Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

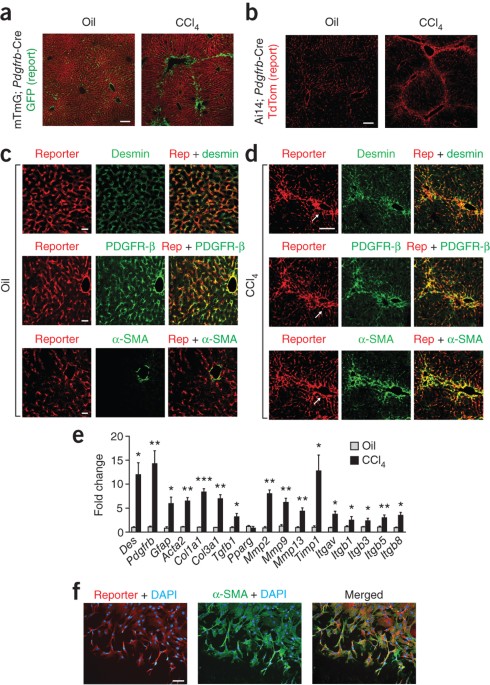
ABSTRACT Myofibroblasts are the major source of extracellular matrix components that accumulate during tissue fibrosis, and hepatic stellate cells (HSCs) are believed to be the major source
of myofibroblasts in the liver. To date, robust systems to genetically manipulate these cells have not been developed. We report that Cre under control of the promoter of _Pdgfrb_
(_Pdgfrb-_Cre) inactivates _loxP_-flanked genes in mouse HSCs with high efficiency. We used this system to delete the gene encoding αv integrin subunit because various αv-containing
integrins have been suggested as central mediators of fibrosis in multiple organs. Such depletion protected mice from carbon tetrachloride–induced hepatic fibrosis, whereas global loss of
β3, β5 or β6 integrins or conditional loss of β8 integrins in HSCs did not. We also found that _Pdgfrb-_Cre effectively targeted myofibroblasts in multiple organs, and depletion of the αv
integrin subunit using this system was protective in other models of organ fibrosis, including pulmonary and renal fibrosis. Pharmacological blockade of αv-containing integrins by a small
molecule (CWHM 12) attenuated both liver and lung fibrosis, including in a therapeutic manner. These data identify a core pathway that regulates fibrosis and suggest that pharmacological
targeting of all αv integrins may have clinical utility in the treatment of patients with a broad range of fibrotic diseases. Access through your institution Buy or subscribe This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per
year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS AN INDUCIBLE
MODEL FOR GENETIC MANIPULATION AND FATE-TRACING OF PDGFRΒ-EXPRESSING FIBROGENIC CELLS IN THE LIVER Article Open access 05 May 2023 AMPK STIMULATION INHIBITS YAP/TAZ SIGNALING TO AMELIORATE
HEPATIC FIBROSIS Article Open access 03 March 2024 ANTI-INTEGRIN ΑV THERAPY IMPROVES CARDIAC FIBROSIS AFTER MYOCARDIAL INFARCTION BY BLUNTING CARDIAC PW1+ STROMAL CELLS Article Open access
09 July 2020 REFERENCES * Gleizes, P.E. et al. TGF-β latency: biological significance and mechanisms of activation. _Stem Cells_ 15, 190–197 (1997). CAS PubMed Google Scholar * Munger,
J.S. et al. Latent transforming growth factor-β: structural features and mechanisms of activation. _Kidney Int._ 51, 1376–1382 (1997). CAS PubMed Google Scholar * Munger, J.S. et al. The
integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. _Cell_ 96, 319–328 (1999). CAS PubMed Google Scholar * Mu, D. et al. The
integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. _J. Cell Biol._ 157, 493–507 (2002). CAS PubMed PubMed Central Google Scholar * Annes, J.P.,
Rifkin, D.B. & Munger, J.S. The integrin αvβ6 binds and activates latent TGFβ3. _FEBS Lett._ 511, 65–68 (2002). CAS PubMed Google Scholar * Aluwihare, P. et al. Mice that lack
activity of αvβ6- and αvβ8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. _J. Cell Sci._ 122, 227–232 (2009). CAS PubMed Google Scholar * Wang, B. et al. Role of
αvβ6 integrin in acute biliary fibrosis. _Hepatology_ 46, 1404–1412 (2007). CAS PubMed Google Scholar * Hahm, K. et al. αv βa6 integrin regulates renal fibrosis and inflammation in Alport
mouse. _Am. J. Pathol._ 170, 110–125 (2007). CAS PubMed PubMed Central Google Scholar * Ma, L.J. et al. Transforming growth factor-β-dependent and -independent pathways of induction of
tubulointerstitial fibrosis in β6−/− mice. _Am. J. Pathol._ 163, 1261–1273 (2003). CAS PubMed PubMed Central Google Scholar * Breuss, J.M., Gillett, N., Lu, L., Sheppard, D. &
Pytela, R. Restricted distribution of integrin β6 mRNA in primate epithelial tissues. _J. Histochem. Cytochem._ 41, 1521–1527 (1993). CAS PubMed Google Scholar * Breuss, J.M. et al.
Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. _J. Cell Sci._ 108, 2241–2251 (1995). CAS PubMed Google Scholar
* Shi, M. et al. Latent TGF-β structure and activation. _Nature_ 474, 343–349 (2011). CAS PubMed PubMed Central Google Scholar * Wipff, P.J., Rifkin, D.B., Meister, J.J. & Hinz, B.
Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. _J. Cell Biol._ 179, 1311–1323 (2007). CAS PubMed PubMed Central Google Scholar * Munger, J.S., Harpel,
J.G., Giancotti, F.G. & Rifkin, D.B. Interactions between growth factors and integrins: latent forms of transforming growth factor-β are ligands for the integrin αvβ1 . _Mol. Biol. Cell_
9, 2627–2638 (1998). CAS PubMed PubMed Central Google Scholar * Asano, Y. et al. Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in
scleroderma fibroblasts. _J. Immunol._ 175, 7708–7718 (2005). CAS PubMed Google Scholar * Asano, Y., Ihn, H., Yamane, K., Jinnin, M. & Tamaki, K. Increased expression of integrin αvβ5
induces the myofibroblastic differentiation of dermal fibroblasts. _Am. J. Pathol._ 168, 499–510 (2006). CAS PubMed PubMed Central Google Scholar * Friedman, S.L. & Arthur, M.J.
Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of
platelet-derived growth factor receptors. _J. Clin. Invest._ 84, 1780–1785 (1989). CAS PubMed PubMed Central Google Scholar * Pinzani, M., Gesualdo, L., Sabbah, G.M. & Abboud, H.E.
Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. _J. Clin. Invest._ 84, 1786–1793 (1989). CAS
PubMed PubMed Central Google Scholar * Wong, L., Yamasaki, G., Johnson, R.J. & Friedman, S.L. Induction of β-platelet–derived growth factor receptor in rat hepatic lipocytes during
cellular activation _in vivo_ and in culture. _J. Clin. Invest._ 94, 1563–1569 (1994). CAS PubMed PubMed Central Google Scholar * Pinzani, M. et al. Expression of platelet-derived growth
factor and its receptors in normal human liver and during active hepatic fibrogenesis. _Am. J. Pathol._ 148, 785–800 (1996). CAS PubMed PubMed Central Google Scholar * Ikura, Y. et al.
Expression of platelet-derived growth factor and its receptor in livers of patients with chronic liver disease. _J. Gastroenterol._ 32, 496–501 (1997). CAS PubMed Google Scholar * Coin,
P.G. et al. Lipopolysaccharide up-regulates platelet-derived growth factor (PDGF) α-receptor expression in rat lung myofibroblasts and enhances response to all PDGF isoforms. _J. Immunol._
156, 4797–4806 (1996). CAS PubMed Google Scholar * Bonner, J.C. Regulation of PDGF and its receptors in fibrotic diseases. _Cytokine Growth Factor Rev._ 15, 255–273 (2004). CAS PubMed
Google Scholar * Chen, Y.T. et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. _Kidney
Int._ 80, 1170–1181 (2011). CAS PubMed Google Scholar * Foo, S.S. et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. _Cell_ 124, 161–173 (2006). CAS
PubMed Google Scholar * de Leeuw, A.M., McCarthy, S.P., Geerts, A. & Knook, D.L. Purified rat liver fat-storing cells in culture divide and contain collagen. _Hepatology_ 4, 392–403
(1984). CAS PubMed Google Scholar * Friedman, S.L., Roll, F.J., Boyles, J. & Bissell, D.M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. _Proc. Natl.
Acad. Sci. USA_ 82, 8681–8685 (1985). CAS PubMed PubMed Central Google Scholar * Muzumdar, M.D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter
mouse. _Genesis_ 45, 593–605 (2007). CAS PubMed Google Scholar * Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. _Nat.
Neurosci._ 13, 133–140 (2010). CAS PubMed Google Scholar * Abe, M. et al. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1
promoter-luciferase construct. _Anal. Biochem._ 216, 276–284 (1994). CAS PubMed Google Scholar * Hynes, R.O. Integrins: bidirectional, allosteric signaling machines. _Cell_ 110, 673–687
(2002). CAS PubMed Google Scholar * Zhu, J. et al. β8 integrins are required for vascular morphogenesis in mouse embryos. _Development_ 129, 2891–2903 (2002). CAS PubMed Google Scholar
* Fässler, R. & Meyer, M. Consequences of lack of β1 integrin gene expression in mice. _Genes Dev._ 9, 1896–1908 (1995). PubMed Google Scholar * Stephens, L.E. et al. Deletion of β1
integrins in mice results in inner cell mass failure and peri-implantation lethality. _Genes Dev._ 9, 1883–1895 (1995). CAS PubMed Google Scholar * Abraham, S., Kogata, N., Fässler, R.
& Adams, R.H. Integrin β1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. _Circ. Res._ 102, 562–570 (2008). CAS PubMed Google Scholar * Hinz, B. et
al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. _Am. J. Pathol._ 180, 1340–1355 (2012). CAS PubMed PubMed Central Google Scholar * Armulik,
A., Genové, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. _Dev. Cell_ 21, 193–215 (2011). CAS PubMed Google Scholar
* Patsenker, E. et al. Pharmacological inhibition of integrin αvβ3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis. _Hepatology_ 50, 1501–1511 (2009). CAS
PubMed Google Scholar * Ignotz, R.A. & Massagué, J. Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular
matrix. _J. Biol. Chem._ 261, 4337–4345 (1986). CAS PubMed Google Scholar * Roberts, A.B. et al. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis _in vivo_
and stimulation of collagen formation _in vitro_. _Proc. Natl. Acad. Sci. USA_ 83, 4167–4171 (1986). CAS PubMed PubMed Central Google Scholar * Leask, A. & Abraham, D.J. TGF-β
signaling and the fibrotic response. _FASEB J._ 18, 816–827 (2004). CAS PubMed Google Scholar * Lacy-Hulbert, A. et al. Ulcerative colitis and autoimmunity induced by loss of myeloid αv
integrins. _Proc. Natl. Acad. Sci. USA_ 104, 15823–15828 (2007). CAS PubMed PubMed Central Google Scholar * Proctor, J.M., Zang, K., Wang, D., Wang, R. & Reichardt, L.F. Vascular
development of the brain requires β8 integrin expression in the neuroepithelium. _J. Neurosci._ 25, 9940–9948 (2005). CAS PubMed PubMed Central Google Scholar * Hodivala-Dilke, K.M. et
al. β3-integrin–deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. _J. Clin. Invest._ 103, 229–238 (1999). CAS PubMed PubMed Central
Google Scholar * Huang, X., Griffiths, M., Wu, J., Farese, R.V. Jr. & Sheppard, D. Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. _Mol. Cell.
Biol._ 20, 755–759 (2000). CAS PubMed PubMed Central Google Scholar * Huang, X.Z. et al. Inactivation of the integrin β6 subunit gene reveals a role of epithelial integrins in regulating
inflammation in the lung and skin. _J. Cell Biol._ 133, 921–928 (1996). CAS PubMed Google Scholar * Henderson, N.C. et al. Galectin-3 regulates myofibroblast activation and hepatic
fibrosis. _Proc. Natl. Acad. Sci. USA_ 103, 5060–5065 (2006). CAS PubMed PubMed Central Google Scholar * Henderson, N.C. et al. Galectin-3 expression and secretion links macrophages to
the promotion of renal fibrosis. _Am. J. Pathol._ 172, 288–298 (2008). CAS PubMed PubMed Central Google Scholar * McCarty, J.H. et al. Selective ablation of αv integrins in the central
nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. _Development_ 132, 165–176 (2005). CAS PubMed Google Scholar * Nagarajan, S.R. et al.
R-isomers of Arg-Gly-Asp (RGD) mimics as potent αvβ3 inhibitors. _Bioorg. Med. Chem._ 15, 3783–3800 (2007). CAS PubMed Google Scholar * Shannon, K.E. et al. Anti-metastatic properties of
RGD-peptidomimetic agents S137 and S247. _Clin. Exp. Metastasis_ 21, 129–138 (2004). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by a Wellcome
Trust Intermediate Clinical Fellowship (ref. 085187) to N.C.H., National Institutes of Health grants HL102292, HL53949 and AI077439 (to D.S.), a University of California, San Francisco
(UCSF) Liver Center Tool and Technology grant (to N.C.H) and P30 DK026743 (UCSF Liver Center). We thank K. Thorn at the UCSF Nikon Imaging Center for assistance with image analysis. We also
thank C. Her, N. Wu, S. Huling, D. Rodrigues and R. Aucott for expert technical assistance. We also acknowledge the contribution of M. Singh (chemical synthesis of compounds CWHM 12 and CWHM
96), D. Tajfirouz, S. Freeman and M. Yates at Saint Louis University for technical assistance in conducting integrin functional assays to characterize compound activities. L. Reichardt
(UCSF) provided _Itgb8_flox/flox mice and R. Hynes (Massachusetts Institute of Technology) provided _Itgb3_−/− mice on 129/svJae background. S. Violette (Biogen Idec) provided antibody to
αvβ6 (human/mouse chimeric 2A1), W. Stallcup (Sanford-Burnham Medical Research Institute) provided antibody to PDGFR-β and H. Yagita (Juntendo University) provided antibody to αv integrin
(clone RMV-7). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Medicine, Lung Biology Center, University of California, San Francisco, San Francisco, California, USA Neil C
Henderson, Yoshio Katamura, Marilyn M Giacomini, Juan D Rodriguez & Dean Sheppard * Medical Research Council Centre for Inflammation Research, The Queen's Medical Research
Institute, University of Edinburgh, Edinburgh, UK Neil C Henderson, Antonella Pellicoro & John P Iredale * Department of Pediatrics, University of California, San Francisco, San
Francisco, California, USA Thomas D Arnold * Department of Cancer Biology, University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA Joseph H McCarty * Department of Immunology,
Genetics and Pathology, Uppsala University, Uppsala, Sweden Elisabeth Raschperger & Christer Betsholtz * Department of Medical Biochemistry and Biophysics, Karolinska Institutet,
Stockholm, Sweden Elisabeth Raschperger & Christer Betsholtz * Center for World Health and Medicine, Saint Louis University, Edward A. Doisy Research Center, St. Louis, Missouri, USA
Peter G Ruminski, David W Griggs & Michael J Prinsen * Department of Medicine, The Liver Center, University of California, San Francisco, San Francisco, California, USA Jacquelyn J Maher
* Department of Pediatrics, Program of Developmental Immunology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA Adam Lacy-Hulbert * Department of
Tissue Morphogenesis, Faculty of Medicine, Max Planck Institute for Molecular Biomedicine, University of Münster, Münster, Germany Ralf H Adams Authors * Neil C Henderson View author
publications You can also search for this author inPubMed Google Scholar * Thomas D Arnold View author publications You can also search for this author inPubMed Google Scholar * Yoshio
Katamura View author publications You can also search for this author inPubMed Google Scholar * Marilyn M Giacomini View author publications You can also search for this author inPubMed
Google Scholar * Juan D Rodriguez View author publications You can also search for this author inPubMed Google Scholar * Joseph H McCarty View author publications You can also search for
this author inPubMed Google Scholar * Antonella Pellicoro View author publications You can also search for this author inPubMed Google Scholar * Elisabeth Raschperger View author
publications You can also search for this author inPubMed Google Scholar * Christer Betsholtz View author publications You can also search for this author inPubMed Google Scholar * Peter G
Ruminski View author publications You can also search for this author inPubMed Google Scholar * David W Griggs View author publications You can also search for this author inPubMed Google
Scholar * Michael J Prinsen View author publications You can also search for this author inPubMed Google Scholar * Jacquelyn J Maher View author publications You can also search for this
author inPubMed Google Scholar * John P Iredale View author publications You can also search for this author inPubMed Google Scholar * Adam Lacy-Hulbert View author publications You can also
search for this author inPubMed Google Scholar * Ralf H Adams View author publications You can also search for this author inPubMed Google Scholar * Dean Sheppard View author publications
You can also search for this author inPubMed Google Scholar CONTRIBUTIONS N.C.H. and D.S. conceived and designed the project. N.C.H. performed the experiments with assistance from T.D.A.,
Y.K., M.M.G., J.D.R. and A.P.; J.H.M. contributed reagents; P.G.R., D.W.G. and M.J.P. designed and synthesized the small molecule αv integrin inhibitor (CWHM 12) and performed the
ligand-binding studies to characterize the _in vitro_ potency of CWHM 12; J.J.M. and J.P.I. contributed reagents and provided substantial intellectual contribution; E.R. and C.B. contributed
_Pdgfrb_-BAC-eGFP knock-in reporter mice; A.L.-H. contributed _Itgav_flox/flox mice; R.H.A. contributed _Pdgfrb_-Cre mice; N.C.H., T.D.A., Y.K., M.M.G. and D.S. analyzed data and N.C.H.,
J.P.I. and D.S. wrote the manuscript. CORRESPONDING AUTHORS Correspondence to Neil C Henderson or Dean Sheppard. ETHICS DECLARATIONS COMPETING INTERESTS P.G.R. and D.W.G. hold equity in
Antegrin Therapeutics, LLC. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–8 and Supplementary Methods (PDF 4801 kb) RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Henderson, N., Arnold, T., Katamura, Y. _et al._ Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in
several organs. _Nat Med_ 19, 1617–1624 (2013). https://doi.org/10.1038/nm.3282 Download citation * Received: 03 April 2012 * Accepted: 17 June 2013 * Published: 10 November 2013 * Issue
Date: December 2013 * DOI: https://doi.org/10.1038/nm.3282 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative