Rab11_-independent transport mechanism. Such a recycling independent transport has been reported for mammalian cells and was named ‘Cadherin flow’.
This flow was shown to move E-cad within the lateral PM in apical direction41. To test whether a Cadherin flow exists in the follicular epithelium we analysed PM-labelled DE-cad
distribution in the absence of endocytosis. We blocked endocytosis by using a temperature-sensitive mutation of _Dynamin_ (_shibire__ts1_), a critical regulator of endocytic vesicle
scission42. Ovaries were briefly incubated with the antibody and basolateral localization of labelled DE-cad was confirmed (Fig. 6a). Strikingly, after 60 min at restrictive temperature
almost all DE-cad accumulated at the ZA (Fig. 6b). Thus, lateral DE-cad is efficiently transported from the basal to the apical part of the lateral PM in absence of endocytosis. This
indicates the existence of an apically directed DE-cad flow, which mediates ZA localization. To characterize the dynamics of the DE-cad flow we measured the extent of the apical movement of
basolaterally labelled DE-cad at different time points in _shibire_ mutants. Within the first 8 min DE-cad reached the middle region of the PM indicating fast membrane flow in the basal half
of the lateral PM (Supplementary Fig. 5). Interestingly, DE-cad reaches the ZA only after 30 min. This indicates that the DE-cad flow is slower in the apical half of the PM. The Cadherin
flow in mammalian cells is inhibited when actin filaments are disrupted41. We therefore performed DE-cad labelling experiments in _shibire_ ovaries in the presence of cytochalasin D. F-actin
disruption abolished the accumulation of DE-cad at the ZA resulting in homogenous distribution along the lateral PM (Fig. 6c). This indicates that the apically directed DE-cad flow is
dependent on actin filaments. Thus, three mechanisms facilitate the accumulation of DE-cad at the ZA: transport of newly synthesized DE-cad, targeted recycling of endocytosed protein and an
apically directed DE-cad flow within the lateral PM. DISCUSSION Our study reveals that vesicles with newly synthesized DE-cad leave the Golgi by two different pathways: lateral and
apicolateral exocytosis (Fig. 7a). _Rab11_ functionally distinguishes these two pathways as the gene is central for apicolateral but dispensable for lateral exocytosis. The lateral pathway
could be common to all transmembrane proteins facilitating cell–cell adhesion including Fas2 and Fas3. Lateral adhesion proteins underlie a dynamic turnover. After internalization they are
delivered to the early endosome in a _Rab5_-dependent process. Within the early endosome they are sorted to a large extent for return to the PM8,11,43. Commonly, recycling occurs either via
direct recycling mediated by Rab4 or via the recycling endosome, which is regulated by Rab11 (ref. 18). It is likely that DE-cad is recycled by both Rab4- and Rab11-dependent mechanisms. We
identify _RabX1_ as a critical new component for DE-cad recycling, and our data place its function in between the early and the recycling endosome. In _RabX1_ mutants endocytosed DE-cad
protein is not properly recycled but accumulates together with Rab5 and Rab11 in a large compartment (Fig. 7c). _RabX1_ mutants are viable, which implies that recycling is not completely
blocked, and consistent with this we observed a normal Rab4 distribution in _RabX1_ mutants. It seems therefore likely that in _RabX1_ mutants Rab11-mediated DE-cad recycling is blocked,
while Rab4 mediated recycling is still functional. Two compartments, the recycling endosome and the Golgi, provide DE-cad for ZA accumulation. It is unclear whether an interchange of DE-cad
vesicles between these two compartments exists in the follicular epithelium. For instance it is possible that DE-cad, which leaves the Golgi is first transported to the recycling endosome,
where it undergoes a sorting step for ZA delivery. However, the phenotypic differences between _RabX1_ and _Rab11_ mutant cells argue against this hypothesis. Our data indicate that _RabX1_
mutants specifically affect DE-cad recycling, whereas _Rab11_ mutants block apicolateral exocytosis of DE-cad but do not prevent recycling (probably because Rab11-mediated recycling is
completely replaced by Rab4 mediated recycling). If newly synthesized DE-cad had to pass the recycling endosome, then it should accumulate there together with the endocytosed DE-cad in
_RabX1_ mutants, and this would lead to severe ZA defects. However, _RabX1_ cells show only mild defects in comparison with _Rab11_ cells arguing that _de novo_ synthesized DE-cad does not
pass the recycling endosome. It remains to be determined whether endocytosed DE-cad traffics to the Golgi for a sorting step for ZA delivery. The exocyst complex is central for targeting
DE-cad to the ZA. Biochemical studies indicate that Rab11 protein recruits the exocyst complex to DE-cad vesicles for their targeted fusion with the ZA44,45,46,47. It has been shown that in
exocyst mutants endocytosed DE-cad does not traffic to the ZA11. Our analysis shows that exocyst mutant cells form, like Rab11 cells, numerous small AJ aggregates indicating defects in
apicolateral exocytosis of newly synthesized DE-cad. Thus, exocyst mutants combine recycling and apicolateral exocytosis defects supporting the idea that the exocyst complex targets both
vesicles with newly synthesized and with endocytosed DE-cad to the ZA. Lateral DE-cad reaches the ZA not only by targeted recycling but also by an actin-dependent basal to apical DE-cad flow
within the PM. Notably, after disruption of the flow, DE-cad spreads homogeneously within the lateral PM but neither enters the apical nor the basal PM domain (Fig. 6c). This indicates that
the lateral PM is enclosed by barriers, which prevent the exit of DE-cad. The apical barrier is likely to be formed by the ZA itself. In _Rab11_ mutant clones the ZA is not maintained and
the membrane flow transports DE-cad into the apical domain (Fig. 7b and Fig. 5g, arrow). Interestingly, in _Rab11_ cells neither the lateral marker Fas3 moves into the apical PM nor the
apical marker aPKC redistributes into the lateral PM (Supplementary Fig. 6). This indicates that there is no free exchange between apical and lateral proteins in _Rab11_ cells. Hence, the
flow seems to be specific for DE-cad and does not transport other adhesion proteins. Analysis of the cadherin flow in mammalian cells suggested that VE-cadherin is moving as a trans dimer in
apical direction41. In our experiments, DE-cad was labelled with an antibody that binds to the cadherin domains32, which is likely to interfere with trans interactions. This suggests that
trans interactions are no pre-requisite for the Cadherin flow in the follicular epithelium. Interestingly, our analysis of the dynamics of DE-cad movement indicates that the flow is faster
in the basal region of the lateral PM than in its apical part. This could be explained by different mechanisms driving the flow in the basal and apical regions of the lateral PM. It is also
possible that there is more resistance in the apical part slowing down the velocity of the flow. Moreover, the actin cytoskeleton, which provides routes for the cadherin flow41 might be
organized differently in basal and apical regions of the lateral PM. The finding that various pathways control DE-cad distribution raises the question why E-cad localization underlies such
complex regulation. The _Rab11_ phenotype indicates that apicolateral exocytosis is essential for epithelial cell shape as mutant cells lose their ZA and flatten. E-cad localization to the
lateral PM is important for the assembly of a contractile lateral actin cortex7. Interestingly, a balance of tension of the actin network of the ZA versus the actin network of lateral AJs
has been identified in mammalian epithelial cells. This balance is important for epithelial integrity, and cells extrude from the epithelium when it is disturbed7. The ratio between
apicolateral and lateral DE-cad exocytosis is a first step in regulating E-cad distribution between the lateral and apicolateral PM, which is likely to impinge on the tension balance. The
ratio between apicolateral and lateral DE-cad levels is also regulated by transporting lateral DE-cad to the ZA. This is mediated by targeted recycling and by the Cadherin flow. Analysis of
_shibire_ mutants shows that the flow is able to localize DE-cad efficiently in the absence of targeted recycling. This flow could be important for the establishment of the ZA during MET. In
follicle stem cells and their mesenchymal precursors DE-cad localizes broadly along the lateral PM. Only after MET completion, DE-cad concentrates apicolaterally to establish the ZA24,48.
This redistribution could be supported by the Cadherin flow. The maintenance of the ZA is facilitated by targeted recycling. When targeted recycling is blocked, like in _RabX1_ mutants,
cells show ZA and cell shape defects. This indicates that the DE-cad flow cannot fully compensate the loss of the targeted recycling pathway. The possibility of differentially activating the
various pathways controlling DE-cad localization provides an intriguing tool for epithelial cell shape changes. For instance, shutdown of lateral exocytosis in favour of enhanced
apicolateral exocytosis could promote shrinking of the lateral PM and simultaneous expansion of the ZA, a process necessary to form squamous epithelia. By contrast, enhanced lateral
exocytosis might support the expansion of the lateral PM when epithelial cells adopt a columnar shape. Thus, modulation of the activities of the different pathways could drive epithelial
morphogenesis. Disturbances of the pathways in differentiated epithelial tissues will compromise cell–cell adhesion and tissue architecture. It is for example tempting to speculate that loss
of apicolateral exocytosis combined with an unrestricted Cadherin flow disturbs the organization of membrane domains in tumours, similar to our observations in _Rab11_ cells. It will be
important to explore how such misregulations contribute to disease processes like organ fibrosis and metastasis. METHODS _DROSOPHILA_ GENETICS Following stocks were used: _w; traffic
jam_-Gal4 (ref. 37; provided by J. Bennecke), GR1-Gal4/TM3_Ser_49 (provided by S. Roth), w;; _Rab11__dFRT_/TM3_Sb_50_, w;;_ FRT5377_, Hrb98DE::GFP_ (all provided by R.S. Cohen), _w; Rab5__2_
FRT40A/CyO51 (provided by A. Guichet), _yw;;_ UAS-YFP_-RabX1_ (ref. 52), _yw; RabX1__KG06805_/CyO_, w;_ Df(2R)BSC661/SM6a_, Shibire__TS1_ (ref. 53, all from Bloomington _Drosophila_ Stock
Centre), w; FRTG13 _sec6__Ex15_/CyO54 (provided by Y. Bellaiche), _Rab5_ RNAi (Vienna _Drosophila_ Resource Center (VDRC) KK 103945 and VDRC GD 34096), _Rab11_ RNAi (TRiP library, VALIUM10,
BDSC), UAS-HA-_Rab11_ (insertions on second and third chromosome), UAS-HA-_Rab5_ (insertions on second and third chromosome), _w;_ neoFRT42D _RabX1__KG06805_, _hs-FLP; rab5__2_ FRT40A
_RabX1__KG06805_/CyO_, w;_ GFP FRT40A _RabX1__KG06805_/CyO_, w;;_GR1-_Gal4_ UAS-FLP/TM3_Ser_ (all from this work). _Genotypes of females generated for genetic epistasis studies_. Figure 1c:
hs-FLP_; rab5__2_ FRT40A_/GFP_ FRT40A; _Rab11__dFRT_/FRT5377_, Hrb98DE::_GFP Figure 4a: hs-FLP; rab52 FRT40A RabX1KG06805/GFP FRT40A RabX1KG06805 Figure 4b: _w;_ FRT42D
_RabX1__KG06805_/FRT42D _GFP_; GR1-Gal4 UAS-FLP/UAS_-Rab5_-RNAi. Figure 5a,b,e: hs-FLP; FRT42D RabX1KG06805/FRT42D RabX1KG06805; Rab11dFRT/FRT5377, Hrb98DE::GFP. Supplementary Figure 1g: _w;
traffic jam_-Gal4/UAS_-Rab5_-RNAi; UAS_-Rab11_-RNAi. _Generation of a FRT42D RabX1__KG06805__ stock_. We first removed an unrelated P-element insertion on the first chromosome of the
original stock. The second chromosome carrying the insertion in the RabX1 locus harboured an additional unrelated lethal mutation, which was removed by recombining the P-element in _RabX1_
with neoFRT42D. _Genotypes of females generated for rescue experiments_. Supplementary Fig. 3c,e: _w;_ FRT42D _RabX1__KG06805_/FRT42D GFP; GR1-Gal4 UAS-FLP/TM3_Ser_ Supplementary Figure
3d,f,: _w;_ FRT42D _RabX1__KG06805_/FRT42D GFP; GR1-Gal4 UAS-FLP/UAS-YFP_-RabX1._ GENERATION OF GENETIC MOSAICS AND RNAI INDUCTION Genetic mosaics were generated by using either a heat-shock
(hs) or a UAS/Gal4 inducible Flippase (FLP). To induce the hs-FLP for homozygous mutant cell clones heat shocks were applied during pupal stages by placing the vials in 37 °C water bath for
1 h once a day until hatching. Females were dissected 24 to 48 h after hatching. UAS-FLP was induced in follicular epithelium using a GR1-Gal4 UAS-FLP recombinant chromosome. Crosses with
this chromosome were raised at room temperature to avoid excessive clone induction. For RNAi knockdowns in the follicular epithelium UAS-inducible RNAi transgenes were driven by either
_traffic jam_-Gal4 or GR1-Gal4. Crosses were raised at room temperature. IMMUNOHISTOLOGY Ovaries were dissected in Schneider’s medium (Gibco LifeTechnologies) and separated in their anterior
part using fine needles. After fixation in 4% paraformaldehyde in PBS for 10 min. at room temperature, ovaries were washed and permeabilized with 0.1% TritonX-100 in PBS. All primary
antibodies were diluted in 0.1% Triton/PBS and incubated for 3 h at room temperature. Secondary antibodies were incubated for 2 h. Ovaries were then stained with 0.5 μg ml−1 DAPI for 5 min,
washed and mounted in Vectashield (Vector labs). Primary antibodies were used at the following concentrations: mouse anti-Armadillo 1:100 (DSHB, clone N2 7A1, concentrate), rat
anti-DE-cadherin 1:50 (DSHB, clone DCAD2, concentrate), mouse anti-Discs large 1:100 (DSHB, clone 4F3, concentrate), goat anti-GFP-FITC 1:200 (GeneTex, GTX26662), mouse anti-Fas3 1:200
(DSHB, clone 7G10, concentrate), mouse anti-Fas2 1:200 (DSHB, clone1D4, concentrate), rabbit anti-GM130 1:100 (Abcam, ab30637), guinea pig anti-Sec6 1:1,000 (provided by U. Tepass47), mouse
anti-Rab11 1:100 (BD, catalogue number 610657); rabbit anti-Rab5 1:250 (Abcam, ab31261), rabbit anti-aPKC 1:200 (Santa Cruz Biotechnologies, cs-216), mouse anti-HA 1:100 (Santa Cruz
Biotechnologies, sc-7379), rat anti-HA 1:100 (Roche, clone 3F10, catalogue number 1867423), rabbit anti-Rab4 1:200 (Abcam, ab78970). Alexa fluorophores coupled secondary antibodies
(Invitrogen) were used in a 1:200 dilution. Images were acquired using Leica LSM SP5 DS and SP5 MP confocal microscopes using × 63 oil immersion magnification at a resolution of 1,024 ×
1,024 then adjusted for gamma, edited and assembled with Adobe Photoshop CS3. ANALYSIS OF DE-CAD DISTRIBUTION IN LIVING OVARIES For the analysis of lateral DE-cad exocytosis living ovaries
were dissected in Schneiders medium and ovarioles separated in their anterior part with fine insect needles. Subsequently, ovaries were incubated in DE-cad solution (DE-cad antibody (1:50)
in Schneider’s medium containing 10% fetal calf serum, 100 μg ml−1 bovine insulin (Sigma)) for 10 min at room temperature. After washing, ovaries were fixed and permeabilized followed by
incubation with anti-Arm (Fig. 2a) or anti-Dlg (Fig. 2b) and anti-rat Alexa488 (to detect DE-cad) for two hours. The ovaries were washed and incubated with anti-mouse Alexa568 antibody to
detect Arm or Dlg. To follow DE-cad endocytosis and redistribution living ovaries harbouring _Rab11_ or _RabX1_ single or _RabX1 Rab11_ double-mutant clones (Figs 1d and 5c–e) were
continuously incubated in DE-cad solution for 3 h, then washed, fixed and permeabilized. Subsequently, ovaries were incubated with anti-GFP-FITC (to label the clones), anti-GM130 (Fig. 1d)
or anti-Arm (Fig. 5c–e) and anti-rat Alexa568 antibodies (to detect DE-cad) for 2 h. Then ovaries were washed and incubated with anti-rabbit Alexa647 (for GM130) or chicken anti-mouse
Alexa647 (for Arm) antibodies. To analyse DE-cad distribution in the absence of endocytosis (Fig. 6a,b) _shibire__TS1_ homozygous mutant flies were raised at 18 °C. Ovaries were dissected
and opened in Schneider’s medium and preincubated in a water bath at restrictive temperature (32 °C) for 20 min. Then DE-cad incubation was performed for 2 min using prewarmed (32 °C) DE-cad
solution at 32 °C. After washing, ovaries were incubated for 60 min in prewarmed incubation solution (Schneider’s medium containing 10% fetal calf serum, 100 μg ml−1 Insulin) at 32 °C to
allow DE-cad redistribution. After washing, fixation and permeabilization ovaries were incubated with anti-Arm and anti-rat Alexa488 (to detect DE-cad) antibodies for 2 h. The ovaries were
then washed and incubated with anti-mouse Alexa568. To analyse the movement of PM-labelled DE-cad along the lateral membrane DE-cad (Supplementary Fig. 5) incubation was performed for 2 min
in prewarmed DE-cad solution at 32 °C. After washing, ovaries were incubated for 1, 3, 5, 8, 12, 19, 25 and 30 min in incubation solution at 32 °C to allow DE-cad redistribution. After
washing, fixation and permeabilization, DE-cad was detected and Arm counterstaining performed (see above). After image acquisition the membrane length from five randomly selected cells was
measured. The distance between the most apical Arm signal and the most basal DE-cad signal revealed the total lateral PM length. Subsequently, the length of DE-cad signal along the lateral
membrane was measured. The percentages of DE-cad signal along the total membrane lengths was determined and plotted against the incubation times. To analyse DE-cad distribution when actin
polymerization is inhibited (Fig. 6c) ovaries were preincubated with 100 μg ml−1 cytochalasin D (Sigma) in incubation solution for 20 min at restrictive temperature. Subsequently, ovaries
were incubated for 2 min in DE-cad solution. After washing, the ovaries were incubated in incubation solution containing cytochalasin D in a final concentration of 100 μg ml−1 at 32 °C for
60 min. After washing, fixation and permeabilization, DE-cad was detected and Arm counterstaining performed (see above). GENERATION OF TRANSGENES HA-Rab11 and HA-Rab5 transgenic flies were
generated by cloning the open reading frame into the pUASp2 vector. Germline transformation was performed after standard procedure. cDNAs were obtained from _Drosophila_ Genomics Resource
Center (GM06568 for Rab11 and GH24702 for Rab5). cDNAs were amplified using the following primers: 5′- TGGATCCATGGGTGCAAGAGAAGACG -3′ 5′- GTCTAGATCACTGACAGCACTGTTTGCG -3′ (Rab11) and 5′-
CGAATTCATGGCAACCACTCC -3′ 5′- TTTCTAGATCACTTGCAGCAGTTGTTCG -3′ (Rab5). The PCR product was cloned (BamHI and XbaI for Rab11 and EcoRI and XbaI for Rab5) into HA-tag carrying pUASp2 vector
and sequenced resulting in Rab proteins with an N-terminal HA-tag. IMAGE ANALYSIS _Quantification of vertices and cell areas_. To count vertices and measure cell areas we developed a MATLAB
script (Version: 8.5.0.197613 (MathWorks, R2015a)), which uses standard image process functions to highlight the cell boundaries on binary images. After boundaries recognition, the vertices
were counted using the connectivity with the neighbour cells. The cells at the edges of the images were removed from the analysis. We only considered cells with a maximum of 8 vertices. We
calculated the cell area using the ‘regionprops’ MATLAB function. _Quantification of vesicle co-localization_. To identify co-localizing vesicles ImageJ/Fiji script was developed. The source
images are segmented using a low sigma Gaussian filter to highlight the vesicles in the green and in the red channel. Thresholds were applied to both channels to detect the vesicles. For
each channel a binary image was created, and to separate fused vesicles the ‘erode’ function was applied. After vesicle detection, the ‘analyse particles’ tool was used to count the number
of vesicles in the green and in the red channel. Overlapping vesicles were highlighted in yellow using the ‘image calculator’ plugin and counted using the ‘analyse particles’ tool. The batch
mode was set on true to reduce RAM usage and to speed up the image analysis. To preferentially restrict the analysis to the vesicles, a minimum and maximum vesicle size area was set.
Moreover, the circularity of the particles was considered for the analysis of some of the images. _Quantification of Arm aggregates and Golgi overlap_. An ImageJ/Fiji script was developed to
analyse the overlapping area between the Golgi and Arm aggregates. The source images were segmented using adapted Gaussian filters to highlight the Golgi and Arm signal and to reduce the
image background. Thresholds were applied to detect the Golgi and Arm aggregates and for each channel a binary image was created. To count and measure the area of the Golgi and Arm
aggregates the ‘analyse particles’ tool was used. The overlapping regions were detected using the ‘_image calculator_’ plugin, and the co-localizing areas were measured using the ‘analyse
particles’ tool. To restrict the analysis to the mutant cell clones a manual approach was used to remove detected objects form wild-type cells. The batch mode was set on true to reduce RAM
usage and to speed up the image analysis. To restrict the detection to the Golgi and to Arm aggregates minimum and maximum size area was applied to both channels during the image analysis.
ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Woichansky, I. _et al_. Three mechanisms control E-cadherin localization to the zonula adherens. _Nat. Commun._ 7:10834 doi:
10.1038/ncomms10834 (2016). REFERENCES * Niessen, C. M., Leckband, D. & Yap, A. S. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of
morphogenetic regulation. _Physiol. Rev._ 91, 691–731 (2011). Article CAS PubMed Google Scholar * Harris, T. J. & Tepass, U. Adherens junctions: from molecules to morphogenesis.
_Nat. Rev. Mol. Cell Biol._ 11, 502–514 (2010). Article CAS PubMed Google Scholar * Takeichi, M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. _Nat.
Rev. Mol. Cell Biol._ 15, 397–410 (2014). Article CAS PubMed Google Scholar * Schmalhofer, O., Brabletz, S. & Brabletz, T. E-cadherin, beta-catenin, and ZEB1 in malignant progression
of cancer. _Cancer Metastasis Rev._ 28, 151–166 (2009). Article CAS PubMed Google Scholar * Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. _J. Clin.
Invest._ 119, 1420–1428 (2009). Article CAS PubMed PubMed Central Google Scholar * Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in
development and disease. _Cell_ 139, 871–890 (2009). Article CAS PubMed Google Scholar * Wu, S. K. et al. Cortical F-actin stabilization generates apical-lateral patterns of junctional
contractility that integrate cells into epithelia. _Nat. Cell Biol._ 16, 167–178 (2014). Article CAS PubMed Google Scholar * Kowalczyk, A. P. & Nanes, B. A. Adherens junction
turnover: regulating adhesion through cadherin endocytosis, degradation, and recycling. _Subcell Biochem._ 60, 197–222 (2012). Article CAS PubMed PubMed Central Google Scholar * Baum,
B. & Georgiou, M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. _J. Cell Biol._ 192, 907–917 (2011). Article CAS PubMed PubMed Central
Google Scholar * Wirtz-Peitz, F. & Zallen, J. A. Junctional trafficking and epithelial morphogenesis. _Curr. Opin. Genet. Dev._ 19, 350–356 (2009). Article CAS PubMed PubMed Central
Google Scholar * Langevin, J. et al. _Drosophila_ exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. _Dev. Cell_ 9,
365–376 (2005). Article CAS PubMed Google Scholar * Blankenship, J. T., Fuller, M. T. & Zallen, J. A. The _Drosophila_ homolog of the Exo84 exocyst subunit promotes apical
epithelial identity. _J. Cell Sci._ 120, 3099–3110 (2007). Article CAS PubMed Google Scholar * Georgiou, M., Marinari, E., Burden, J. & Baum, B. Cdc42, Par6, and aPKC regulate
Arp2/3-mediated endocytosis to control local adherens junction stability. _Curr. Biol._ 18, 1631–1638 (2008). Article CAS PubMed Google Scholar * Leibfried, A., Fricke, R., Morgan, M.
J., Bogdan, S. & Bellaiche, Y. _Drosophila_ Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. _Curr. Biol._ 18, 1639–1648 (2008). Article
CAS PubMed Google Scholar * Stenmark, H. Rab GTPases as coordinators of vesicle traffic. _Nat. Rev. Mol. Cell Biol._ 10, 513–525 (2009). Article CAS PubMed Google Scholar *
Hutagalung, A. H. & Novick, P. J. Role of Rab GTPases in membrane traffic and cell physiology. _Physiol. Rev._ 91, 119–149 (2011). Article CAS PubMed Google Scholar * Wandinger-Ness,
A. & Zerial, M. Rab proteins and the compartmentalization of the endosomal system. _Cold Spring Harb. Perspect. Biol._ 6, a022616 (2014). Article PubMed PubMed Central Google Scholar
* Scott, C. C., Vacca, F. & Gruenberg, J. Endosome maturation, transport and functions. _Semin. Cell Dev. Biol._ 31, 2–10 (2014). Article CAS PubMed Google Scholar * Miranda, K. C.
et al. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. _J. Biol. Chem._ 276, 22565–22572 (2001). Article CAS
PubMed Google Scholar * Lock, J. G. & Stow, J. L. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. _Mol. Biol. Cell_ 16, 1744–1755 (2005).
Article CAS PubMed PubMed Central Google Scholar * Grindstaff, K. K. et al. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the
basal-lateral membrane in epithelial cells. _Cell_ 93, 731–740 (1998). Article CAS PubMed Google Scholar * Yeaman, C., Grindstaff, K. K. & Nelson, W. J. Mechanism of recruiting
Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. _J. Cell Sci._ 117, 559–570 (2004). Article CAS PubMed Google Scholar * Tanentzapf, G.,
Smith, C., McGlade, J. & Tepass, U. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. _J. Cell Biol._
151, 891–904 (2000). Article CAS PubMed PubMed Central Google Scholar * Franz, A. & Riechmann, V. Stepwise polarisation of the _Drosophila_ follicular epithelium. _Dev. Biol._ 338,
136–1347 (2010). Article CAS PubMed Google Scholar * Nystul, T. & Spradling, A. Regulation of epithelial stem cell replacement and follicle formation in the _Drosophila_ ovary.
_Genetics_ 184, 503–515 (2010). Article CAS PubMed PubMed Central Google Scholar * Horne-Badovinac, S. & Bilder, D. Mass transit: epithelial morphogenesis in the _Drosophila_ egg
chamber. _Dev. Dyn._ 232, 559–574 (2005). Article CAS PubMed Google Scholar * Berns, N., Woichansky, I., Friedrichsen, S., Kraft, N. & Riechmann, V. A genome-scale in vivo RNAi
analysis of epithelial development in _Drosophila_ identifies new proliferation domains outside of the stem cell niche. _J. Cell Sci._ 127, 2736–2748 (2014). Article CAS PubMed Google
Scholar * Chen, Y. T., Stewart, D. B. & Nelson, W. J. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane
targeting of E-cadherin in polarized MDCK cells. _J. Cell Biol._ 144, 687–699 (1999). Article CAS PubMed PubMed Central Google Scholar * Xu, J., Lan, L., Bogard, N., Mattione, C. &
Cohen, R. S. Rab11 is required for epithelial cell viability, terminal differentiation, and suppression of tumor-like growth in the _Drosophila_ egg chamber. _PLoS ONE_ 6, e20180 (2011).
Article ADS CAS PubMed PubMed Central Google Scholar * Morrison, H. A. et al. Regulation of early endosomal entry by the _Drosophila_ tumor suppressors Rabenosyn and Vps45. _Mol. Biol.
Cell_ 19, 4167–4176 (2008). Article CAS PubMed PubMed Central Google Scholar * Oda, H., Uemura, T., Harada, Y., Iwai, Y. & Takeichi, M. A _Drosophila_ homolog of cadherin
associated with armadillo and essential for embryonic cell-cell adhesion. _Dev. Biol._ 165, 716–726 (1994). Article CAS PubMed Google Scholar * Oda, H. & Tsukita, S. Nonchordate
classic cadherins have a structurally and functionally unique domain that is absent from chordate classic cadherins. _Dev. Biol._ 216, 406–422 (1999). Article CAS PubMed Google Scholar *
Izumi, G. et al. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. _J. Cell Biol._ 166, 237–248 (2004). Article CAS PubMed PubMed
Central Google Scholar * Kondylis, V. & Rabouille, C. The Golgi apparatus: lessons from _Drosophila_. _FEBS Lett._ 583, 3827–3838 (2009). Article CAS PubMed Google Scholar *
Mahowald, A. P. Ultrastructural observations on oogenesis in _Drosophila_. _J. Morphol._ 137, 29–48 (1972). Article CAS PubMed Google Scholar * Oshima, K. & Fehon, R. G. Analysis of
protein dynamics within the septate junction reveals a highly stable core protein complex that does not include the basolateral polarity protein Discs large. _J. Cell Sci._ 124, 2861–2871
(2011). Article CAS PubMed PubMed Central Google Scholar * Olivieri, D., Sykora, M. M., Sachidanandam, R., Mechtler, K. & Brennecke, J. An in vivo RNAi assay identifies major
genetic and cellular requirements for primary piRNA biogenesis in _Drosophila_. _Embo J_ 29, 3301–3317 (2010). Article CAS PubMed PubMed Central Google Scholar * Chan, C. C. et al.
Systematic discovery of Rab GTPases with synaptic functions in _Drosophila_. _Curr. Biol._ 21, 1704–1715 (2011). Article CAS PubMed PubMed Central Google Scholar * Lu, H. & Bilder,
D. Endocytic control of epithelial polarity and proliferation in _Drosophila_. _Nat. Cell Biol._ 7, 1132–1139 (2005). Article CAS Google Scholar * Vaccari, T. & Bilder, D. The
_Drosophila_ tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. _Dev. Cell_ 9, 687–698 (2005). Article CAS PubMed Google Scholar * Kametani,
Y. & Takeichi, M. Basal-to-apical cadherin flow at cell junctions. _Nat. Cell Biol._ 9, 92–98 (2007). Article CAS PubMed Google Scholar * Hill, E., van Der Kaay, J., Downes, C. P.
& Smythe, E. The role of dynamin and its binding partners in coated pit invagination and scission. _J. Cell Biol._ 152, 309–323 (2001). Article CAS PubMed PubMed Central Google
Scholar * Gomez, J. M., Wang, Y. & Riechmann, V. Tao controls epithelial morphogenesis by promoting Fasciclin 2 endocytosis. _J. Cell Biol._ 199, 1131–1143 (2012). Article CAS PubMed
PubMed Central Google Scholar * Folsch, H., Pypaert, M., Maday, S., Pelletier, L. & Mellman, I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally
distinct membrane domains. _J. Cell Biol._ 163, 351–362 (2003). Article PubMed PubMed Central Google Scholar * Prigent, M. et al. ARF6 controls post-endocytic recycling through its
downstream exocyst complex effector. _J. Cell Biol._ 163, 1111–1121 (2003). Article CAS PubMed PubMed Central Google Scholar * Zhang, X. M., Ellis, S., Sriratana, A., Mitchell, C. A.
& Rowe, T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. _J. Biol. Chem._ 279, 43027–43034 (2004). Article CAS PubMed Google Scholar * Beronja, S. et al. Essential
function of _Drosophila_ Sec6 in apical exocytosis of epithelial photoreceptor cells. _J. Cell Biol._ 169, 635–646 (2005). Article CAS PubMed PubMed Central Google Scholar * Kronen, M.
R., Schoenfelder, K. P., Klein, A. M. & Nystul, T. G. Basolateral junction proteins regulate competition for the follicle stem cell niche in the _Drosophila_ ovary. _PLoS ONE_ 9, e101085
(2014). Article ADS PubMed PubMed Central Google Scholar * Gupta, T. & Schüpbach, T. Cct1, a phosphatidylcholine biosynthesis enzyme, is required for _Drosophila_ oogenesis and
ovarian morphogenesis. _Development_ 130, 6075–6087 (2003). Article CAS PubMed Google Scholar * Bogard, N., Lan, L., Xu, J. & Cohen, R. S. Rab11 maintains connections between
germline stem cells and niche cells in the _Drosophila_ ovary. _Development_ 134, 3413–3418 (2007). Article CAS PubMed Google Scholar * Wucherpfennig, T., Wilsch-Brauninger, M. &
Gonzalez-Gaitan, M. Role of _Drosophila_ Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. _J. Cell Biol._ 161, 609–624 (2003). Article CAS PubMed
PubMed Central Google Scholar * Zhang, J. et al. Thirty-one flavors of _Drosophila_ rab proteins. _Genetics_ 176, 1307–1322 (2007). Article CAS PubMed PubMed Central Google Scholar *
van der Bliek, A. M. & Meyerowitz, E. M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. _Nature_ 351, 411–414 (1991). Article ADS CAS
PubMed Google Scholar * Murthy, M. et al. Sec6 mutations and the Drosophila _exocyst_ complex. _J. Cell Sci._ 118, 1139–1150 (2005). Article CAS PubMed Google Scholar Download
references ACKNOWLEDGEMENTS We thank R.S. Cohen, A. Guichet, Y. Bellaiche, U. Tepass, T. Schwarz, Bloomington stock centre, VDRC, TRiP and Developmental Studies Hybridoma Bank for fly stocks
and antibodies. We are grateful to M. Carl for helpful comments on this manuscript. We acknowledge support of the Core Facility Live Cell Imaging Mannheim at the CBTM (DFG INST 91027/9-1
FUGG, DFG INST 91027/10-1 FUGG). The project was funded by grants from the German Research Foundation (DFG, RI 1225/2-1) and the German Cancer Aid (Deutsche Krebshilfe, 110208). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Cell and Molecular Biology and Division of Signaling and Functional Genomics at the German Cancer Research Center (DKFZ), Medical Faculty
Mannheim, Heidelberg University, Ludolf-Krehl-Strasse 13-17, Mannheim, D-68167, Germany Innokenty Woichansky, Nicola Berns & Veit Riechmann * Heidelberg University, COS and Nikon
Imaging Center at the University of Heidelberg, Heidelberg, D-69120, Bioquant, Germany Carlo Antonio Beretta * Excellenzcluster CellNetworks, University of Heidelberg, Heidelberg, D-69120,
Germany Carlo Antonio Beretta Authors * Innokenty Woichansky View author publications You can also search for this author inPubMed Google Scholar * Carlo Antonio Beretta View author
publications You can also search for this author inPubMed Google Scholar * Nicola Berns View author publications You can also search for this author inPubMed Google Scholar * Veit Riechmann
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS I.W. performed the experiments. C.B. implemented automatic computer-based quantification.
N.B. identified the _Rab5_ and _Rab11_ phenotypes. V.R conceived the project and wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Veit Riechmann. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures 1-6 and Supplementary Tables 1-3 (PDF 1749 kb)
RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission
from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Woichansky, I., Beretta, C., Berns, N. _et al._ Three mechanisms control E-cadherin localization to the zonula adherens. _Nat Commun_ 7, 10834 (2016).
https://doi.org/10.1038/ncomms10834 Download citation * Received: 09 July 2015 * Accepted: 25 January 2016 * Published: 10 March 2016 * DOI: https://doi.org/10.1038/ncomms10834 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative