A human-specific as3mt isoform and borcs7 are molecular risk factors in the 10q24. 32 schizophrenia-associated locus
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

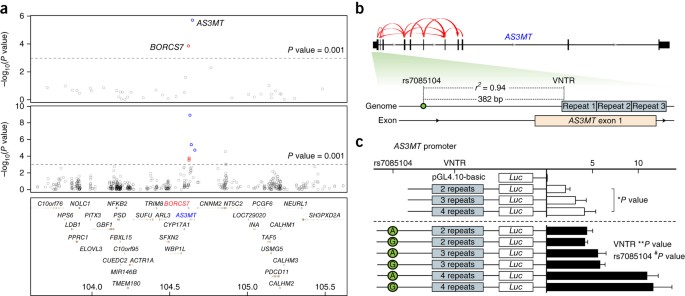
ABSTRACT Genome-wide association studies (GWASs) have reported many single nucleotide polymorphisms (SNPs) associated with psychiatric disorders, but knowledge is lacking regarding molecular
mechanisms. Here we show that risk alleles spanning multiple genes across the 10q24.32 schizophrenia-related locus are associated in the human brain selectively with an increase in the
expression of both BLOC-1 related complex subunit 7 (_BORCS7_) and a previously uncharacterized, human-specific arsenite methyltransferase (_AS3MT_) isoform (_AS3MT_d2d3), which lacks
arsenite methyltransferase activity and is more abundant in individuals with schizophrenia than in controls. Conditional-expression analysis suggests that _BORCS7_ and _AS3MT_d2d3 signals
are largely independent. GWAS risk SNPs across this region are linked with a variable number tandem repeat (VNTR) polymorphism in the first exon of _AS3MT_ that is associated with the
expression of _AS3MT_d2d3 in samples from both Caucasians and African Americans. The VNTR genotype predicts promoter activity in luciferase assays, as well as DNA methylation within the
_AS3MT_ gene. Both _AS3MT_d2d3 and _BORCS7_ are expressed in adult human neurons and astrocytes, and they are upregulated during human stem cell differentiation toward neuronal fates. Our
results provide a molecular explanation for the prominent 10q24.32 locus association, including a novel and evolutionarily recent protein that is involved in early brain development and
confers risk for psychiatric illness. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through
your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant
access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions *
Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A MISSENSE VARIANT IN _NDUFA6_ CONFERS SCHIZOPHRENIA RISK BY AFFECTING YY1 BINDING AND _NAGA_ EXPRESSION
Article 30 April 2021 INTEGRATIVE ANALYSES PRIORITIZE _GNL3_ AS A RISK GENE FOR BIPOLAR DISORDER Article 21 August 2020 THE MOLECULAR PATHOLOGY OF SCHIZOPHRENIA: AN OVERVIEW OF EXISTING
KNOWLEDGE AND NEW DIRECTIONS FOR FUTURE RESEARCH Article Open access 06 March 2023 REFERENCES * Prince, M. et al. No health without mental health. _Lancet_ 370, 859–877 (2007). Article
Google Scholar * O'Donovan, M.C. et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. _Nat. Genet._ 40, 1053–1055 (2008). Article CAS
Google Scholar * Stefansson, H. et al. Common variants conferring risk of schizophrenia. _Nature_ 460, 744–747 (2009). Article CAS Google Scholar * Schizophrenia Working Group of the
Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. _Nature_ 511, 421–427 (2014). * Psychiatric GWAS Consortium Bipolar Disorder Working
Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near _ODZ4_. _Nat. Genet._ 43, 977–983 (2011). * Ripke, S. et al. Genome-wide
association analysis identifies 13 new risk loci for schizophrenia. _Nat. Genet._ 45, 1150–1159 (2013). Article CAS Google Scholar * Cross-Disorder Group of the Psychiatric Genomics
Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. _Lancet_ 381, 1371–1379 (2013). * Shi, J. et al. Common variants on
chromosome 6p22.1 are associated with schizophrenia. _Nature_ 460, 753–757 (2009). Article CAS Google Scholar * Shi, Y. et al. Common variants on 8p12 and 1q24.2 confer risk of
schizophrenia. _Nat. Genet._ 43, 1224–1227 (2011). Article CAS Google Scholar * Rietschel, M. et al. Association between genetic variation in a region on chromosome 11 and schizophrenia
in large samples from Europe. _Mol. Psychiatry_ 17, 906–917 (2012). Article CAS Google Scholar * Ripke, S. et al. Genome-wide association study identifies five new schizophrenia loci.
_Nat. Genet._ 43, 969–976 (2011). Article CAS Google Scholar * Huffaker, S.J. et al. A primate-specific, brain isoform of _KCNH2_ affects cortical physiology, cognition, neuronal
repolarization and risk of schizophrenia. _Nat. Med._ 15, 509–518 (2009). Article CAS Google Scholar * Tao, R. et al. Expression of _ZNF804A_ in human brain and alterations in
schizophrenia, bipolar disorder, and major depressive disorder: a novel transcript fetally regulated by the psychosis risk variant rs1344706. _JAMA Psychiatry_ 71, 1112–1120 (2014). Article
Google Scholar * Law, A.J. et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. _Proc. Natl. Acad. Sci. USA_
103, 6747–6752 (2006). Article CAS Google Scholar * Wedenoja, J. et al. Replication of association between working memory and Reelin, a potential modifier gene in schizophrenia. _Biol.
Psychiatry_ 67, 983–991 (2010). Article CAS Google Scholar * Zhang, J. et al. A _cis_-phase interaction study of genetic variants within the _MAOA_ gene in major depressive disorder.
_Biol. Psychiatry_ 68, 795–800 (2010). Article CAS Google Scholar * Prata, D.P. et al. Altered effect of dopamine transporter 3′UTR VNTR genotype on prefrontal and striatal function in
schizophrenia. _Arch. Gen. Psychiatry_ 66, 1162–1172 (2009). Article CAS Google Scholar * Wood, T.C. et al. Human arsenic methyltransferase (_AS3MT_) pharmacogenetics: gene resequencing
and functional genomics studies. _J. Biol. Chem._ 281, 7364–7373 (2006). Article CAS Google Scholar * Valluy, J. et al. A coding-independent function of an alternative _Ube3a_ transcript
during neuronal development. _Nat. Neurosci._ 18, 666–673 (2015). Article CAS Google Scholar * Pu, J. et al. BORC, a multisubunit complex that regulates lysosome positioning. _Dev. Cell_
33, 176–188 (2015). Article CAS Google Scholar * Lonsdale, J. et al. & GTEx Consortium. The genotype-tissue expression (GTEx) project. _Nat. Genet._ 45, 580–585 (2013). Article CAS
Google Scholar * Harrison, P.J. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. _Psychopharmacology (Berl.)_ 174,
151–162 (2004). Article CAS Google Scholar * Oberdoerffer, S. A conserved role for intragenic DNA methylation in alternative pre-mRNA splicing. _Transcription_ 3, 106–109 (2012). Article
Google Scholar * Jaffe, A.E. et al. Mapping DNA methylation across development, genotype and schizophrenia in the human frontal cortex. _Nat. Neurosci._ 19, 40–47 (2016). Article CAS
Google Scholar * Geng, Z. et al. Effects of selenium on the structure and function of recombinant human S-adenosyl-L-methionine dependent arsenic (+3 oxidation state) methyltransferase in
_E. coli_. _J. Biol. Inorg. Chem._ 14, 485–496 (2009). Article CAS Google Scholar * Antonelli, R., Shao, K., Thomas, D.J., Sams, R. II & Cowden, J. _AS3MT_, _GSTO_, and _PNP_
polymorphisms: impact on arsenic methylation and implications for disease susceptibility. _Environ. Res._ 132, 156–167 (2014). Article CAS Google Scholar * Rodrigues, E.G. et al. _GSTO_
and _AS3MT_ genetic polymorphisms and differences in urinary arsenic concentrations among residents in Bangladesh. _Biomarkers_ 17, 240–247 (2012). Article CAS Google Scholar * Agusa, T.,
Fujihara, J., Takeshita, H. & Iwata, H. Individual variations in inorganic arsenic metabolism associated with _AS3MT_ genetic polymorphisms. _Int. J. Mol. Sci._ 12, 2351–2382 (2011).
Article CAS Google Scholar * Lin, S. et al. A novel S-adenosyl-L-methionine:arsenic(III) methyltransferase from rat liver cytosol. _J. Biol. Chem._ 277, 10795–10803 (2002). Article CAS
Google Scholar * Tyler, C.R. & Allan, A.M. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. _Curr. Environ. Health Rep._
1, 132–147 (2014). Article Google Scholar * Ghiani, C.A. et al. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in
neurite outgrowth. _Mol. Psychiatry_ 15, 204–215 (2010). Article CAS Google Scholar * Morris, D.W. et al. Dysbindin (_DTNBP1_) and the biogenesis of lysosome-related organelles complex 1
(BLOC-1): main and epistatic gene effects are potential contributors to schizophrenia susceptibility. _Biol. Psychiatry_ 63, 24–31 (2008). Article CAS Google Scholar * Jaffe, A.E. et al.
Developmental regulation of human cortex transcription and its clinical relevance at single base resolution. _Nat. Neurosci._ 18, 154–161 (2015). Article CAS Google Scholar * Kunii, Y. et
al. Revisiting DARPP-32 in postmortem human brain: changes in schizophrenia and bipolar disorder and genetic associations with t-DARPP-32 expression. _Mol. Psychiatry_ 19, 192–199 (2014).
Article CAS Google Scholar * Lipska, B.K. et al. Critical factors in gene expression in postmortem human brain: Focus on studies in schizophrenia. _Biol. Psychiatry_ 60, 650–658 (2006).
Article CAS Google Scholar * Trapnell, C., Pachter, L. & Salzberg, S.L. TopHat: discovering splice junctions with RNA-Seq. _Bioinformatics_ 25, 1105–1111 (2009). Article CAS Google
Scholar * Liao, Y., Smyth, G.K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. _Bioinformatics_ 30, 923–930 (2014).
Article CAS Google Scholar * Scharpf, R.B., Irizarry, R.A., Ritchie, M.E., Carvalho, B. & Ruczinski, I. Using the R Package crlmm for Genotyping and Copy Number Estimation. _J. Stat.
Softw._ 40, 1–32 (2011). Article Google Scholar * Ioannidis, J.P., Ntzani, E.E., Trikalinos, T.A. & Contopoulos-Ioannidis, D.G. Replication validity of genetic association studies.
_Nat. Genet._ 29, 306–309 (2001). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank A. Deep-Soboslay (Lieber Institute for Brain Development) for her tireless
efforts in clinical diagnosis and demographic characterization; R. Zielke, R.D. Vigorito and R.M. Johnson (National Institute of Child Health and Human Development Brain and Tissue Bank for
Developmental Disorders at the University of Maryland) for their provision of fetal, pediatric and adolescent brain tissue specimens; X. Xiao (Johns Hopkins Bloomberg School of Public
Health) for her technical assistance. This work was supported by funding from the Lieber Institute for Brain Development and the Maltz Research Laboratories, and from a Senior Investigator
grant from the Brain Behavior Research Foundation (J.E.K.). The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the US National
Institutes of Health. Additional funds were provided by the NCI, NHGRI, NHLBI, NIDA, NIMH and the NINDS. Donors were enrolled at Biospecimen Source Sites funded by NCI\SAIC-Frederick, Inc.
(SAIC-F) subcontracts to the National Disease Research Interchange (10XS170), Roswell Park Cancer Institute (10XS171) and Science Care, Inc. (X10S172). The Laboratory, Data Analysis and
Coordinating Center (LDACC) were funded through a contract (HHSN268201000029C) to the Broad Institute, Inc. Biorepository operations were funded through an SAIC-F subcontract to Van Andel
Institute (10ST1035). Additional data repository and project management were provided by SAIC-F (HHSN261200800001E). The Brain Bank was supported by supplements to University of Miami grants
DA006227 & DA033684, and to contract N01MH000028. Statistical Methods development grants were made to the University of Geneva (MH090941 & MH101814), the University of Chicago
(MH090951, MH090937, MH101820, MH101825), the University of North Carolina at Chapel Hill (MH090936 & MH101819), Harvard University (MH090948), Stanford University (MH101782), Washington
University St. Louis (MH101810) and the University of Pennsylvania (MH101822). The data used for the analyses described in this manuscript were obtained from dbGaP accession number
phs000424.v6.p1 on October 6, 2015. Data were generated as part of the CommonMind Consortium supported by funding from Takeda Pharmaceuticals Company Limited, F. Hoffman-La Roche, Ltd. and
NIH grants R01MH085542, R01MH093725, P50MH066392, P50MH080405, R01MH097276, RO1-MH-075916, P50M096891, P50MH084053S1, R37MH057881 and R37MH057881S1, HHSN271201300031C, AG02219, AG05138 and
MH06692. Brain tissue for the study was obtained from the following brain-bank collections: the Mount Sinai NIH Brain and Tissue Repository, the University of Pennsylvania Alzheimer's
Disease Core Center, the University of Pittsburgh NeuroBioBank and Brain and Tissue Repositories and the NIMH Human Brain Collection Core. CMC Leadership: P. Sklar, J. Buxbaum (Icahn School
of Medicine at Mount Sinai), B. Devlin, D. Lewis (University of Pittsburgh), R. Gur, C.-G. Hahn (University of Pennsylvania), K. Hirai, H. Toyoshiba (Takeda Pharmaceuticals Company, Ltd.),
E. Domenici, L. Essioux (F. Hoffman-La Roche, Ltd.), L. Mangravite, M. Peters (Sage Bionetworks), T. Lehner, B. Lipska (NIMH). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Lieber Institute
for Brain Development, Johns Hopkins Medical Campus, Baltimore, Maryland, USA Ming Li, Andrew E Jaffe, Richard E Straub, Ran Tao, Joo Heon Shin, Yanhong Wang, Qiang Chen, Chao Li, Yankai
Jia, Kazutaka Ohi, Brady J Maher, Joshua G Chenoweth, Daniel J Hoeppner, Huijun Wei, Thomas M Hyde, Ronald McKay, Joel E Kleinman & Daniel R Weinberger * Department of Mental Health,
Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA Andrew E Jaffe * Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, Baltimore,
Maryland, USA Brady J Maher, Thomas M Hyde, Joel E Kleinman & Daniel R Weinberger * Department of Neuroscience, Johns Hopkins School of Medicine, Baltimore, Maryland, USA Brady J Maher
& Daniel R Weinberger * AstraZeneca Neuroscience, Innovative Medicines and Early Development Biotech Unit, Cambridge, Massachusetts, USA Nicholas J Brandon & Alan Cross * Department
of Neurology, Johns Hopkins School of Medicine, Baltimore, Maryland, USA Thomas M Hyde & Daniel R Weinberger * McKusick Nathans Institute of Genetic Medicine, Johns Hopkins School of
Medicine, Baltimore, Maryland, USA Daniel R Weinberger Authors * Ming Li View author publications You can also search for this author inPubMed Google Scholar * Andrew E Jaffe View author
publications You can also search for this author inPubMed Google Scholar * Richard E Straub View author publications You can also search for this author inPubMed Google Scholar * Ran Tao
View author publications You can also search for this author inPubMed Google Scholar * Joo Heon Shin View author publications You can also search for this author inPubMed Google Scholar *
Yanhong Wang View author publications You can also search for this author inPubMed Google Scholar * Qiang Chen View author publications You can also search for this author inPubMed Google
Scholar * Chao Li View author publications You can also search for this author inPubMed Google Scholar * Yankai Jia View author publications You can also search for this author inPubMed
Google Scholar * Kazutaka Ohi View author publications You can also search for this author inPubMed Google Scholar * Brady J Maher View author publications You can also search for this
author inPubMed Google Scholar * Nicholas J Brandon View author publications You can also search for this author inPubMed Google Scholar * Alan Cross View author publications You can also
search for this author inPubMed Google Scholar * Joshua G Chenoweth View author publications You can also search for this author inPubMed Google Scholar * Daniel J Hoeppner View author
publications You can also search for this author inPubMed Google Scholar * Huijun Wei View author publications You can also search for this author inPubMed Google Scholar * Thomas M Hyde
View author publications You can also search for this author inPubMed Google Scholar * Ronald McKay View author publications You can also search for this author inPubMed Google Scholar *
Joel E Kleinman View author publications You can also search for this author inPubMed Google Scholar * Daniel R Weinberger View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS M.L. and D.R.W. designed the study and interpreted the results. M.L., A.E.J., R.T., J.H.S., C.L., Y.J., K.O. and R.E.S. carried out RNA-seq analysis.
T.M.H. and J.E.K. organized and carried out subject recruitment, phenotype analysis and biological-material collection. M.L., Q.C. and A.E.J. performed genotyping and imputation. M.L.
conducted _in vitro_ functional assays, molecular cloning, cell line experiments, western blot, immunofluorescence and preparation of recombinant proteins, and M.L. and D.R.W. analyzed those
data. M.L., R.T. and C.L. performed RT-qPCR. B.J.M., D.J.H., N.J.B. and A.C. contributed to design and analysis of protein-expression experiments. M.L., Y.W., J.G.C. and R.M. performed the
iPSC experiments. H.W. carried out enzymatic assay analysis. M.L. and D.R.W. drafted the manuscript, and all authors contributed to the final version of the paper. CORRESPONDING AUTHOR
Correspondence to Daniel R Weinberger. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES
Supplementary Figures 1–15 and Supplementary Tables 1–13 (PDF 4154 kb) SUPPLEMENTARY DATASET 1 Demographic characteristics of individuals in RNA-sequencing and the paths to RNA-sequencing
.bam file and expression metrics (CSV 372 kb) SOURCE DATA SOURCE DATA TO FIG. 1 SOURCE DATA TO FIG. 2 SOURCE DATA TO FIG. 3 SOURCE DATA TO FIG. 4 SOURCE DATA TO FIG. 5 RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Li, M., Jaffe, A., Straub, R. _et al._ A human-specific _AS3MT_ isoform and _BORCS7_ are molecular risk factors in the 10q24.32
schizophrenia-associated locus. _Nat Med_ 22, 649–656 (2016). https://doi.org/10.1038/nm.4096 Download citation * Received: 08 August 2015 * Accepted: 05 April 2016 * Published: 09 May 2016
* Issue Date: June 2016 * DOI: https://doi.org/10.1038/nm.4096 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative