Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

During development, visual photoreceptors, bipolar cells and other neurons establish connections within the retina enabling the eye to process visual images over approximately 7 log units of
illumination1. Within the retina, cells that respond to light increment and light decrement are separated into ON- and OFF-pathways. Hereditary diseases are known to disturb these retinal
pathways, causing either progressive degeneration or stationary deficits2. Congenital stationary night blindness (CSNB) is a group of stable retinal disorders that are characterized by
abnormal night vision. Genetic subtypes of CSNB have been defined and different disease actions have been postulated3,4,5. The molecular bases have been elucidated in several subtypes,
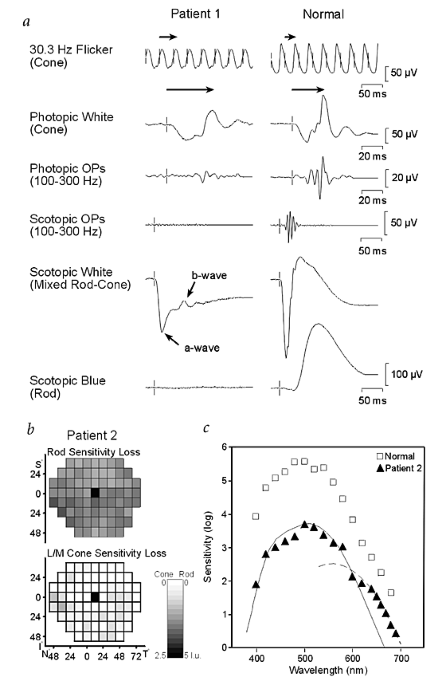
providing a better understanding of the disease mechanisms and developmental retinal neurobiology2. Here we have studied 22 families with 'complete' X-linked CSNB (CSNB1; MIM 310500; ref. 4)
in which affected males have night blindness, some photopic vision loss and a defect of the ON-pathway. We have found 14 different mutations, including 1 founder mutation in 7 families from
the United States, in a novel candidate gene, NYX. NYX, which encodes a glycosylphosphatidyl (GPI)-anchored protein called nyctalopin, is a new and unique member of the small leucine-rich
proteoglycan (SLRP) family6. The role of other SLRP proteins suggests that mutant nyctalopin disrupts developing retinal interconnections involving the ON-bipolar cells, leading to the
visual losses seen in patients with complete CSNB.
We thank the patients and their families for participation; K. Boycott, J. Friedman, D. Rancourt, S. Robbins, P. Schnetkamp, W. Stell, M. Walter and R. Winkfein for discussions; and J.
Whitehead, D. Martindale and L. Yong for technical assistance. This research was supported in part by operating grants to N.T.B.-H. from the RP Research Foundation (Foundation Fighting
Blindness), the Medical Research Council of Canada, and the I.D. Bebensee Foundation, and from the Foundation Fighting Blindness to D.G.B., S.G.J. and R.G.W. In addition, R.G.W. was
supported by an unrestricted grant from Research to Prevent Blindness, S.G.J. by NIH grant EY-05627 and D.G.B. by NIH grant EY05235. N.T.B.-H. was also supported by the Roy Allen Endowment,
The Alberta Children's Hospital Foundation and the Department of Ophthalmology, University of Alberta (W.G. Pearce and I.M. MacDonald).
Margaret J. Naylor and Tracy A. Maybaum: These authors contributed equally to this work.
Department of Medical Genetics, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada
N.Torben Bech-Hansen, Margaret J. Naylor, Tracy A. Maybaum & Rebecca L. Sparkes
Department of Biology, and Centre for Environmental Research, University of Victoria, Victoria, British Columbia, Canada
The Netherlands Ophthalmic Research Institute, Amsterdam, The Netherlands
Department of Physiology and Biophysics, University of Calgary, Calgary, Alberta, Canada
Department of Ophthalmology, Children's Hospital, and McGill University, Montreal, Quebec, Canada
Institut fur Humangenetik, Universitats-Klinikum Hamburg-Eppendorf, Hamburg, Germany
Department of Ophthalmology, Emory Eye Center, Atlanta, Georgia, USA
Department of Ophthalmology, SUNY Downstate, Brooklyn Medical Center, Brooklyn, New York
Scheie Eye Institute, University of Pennsylvania, Philadelphia, Pennsylvania, USA
Department of Ophthalmology and Visual Sciences, Texas Tech University Health Sciences Center, Lubbock, Texas, USA
Casey Eye Institute, Oregon Health Sciences University, Portland, Oregon, USA
Anyone you share the following link with will be able to read this content: