Biomolecular condensates: organizers of cellular biochemistry
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

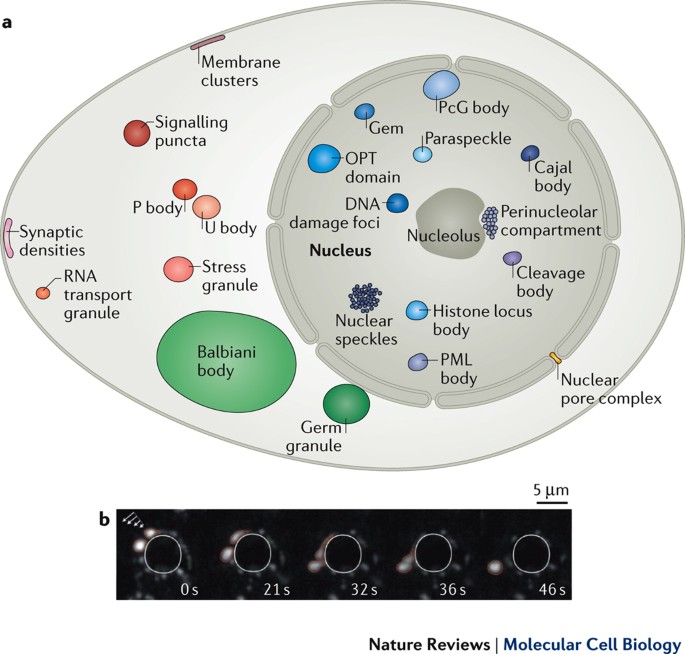
KEY POINTS * In addition to canonical membrane-bound organelles, eukaryotic cells contain numerous membraneless compartments, or biomolecular condensates, that concentrate specific
collections of proteins and nucleic acids. * Biomolecular condensates behave as phase-separated liquids and are enriched in multivalent molecules. * Theoretical concepts from polymer and
physical chemistry regarding the behaviour of multivalent molecules provide a mechanistic framework that can explain a wide range of cellular behaviours exhibited by biomolecular
condensates, including plausible mechanisms by which their assembly, composition, and biochemical and cellular functions can be regulated. ABSTRACT Biomolecular condensates are micron-scale
compartments in eukaryotic cells that lack surrounding membranes but function to concentrate proteins and nucleic acids. These condensates are involved in diverse processes, including RNA
metabolism, ribosome biogenesis, the DNA damage response and signal transduction. Recent studies have shown that liquid–liquid phase separation driven by multivalent macromolecular
interactions is an important organizing principle for biomolecular condensates. With this physical framework, it is now possible to explain how the assembly, composition, physical properties
and biochemical and cellular functions of these important structures are regulated. Access through your institution Buy or subscribe This is a preview of subscription content, access via
your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days
cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A FRAMEWORK FOR UNDERSTANDING THE FUNCTIONS OF BIOMOLECULAR CONDENSATES ACROSS SCALES Article
09 November 2020 BIOMOLECULAR CONDENSATES AS ARBITERS OF BIOCHEMICAL REACTIONS INSIDE THE NUCLEUS Article Open access 15 December 2020 RNA-MEDIATED DEMIXING TRANSITION OF LOW-DENSITY
CONDENSATES Article Open access 27 April 2023 REFERENCES * Mao, Y. S., Zhang, B. & Spector, D. L. Biogenesis and function of nuclear bodies. _Trends Genet._ 27, 295–306 (2011). Article
CAS PubMed PubMed Central Google Scholar * Decker, C. J. & Parker, R. P-Bodies and stress granules: possible roles in the control of translation and mRNA degradation. _Cold Spring
Harb. Perspect. Biol._ 4, a012286 (2012). Article PubMed PubMed Central CAS Google Scholar * Wu, H. Higher-order assemblies in a new paradigm of signal transduction. _Cell_ 153, 287–292
(2013). Article CAS PubMed PubMed Central Google Scholar * Pederson, T. The nucleolus. _Cold Spring Harb. Perspect. Biol._ 3, a000638 (2011). PubMed PubMed Central Google Scholar *
Dundr, M. et al. _In vivo_ kinetics of Cajal body components. _J. Cell Biol._ 164, 831–842 (2004). Article CAS PubMed PubMed Central Google Scholar * Phair, R. D. & Misteli, T. High
mobility of proteins in the mammalian cell nucleus. _Nature_ 404, 604–609 (2000). Article CAS PubMed Google Scholar * Weidtkamp-Peters, S. et al. Dynamics of component exchange at PML
nuclear bodies. _J. Cell Sci._ 121, 2731–2743 (2008). Article CAS PubMed Google Scholar * Platani, M., Goldberg, I., Swedlow, J. R. & Lamond, A. I. _In vivo_ analysis of Cajal body
movement, separation, and joining in live human cells. _J. Cell Biol._ 151, 1561–1574 (2000). Article CAS PubMed PubMed Central Google Scholar * Shaw, P. J. & Jordan, E. G. The
nucleolus. _Annu. Rev. Cell Dev. Biol._ 11, 93–121 (1995). Article CAS PubMed Google Scholar * Fu, L. et al. Nuclear aggresomes form by fusion of PML-associated aggregates. _Mol. Biol.
Cell_ 16, 4905–4917 (2005). Article CAS PubMed PubMed Central Google Scholar * Chen, Y.-C. M., Kappel, C., Beaudouin, J., Eils, R. & Spector, D. L. Live cell dynamics of
promyelocytic leukemia nuclear bodies upon entry into and exit from mitosis. _Mol. Biol. Cell_ 19, 3147–3162 (2008). Article CAS PubMed PubMed Central Google Scholar * Dellaire, G.,
Ching, R. W., Dehghani, H., Ren, Y. & Bazett-Jones, D. P. The number of PML nuclear bodies increases in early S phase by a fission mechanism. _J. Cell Sci._ 119, 1026–1033 (2006).
Article CAS PubMed Google Scholar * Brangwynne, C. P., Mitchison, T. J. & Hyman, A. A. Active liquid-like behavior of nucleoli determines their size and shape in _Xenopus laevis_
oocytes. _Proc. Natl Acad. Sci. USA_ 108, 4334–4339 (2011). DEMONSTRATES THAT NUCLEOLI, SIMILAR TO P GRANULES, BEHAVE AS PHASE-SEPARATED LIQUIDS, INDICATING THE GENERALITY OF THE BEHAVIOUR.
Article CAS PubMed PubMed Central Google Scholar * Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. _Science_ 324,
1729–1732 (2009). SHOWS THAT P GRANULES BEHAVE AS PHASE-SEPARATED LIQUIDS, PROVIDING A PHYSICAL MECHANISM TO EXPLAIN CONDENSATE FORMATION. Article CAS PubMed Google Scholar * Saha, S. et
al. Polar positioning of phase-separated liquid compartments in cells regulated by an mRNA competition mechanism. _Cell_ 166, 1572–1584 (2016). Article CAS PubMed PubMed Central Google
Scholar * Patel, A. et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. _Cell_ 162, 1066–1077 (2015). Article CAS PubMed Google Scholar *
Altmeyer, M. et al. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). _Nat. Commun._ 6, 8088 (2015). Article CAS PubMed Google Scholar * Hyman, A. A.
& Brangwynne, C. P. Beyond stereospecificity: liquids and mesoscale organization of cytoplasm. _Dev. Cell_ 21, 14–16 (2011). Article CAS PubMed Google Scholar * Hyman, A. A., Weber,
C. A. & Jülicher, F. Liquid–liquid phase separation in biology. _Annu. Rev. Cell Dev. Biol._ 30, 39–58 (2014). Article CAS PubMed Google Scholar * Li, P. et al. Phase transitions in
the assembly of multivalent signalling proteins. _Nature_ 483, 336–340 (2012). THE ASSEMBLY OF MULTIVALENT SIGNALLING PROTEINS CAN PROMOTE PHASE SEPARATION, THUS PROVIDING A MOLECULAR
MECHANISM TO EXPLAIN BIOMOLECULAR CONDENSATE FORMATION AND A BIOCHEMICAL ROUTE TO UNDERSTAND THE PROCESS. Article CAS PubMed PubMed Central Google Scholar * King, O. D., Gitler, A. D.
& Shorter, J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. _Brain Res._ 1462, 61–80 (2012). Article CAS PubMed PubMed Central
Google Scholar * Han, T. W. et al. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. _Cell_ 149, 768–779 (2012). DEMONSTRATES THAT
DISORDERED, LOW COMPLEXITY SEQUENCES FROM PROTEINS IN RNA GRANULES CAN FORM AMYLOID-LIKE FIBRES AND HYDROGELS, PROVIDING A CLOSELY RELATED MECHANISM TO EXPLAIN BIOMOLECULAR CONDENSATE
FORMATION. Article CAS PubMed Google Scholar * Nott, T. J. et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. _Mol. Cell_
57, 936–947 (2015). DISORDERED, LOW COMPLEXITY SEQUENCES CAN PHASE SEPARATE, EXPANDING THE RANGE OF MOLECULES SHOWING THIS BEHAVIOUR. Article CAS PubMed PubMed Central Google Scholar *
Mitrea, D. M. et al. Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. _eLife_ 5, 13571 (2016). Article
Google Scholar * Flory, P. J. _Principles of Polymer Chemistry_ (Cornell Univ. Press, 1953). Google Scholar * Banjade, S. & Rosen, M. K. Phase transitions of multivalent proteins can
promote clustering of membrane receptors. _eLife_ 3, e04123 (2014). SHOWS THAT MULTIVALENCY-DRIVEN PHASE SEPARATION CAN ALSO EXPLAIN THE CLUSTERING OF MEMBRANE RECEPTORS. Article PubMed
Central CAS Google Scholar * Su, X. et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. _Science_ 352, 595–599 (2016). DEMONSTRATES THAT PHASE
SEPARATION OF MULTIVALENT PROTEINS IN THE T CELL RECEPTOR SIGNALLING PATHWAY DRIVES THE FORMATION OF SIGNALLING CLUSTERS IN CELLS; PHASE SEPARATION ALSO PROMOTES ACTIN ASSEMBLY, PROTECTS THE
SIGNALLING MOLECULES FROM INACTIVATION BY PHOSPHATASES AND PROBABLY ACTIVATES THE INTRACELLULAR MAPK CASCADE. Article CAS PubMed PubMed Central Google Scholar * Fromm, S. A. et al. _In
vitro_ reconstitution of a cellular phase-transition process that involves the mRNA decapping machinery. _Angew. Chem. Int. Ed._ 53, 7354–7359 (2014). Article CAS Google Scholar * Zeng,
M. et al. Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. _Cell_ 166, 1163–1175.e12 (2016). Article CAS PubMed PubMed Central
Google Scholar * Banani, S. F. et al. Compositional control of phase-separated cellular bodies. _Cell_ 166, 651–663 (2016). DESCRIBES A SIMPLE MODEL FOR CONTROLLING BIOMOLECULAR
CONDENSATE COMPOSITION. Article CAS PubMed PubMed Central Google Scholar * Foo, C. T. S. W. P., Lee, J. S., Mulyasasmita, W., Parisi-Amon, A. & Heilshorn, S. C. Two-component
protein-engineered physical hydrogels for cell encapsulation. _Proc. Natl Acad. Sci. USA_ 106, 22067–22072 (2009). Article CAS Google Scholar * Mulyasasmita, W., Lee, J. S. &
Heilshorn, S. C. Molecular-level engineering of protein physical hydrogels for predictive sol–gel phase behavior. _Biomacromolecules_ 12, 3406–3411 (2011). Article CAS PubMed PubMed
Central Google Scholar * Brangwynne, C. P., Tompa, P. & Pappu, R. V. Polymer physics of intracellular phase transitions. _Nat. Phys._ 11, 899–904 (2015). Article CAS Google Scholar
* Elbaum-Garfinkle, S. et al. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. _Proc. Natl Acad. Sci. USA_ 112, 7189–7194
(2015). Article CAS PubMed PubMed Central Google Scholar * Molliex, A. et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological
fibrillization. _Cell_ 163, 123–133 (2015). Article CAS PubMed PubMed Central Google Scholar * Burke, K. A., Janke, A. M., Rhine, C. L. & Fawzi, N. L. Residue-by-residue view of _in
vitro_ FUS granules that bind the C-terminal domain of RNA polymerase II. _Mol. Cell_ 60, 231–241 (2015). Article CAS PubMed PubMed Central Google Scholar * Zhang, H. et al. RNA
controls polyQ protein phase transitions. _Mol. Cell_ 60, 220–230 (2015). Article CAS PubMed PubMed Central Google Scholar * Gilks, N. et al. Stress granule assembly is mediated by
prion-like aggregation of TIA-1. _Mol. Biol. Cell_ 15, 5383–5398 (2004). Article CAS PubMed PubMed Central Google Scholar * Decker, C. J., Teixeira, D. & Parker, R. Edc3p and a
glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in _Saccharomyces cerevisiae_. _J. Cell Biol._ 179, 437–449 (2007). Article CAS PubMed PubMed Central
Google Scholar * Reijns, M. A. M., Alexander, R. D., Spiller, M. P. & Beggs, J. D. A role for Q/N-rich aggregation-prone regions in P-body localization. _J. Cell Sci._ 121, 2463–2472
(2008). Article CAS PubMed Google Scholar * Kato, M. et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. _Cell_ 149, 753–767
(2012). Article CAS PubMed PubMed Central Google Scholar * Jiang, H. et al. Phase transition of spindle-associated protein regulate spindle apparatus assembly. _Cell_ 163, 108–122
(2015). Article CAS PubMed PubMed Central Google Scholar * Lin, Y., Protter, D. S. W., Rosen, M. K. & Parker, R. Formation and maturation of phase-separated liquid droplets by
RNA-binding proteins. _Mol. Cell_ 60, 208–219 (2015). Article CAS PubMed PubMed Central Google Scholar * Pak, C. W. et al. Sequence determinants of intracellular phase separation by
complex coacervation of a disordered protein. _Mol. Cell_ 63, 72–85 (2016). Article CAS PubMed PubMed Central Google Scholar * Crick, S. L., Jayaraman, M., Frieden, C., Wetzel, R. &
Pappu, R. V. Fluorescence correlation spectroscopy shows that monomeric polyglutamine molecules form collapsed structures in aqueous solutions. _Proc. Natl Acad. Sci. USA_ 103, 16764–16769
(2006). Article CAS PubMed PubMed Central Google Scholar * Crick, S. L., Ruff, K. M., Garai, K., Frieden, C. & Pappu, R. V. Unmasking the roles of N− and C-terminal flanking
sequences from exon 1 of huntingtin as modulators of polyglutamine aggregation. _Proc. Natl Acad. Sci. USA_ 110, 20075–20080 (2013). Article CAS PubMed PubMed Central Google Scholar *
Das, R. K. & Pappu, R. V. Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues. _Proc. Natl Acad. Sci. USA_
110, 13392–13397 (2013). Article CAS PubMed PubMed Central Google Scholar * Kwon, I. et al. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity
domains. _Cell_ 155, 1049–1060 (2013). Article CAS PubMed PubMed Central Google Scholar * Xiang, S. et al. The LC domain of hnRNPA2 adopts similar conformations in hydrogel polymers,
liquid-like droplets, and nuclei. _Cell_ 163, 829–839 (2015). REFERENCES 16, 35, 43 AND 49 DEMONSTRATE THAT INITIAL PHASE SEPARATION OF DISORDERED PROTEINS IS FOLLOWED OVER TIME BY
MATURATION OR HARDENING INTO FIBROUS SOLIDS, UNITING PHASE SEPARATION AND FIBRE FORMATION MECHANISMS OF BIOMOLECULAR CONDENSATE FORMATION UNDER A COMMON FRAMEWORK. Article CAS PubMed
PubMed Central Google Scholar * Buchan, J. R., Kolaitis, R.-M., Taylor, J. P. & Parker, R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. _Cell_ 153,
1461–1474 (2013). Article CAS PubMed PubMed Central Google Scholar * Conicella, A. E., Zerze, G.H., Mittal, J. & Fawzi, N. L. ALS mutations disrupt phase separation mediated by
α-helical structure in the TDP-43 low-complexity C-terminal domain. _Structure_ 24, 1537–1549 (2016). Article CAS PubMed PubMed Central Google Scholar * Jain, S. et al. ATPase-modulated
stress granules contain a diverse proteome and substructure. _Cell_ 164, 487–498 (2016). Article CAS PubMed PubMed Central Google Scholar * Quiroz, F. G. & Chilkoti, A. Sequence
heuristics to encode phase behaviour in intrinsically disordered protein polymers. _Nat. Mater._ 14, 1164–1171 (2015). Article CAS PubMed PubMed Central Google Scholar * Weber, S. C.
& Brangwynne, C. P. Inverse size scaling of the nucleolus by a concentration-dependent phase transition. _Curr. Biol._ 25, 641–646 (2015). Article CAS PubMed PubMed Central Google
Scholar * Shevtsov, S. P. & Dundr, M. Nucleation of nuclear bodies by RNA. _Nat. Cell Biol._ 13, 167–173 (2011). Article CAS PubMed Google Scholar * Kaiser, T. E., Intine, R. V.
& Dundr, M. _De novo_ formation of a subnuclear body. _Science_ 322, 1713–1717 (2008). Article CAS PubMed Google Scholar * Mao, Y. S., Sunwoo, H., Zhang, B. & Spector, D. L.
Direct visualization of the co-transcriptional assembly of a nuclear body by noncoding RNAs. _Nat. Cell Biol._ 13, 95–101 (2011). Article CAS PubMed Google Scholar * Chung, I.,
Leonhardt, H. & Rippe, K. _De novo_ assembly of a PML nuclear subcompartment occurs through multiple pathways and induces telomere elongation. _J. Cell Sci._ 124, 3603–3618 (2011).
Article CAS PubMed Google Scholar * Berry, J., Weber, S. C., Vaidya, N., Haataja, M. & Brangwynne, C. P. RNA transcription modulates phase transition-driven nuclear body assembly.
_Proc. Natl Acad. Sci. USA_ 112, E5237–E5245 (2015). Article CAS PubMed PubMed Central Google Scholar * Hancock, R. A role for macromolecular crowding effects in the assembly and
function of compartments in the nucleus. _J. Struct. Biol._ 146, 281–290 (2004). Article CAS PubMed Google Scholar * Dellaire, G., Eskiw, C., Dehghani, H., Ching, R. & Bazett-Jones,
D. Mitotic accumulations of PML protein contribute to the re-establishment of PML nuclear bodies in G1. _J. Cell Sci._ 119, 1034–1042 (2006). Article CAS PubMed Google Scholar * Saha, S.
et al. Polar positioning of phase-separated liquid compartments in cells regulated by an mRNA competition mechanism. _Cell_ 166, 1572–1584.e16 (2016). Article CAS PubMed PubMed Central
Google Scholar * Grousl, T. et al. Robust heat shock induces eIF2α-phosphorylation-independent assembly of stress granules containing eIF3 and 40S ribosomal subunits in budding yeast,
_Saccharomyces cerevisiae_. _J. Cell Sci._ 122, 2078–2088 (2009). Article CAS PubMed Google Scholar * Buchan, J. R. & Parker, R. Eukaryotic stress granules: the ins and outs of
translation. _Mol. Cell_ 36, 932–941 (2009). Article CAS PubMed PubMed Central Google Scholar * Hoyle, N. P., Castelli, L. M., Campbell, S. G., Holmes, L. E. A. & Ashe, M. P.
Stress-dependent relocalization of translationally primed mRNPs to cytoplasmic granules that are kinetically and spatially distinct from P-bodies. _J. Cell Biol._ 179, 65–74 (2007). Article
CAS PubMed PubMed Central Google Scholar * Louria-Hayon, I. et al. The promyelocytic leukemia protein protects p53 from Mdm2-mediated inhibition and degradation. _J. Biol. Chem._ 278,
33134–33141 (2003). Article CAS PubMed Google Scholar * Ishov, A. M. et al. PML is critical for ND10 formation and recruits the PML-interacting protein Daxx to this nuclear structure
when modified by SUMO-1. _J. Cell Biol._ 147, 221–234 (1999). Article CAS PubMed PubMed Central Google Scholar * Hamill, D. R., Severson, A. F., Carter, J. C. & Bowerman, B.
Centrosome maturation and mitotic spindle assembly in _C. elegans_ require SPD-5, a protein with multiple coiled-coil domains. _Dev. Cell_ 3, 673–684 (2002). Article CAS PubMed Google
Scholar * Kedersha, N. L., Gupta, M., Li, W., Miller, I. & Anderson, P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2α to the assembly of mammalian stress
granules. _J. Cell Biol._ 147, 1431–1442 (1999). Article CAS PubMed PubMed Central Google Scholar * Kroschwald, S. et al. Promiscuous interactions and protein disaggregases determine
the material state of stress-inducible RNP granules. _eLife_ 4, e06807 (2015). Article PubMed PubMed Central Google Scholar * Feric, M. et al. Coexisting liquid phases underlie nucleolar
subcompartments. _Cell_ 165, 1686–1697 (2016). DEMONSTRATES THE RECONSTITUTION OF MULTILAYERED PHASE-SEPARATED LIQUID STRUCTURES FROM SIMPLE MIXTURES OF RECOMBINANT PROTEINS, SHOWING THAT
SUCH COMPLEXITY CAN BE ACHIEVED IN A SIMPLE FASHION. Article CAS PubMed PubMed Central Google Scholar * Boke, E. et al. Amyloid-like self-assembly of a cellular compartment. _Cell_ 166,
637–650 (2016). Article CAS PubMed PubMed Central Google Scholar * Fändrich, M., Fletcher, M. A. & Dobson, C. M. Amyloid fibrils from muscle myoglobin. _Nature_ 410, 165–166
(2001). Article PubMed Google Scholar * Vitalis, A., Wang, X. & Pappu, R. V. Quantitative characterization of intrinsic disorder in polyglutamine: insights from analysis based on
polymer theories. _Biophys. J._ 93, 1923–1937 (2007). Article CAS PubMed PubMed Central Google Scholar * Halfmann, R. A glass menagerie of low complexity sequences. _Curr. Opin. Struc
Biol._ 38, 9–16 (2016). Article CAS Google Scholar * Watanabe, H. Viscoelasticity and dynamics of entangled polymers. _Prog. Polym. Sci._ 24, 1253–1403 (1999). Article CAS Google
Scholar * Ramaswami, M., Taylor, J. P. & Parker, R. Altered ribostasis: RNA–protein granules in degenerative disorders. _Cell_ 154, 727–736 (2013). Article CAS PubMed Google Scholar
* Li, Y. R., King, O. D., Shorter, J. & Gitler, A. D. Stress granules as crucibles of ALS pathogenesis. _J. Cell Biol._ 201, 361–372 (2013). Article CAS PubMed PubMed Central
Google Scholar * Weber, S. C. & Brangwynne, C. P. Getting RNA and protein in phase. _Cell_ 149, 1188–1191 (2012). Article CAS PubMed Google Scholar * Wolozin, B. Physiological
protein aggregation run amuck: stress granules and the genesis of neurodegenerative disease. _Discov. Med._ 17, 47–52 (2014). PubMed PubMed Central Google Scholar * Aguzzi, A. &
Altmeyer, M. Phase separation: linking cellular compartmentalization to disease. _Trends Cell Biol._ 26, 547–558 (2016). Article CAS PubMed Google Scholar * Alberti, S. & Hyman, A.
A. Are aberrant phase transitions a driver of cellular aging? _BioEssays_ 38, 959–968 (2016). Article CAS PubMed PubMed Central Google Scholar * Oakes, C. C., La Salle, S., Smiraglia,
D. J., Robaire, B. & Trasler, J. M. A unique configuration of genome-wide DNA methylation patterns in the testis. _Proc. Natl Acad. Sci. USA_ 104, 228–233 (2007). Article CAS PubMed
Google Scholar * Feric, M. & Brangwynne, C. P. A nuclear F-actin scaffold stabilizes ribonucleoprotein droplets against gravity in large cells. _Nat. Cell Biol._ 15, 1253–1259 (2013).
Article CAS PubMed PubMed Central Google Scholar * Kaizuka, Y., Douglass, A. D., Varma, R., Dustin, M. L. & Vale, R. D. Mechanisms for segregating T cell receptor and adhesion
molecules during immunological synapse formation in Jurkat T cells. _Proc. Natl Acad. Sci. USA_ 104, 20296–20301 (2007). Article CAS PubMed PubMed Central Google Scholar * Yi, J., Wu,
X. S., Crites, T. & Hammer, J. A. Actin retrograde flow and actomyosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T cells. _Mol. Biol.
Cell_ 23, 834–852 (2012). Article CAS PubMed PubMed Central Google Scholar * Lee, C. F., Brangwynne, C. P., Gharakhani, J., Hyman, A. A. & Jülicher, F. Spatial organization of the
cell cytoplasm by position-dependent phase separation. _Phys. Rev. Lett._ 111, 088101 (2013). Article PubMed CAS Google Scholar * Zwicker, D., Hyman, A. A. & Jülicher, F. Suppression
of Ostwald ripening in active emulsions. _Phys. Rev. E_ 92, 012317 (2015). Article CAS Google Scholar * Hagan, M. F. & Baskaran, A. Emergent self-organization in active materials.
_Curr. Opin. Cell Biol._ 38, 74–80 (2016). Article CAS PubMed Google Scholar * Popkin, G. The physics of life. _Nature_ 529, 16–18 (2016). Article CAS PubMed Google Scholar *
Sanchez, T., Chen, D. T. N., DeCamp, S. J., Heymann, M. & Dogic, Z. Spontaneous motion in hierarchically assembled active matter. _Nature_ 491, 431–434 (2012). Article CAS PubMed
PubMed Central Google Scholar * Wang, J. T. et al. Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in _C. elegans_. _eLife_ 3,
e04591 (2014). Article PubMed PubMed Central Google Scholar * Lang, M. et al. Three-dimensional organization of promyelocytic leukemia nuclear bodies. _J. Cell Sci._ 123, 392–400 (2010).
Article CAS PubMed Google Scholar * Boisvert, F.-M., van Koningsbruggen, S., Navascués, J. & Lamond, A. I. The multifunctional nucleolus. _Nat. Rev. Mol. Cell Biol._ 8, 574–585
(2007). Article CAS PubMed Google Scholar * Monneron, A. & Bernhard, W. Fine structural organization of the interphase nucleus in some mammalian cells. _J. Ultrastruct. Res._ 27,
266–288 (1969). Article CAS PubMed Google Scholar * Hyman, A. A. & Simons, K. Cell biology. Beyond oil and water — phase transitions in cells. _Science_ 337, 1047–1049 (2012).
Article CAS PubMed Google Scholar * Tatomer, D. C. et al. Concentrating pre-mRNA processing factors in the histone locus body facilitates efficient histone mRNA biogenesis. _J. Cell
Biol._ 213, 557–570 (2016). Article CAS PubMed PubMed Central Google Scholar * Strzelecka, M. et al. Coilin-dependent snRNP assembly is essential for zebrafish embryogenesis. _Nat.
Struct. Mol. Biol._ 17, 403–409 (2010). Article CAS PubMed Google Scholar * Novotny, I., Blazikova, M., Stanek, D., Herman, P. & Malinsky, J. _In vivo_ kinetics of U4/U6·U5 tri-snRNP
formation in Cajal bodies. _Mol. Biol. Cell_ 22, 513–523 (2011). Article CAS PubMed PubMed Central Google Scholar * Strulson, C. A., Molden, R. C., Keating, C. D. & Bevilacqua, P.
C. RNA catalysis through compartmentalization. _Nat. Chem._ 4, 941–946 (2012). Article CAS PubMed Google Scholar * Deryusheva, S. & Gall, J. G. Small Cajal body-specific RNAs of
_Drosophila_ function in the absence of Cajal bodies. _Mol. Biol. Cell_ 20, 5250–5259 (2009). Article CAS PubMed PubMed Central Google Scholar * Davis, B. W. et al. Colocalization and
sequential enzyme activity in aqueous biphasic systems: experiments and modeling. _Biophys. J._ 109, 2182–2194 (2015). Article CAS PubMed PubMed Central Google Scholar * Kuznetsova, I.
M., Zaslavsky, B. Y., Breydo, L., Turoverov, K. K. & Uversky, V. N. Beyond the excluded volume effects: mechanistic complexity of the crowded milieu. _Molecules_ 20, 1377–1409 (2015).
Article PubMed PubMed Central CAS Google Scholar * Cai, L.-H., Panyukov, S. & Rubinstein, M. Mobility of nonsticky nanoparticles in polymer liquids. _Macromolecules_ 44, 7853–7863
(2011). Article CAS PubMed PubMed Central Google Scholar * Elbaum-Garfinkle, S. & Brangwynne, C. P. Liquids, fibers, and gels: the many phases of neurodegeneration. _Dev. Cell_ 35,
531–532 (2015). Article CAS PubMed Google Scholar * Good, M. C., Zalatan, J. G. & Lim, W. A. Scaffold proteins: hubs for controlling the flow of cellular information. _Science_ 332,
680–686 (2011). Article CAS PubMed PubMed Central Google Scholar * Castellana, M. et al. Enzyme clustering accelerates processing of intermediates through metabolic channeling. _Nat.
Biotechnol._ 32, 1011–1018 (2014). Article CAS PubMed PubMed Central Google Scholar * O'Connell, J. D., Zhao, A., Ellington, A. D. & Marcotte, E. M. Dynamic reorganization of
metabolic enzymes into intracellular bodies. _Annu. Rev. Cell Dev. Biol._ 28, 89–111 (2012). Article CAS PubMed PubMed Central Google Scholar * Noree, C., Monfort, E., Shiau, A. K.
& Wilhelm, J. E. Common regulatory control of CTP synthase enzyme activity and filament formation. _Mol. Biol. Cell_ 25, 2282–2290 (2014). Article PubMed PubMed Central Google Scholar
* Li, H. et al. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. _Mol. Cell. Biol._ 20, 1784–1796 (2000). Article CAS PubMed PubMed Central Google
Scholar * Eldar, A. & Elowitz, M. B. Functional roles for noise in genetic circuits. _Nature_ 467, 167–173 (2010). Article CAS PubMed PubMed Central Google Scholar * Kshirsagar, M.
& Parker, R. Identification of Edc3p as an enhancer of mRNA decapping in _Saccharomyces cerevisiae_. _Genetics_ 166, 729–739 (2004). Article CAS PubMed PubMed Central Google Scholar
* Dunckley, T. & Parker, R. The DCP2 protein is required for mRNA decapping in _Saccharomyces cerevisiae_ and contains a functional MutT motif. _EMBO J._ 18, 5411–5422 (1999). Article
CAS PubMed PubMed Central Google Scholar * Bernardi, R. & Pandolfi, P. P. Role of PML and the PML-nuclear body in the control of programmed cell death. _Oncogene_ 22, 9048–9057
(2003). Article CAS PubMed Google Scholar * Nakagawa, S., Naganuma, T., Shioi, G. & Hirose, T. Paraspeckles are subpopulation-specific nuclear bodies that are not essential in mice.
_J. Cell Biol._ 193, 31–39 (2011). Article CAS PubMed PubMed Central Google Scholar * Dill, K. A. & Bromberg, S. _Molecular Driving Forces_. (Garland Science, 2011). Google Scholar
* Flory, P. J. Thermodynamics of high polymer solutions. _J. Chem. Phys._ 10, 51 (1942). Article CAS Google Scholar * Griffin, E. E., Odde, D. J. & Seydoux, G. Regulation of the
MEX-5 gradient by a spatially segregated kinase/phosphatase cycle. _Cell_ 146, 955–968 (2011). Article CAS PubMed PubMed Central Google Scholar * Stockmayer, W. Molecular distribution
in condensation polymers. _J. Polymer Sci._ 9, 69–71 (1952). Article CAS Google Scholar * Cohen, R. & Benedek, G. Equilibrium and kinetic theory of polymerization and the sol–gel
transition. _J. Phys. Chem._ 86, 3696–3714 (1982). Article CAS Google Scholar * Huggins, M. L. Solutions of long chain compounds. _J. Chem. Phys._ 9, 440 (1941). Article CAS Google
Scholar * Semenov, A. & Rubinstein, M. Thermoreversible gelation in solutions of associative polymers. 1. Statics. _Macromolecules_ 31, 1373–1385 (1998). Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS The authors thank R. Duronio and C. Weber for discussion and critical comments on the Review. Research on multivalency-driven phase separation is
supported in the Hyman laboratory by the Max Planck Society, and in the Rosen laboratory by the Howard Hughes Medical Institute, the Welch Foundation (I-1544) and a Sara and Frank McKnight
Graduate Fellowship (to S.F.B.). AUTHOR INFORMATION Author notes * Salman F. Banani and Hyun O. Lee: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of
Biophysics and Howard Hughes Medical Institute, University of Texas Southwestern Medical Center, Dallas, 75390, Texas, USA Salman F. Banani & Michael K. Rosen * Max Planck Institute of
Molecular Cell Biology and Genetics, 01307, Dresden, Germany Hyun O. Lee & Anthony A. Hyman Authors * Salman F. Banani View author publications You can also search for this author
inPubMed Google Scholar * Hyun O. Lee View author publications You can also search for this author inPubMed Google Scholar * Anthony A. Hyman View author publications You can also search for
this author inPubMed Google Scholar * Michael K. Rosen View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Anthony
A. Hyman or Michael K. Rosen. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION DRIPPING OF P GRANULES. Movie shows
syncytial germ cell nuclei covered in P granules in the germ line of a GFP::PGL-1 worm. The germ line has been dissected and squashed. P granules appear to drip off of the nuclei, fuse, and
round up. From Brangwynne, C. P. _et al_. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. _Science_ 324, 1729–1732 (2009). Reprinted with
permission from AAAS. (MOV 259 kb) DYNAMICS OF FUS BODIES. Timelapse imaging of stress granules in a live HeLa cell expressing FUS-GFP using high-resolution lightsheet microscopy. Movie
courtesy of H. O. Lee and M. Weigert, MPI-CBG, Dresden, Germany. (MOV 28339 kb) FUSION OF STRESS GRANULES. Expanded and rendered movie of the same cell in Supplemental movie 2, showing
fusion of two stress granules visualized through FUS-GFP. Movie courtesy of H. O. Lee and M. Weigert, MPI-CBG, Dresden, Germany. (AVI 60 kb) FORMATION AND MERGING OF PNEPHRIN CLUSTERS. Alexa
488-labeled His8-pNephrin was attached to a DOPC supported lipid bilayer doped (1%) with Ni2+-NTA lipids, and Nck and N-WASP were added. Movie shows TIRF images acquired every minute.
Initial clusters are small and numerous, but merge over time to make larger structures. Reproduced from Banjade, S. & Rosen, M. K. Phase transitions of multivalent proteins can promote
clustering of membrane receptors. _eLife_ 3, e04123 (2014). (AVI 553 kb) SUPPLEMENTARY INFORMATION S5 (BOX) How are condensed phases different from macromolecular complexes? (PDF 147 kb)
SUPPLEMENTARY INFORMATION S6 (TABLE) Various biomolecular condensates and their functions (PDF 156 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT
SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 GLOSSARY * Cajal bodies Biomolecular condensates in eukaryotic nuclei containing coilin and survival motor neuron
protein (SMN) as well as many factors involved in mRNA splicing. Cajal bodies are thought to have a role in assembling spliceosomal small nuclear ribonucleoproteins. * PML nuclear bodies
Biomolecular condensates in eukaryotic nuclei containing promyelocytic leukaemia (PML), death domain-associated protein (DAXX) and Sp100. PML nuclear bodies are thought to have a role in
apoptotic signalling, antiviral defence and transcriptional regulation. * Entropy A measure of disorder in a given system. Specifically, the number of microstates possible for a given state.
Systems tend to approach states that maximize their entropy. * Free energy The energy available in a thermodynamic system to work. Systems tend to approach states that minimize their free
energy. * Stereospecificity A property of binding reactions whereby the specificity is largely dictated by the complementary geometries of the ligand and receptor molecules. * WW domains
Small (∼5 kDa) modular signalling domains found in numerous proteins that contain two conserved tryptophan residues. WW domains bind to proline-containing peptide motifs. * Cation–pi
interactions Noncovalent interactions between positively charged residues (for example, lysine) and pi electrons in aromatic residues (for example, phenylalanine). * Pi-stacking interactions
Attractive interactions between aromatic rings, such as those found in phenylalanine, tyrosine and tryptophan residues. * Dipolar interactions Interactions between two molecules that are
electrically polarized, wherein the partial positive charge on one interacts with the partial negative charge on the other. * Chemical footprinting Use of a small reactive chemical to modify
solvent-exposed sites in a macromolecule, providing information on the structure of that macromolecule. * Chemical potential The partial molar free energy within a system. Mathematically,
the first derivative of free energy with respect to composition. Systems tend to approach states that dissipate gradients in chemical potential. * Histone locus bodies Biomolecular
condensates in eukaryotic nuclei containing nuclear protein, ataxia-telangiectasia locus (NPAT) and FLICE-associated huge protein (FLASH), and thought to be involved in the processing of
histone mRNAs. * Nuage Biomolecular condensates in metazoan germ cells thought to have a role in maintaining germ cell genomic integrity. This class of compartments includes P granules,
polar granules and mammalian nuages. * Paraspeckles Biomolecular condensates in the mammalian nucleus that contain the long non-coding RNA nuclear paraspeckle assembly transcript 1 (NEAT1)
and a variety of RNA-binding and other proteins. The functions of paraspeckles are not well understood, but include storage of certain RNAs. * Balbiani bodies A transient collection of
proteins, RNA and membrane-bound organelles (endoplasmic reticulum, Golgi and mitochondria) found in primary oocytes of all animals observed to date (flies, frogs, mice and humans). * Small
nuclear ribonucleoprotein A RNA–protein complex that is the primary constituent of spliceosomes, the eukaryotic splicing machinery. * Hammerhead ribozyme A catalytic RNA molecule involved in
RNA cleavage found in organisms ranging from bacteria to mammals. * Partition coefficients Measures the enrichment of chemical species into the condensed phase of a two-phase system.
Mathematically, the partition coefficient is defined as the ratio of concentration of the species in the condensed phase to that in the dilute phase. RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Banani, S., Lee, H., Hyman, A. _et al._ Biomolecular condensates: organizers of cellular biochemistry. _Nat Rev Mol Cell Biol_ 18, 285–298
(2017). https://doi.org/10.1038/nrm.2017.7 Download citation * Published: 22 February 2017 * Issue Date: May 2017 * DOI: https://doi.org/10.1038/nrm.2017.7 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative