Not just amyloid: physiological functions of the amyloid precursor protein family
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Amyloid precursor protein (APP) and the APP-like proteins APLP1 and APLP2 form the mammalian APP gene family. They have important physiological functions in the peripheral and central
nervous systems, some of which are still emerging.
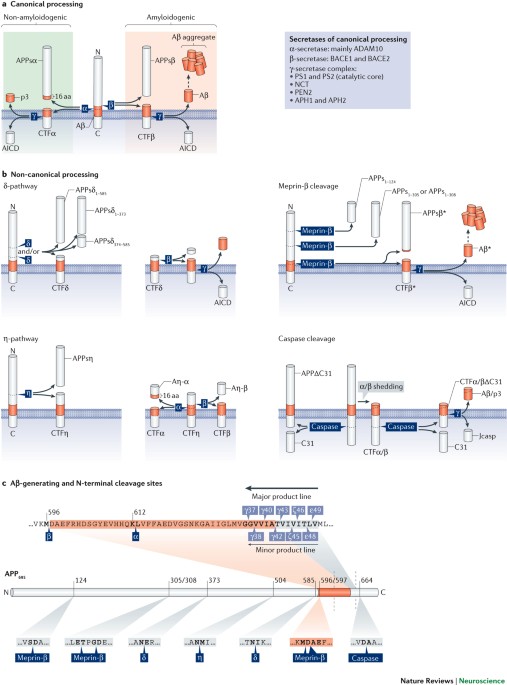
APP family members share a similar structure and have partially overlapping functions. Their processing by canonical and non-canonical secretases results in numerous biologically active
fragments, which mediate distinct and even opposing functions.
Membrane-bound APP family members interact in cis or in trans, which enables them to function as cell-adhesion molecules. Large numbers of extracellular and intracellular binding partners
have been identified, and this Review summarizes those that are involved in physiological pathways in vivo.
Biological functions in which APP family members are involved include nervous system development, the formation and function of the neuromuscular junction, synaptogenesis, dendritic
complexity and spine density, axonal growth and guidance, and synaptic functions, including synaptic plasticity, learning and memory.
α-Secretase cleavage of APP releases the neuroprotective and neurotrophic fragment APPsα. It upregulates protective pathways, inhibits neuronal apoptosis, increases neuronal resistance to
brain injuries and has a crucial role in synaptic plasticity, learning and memory.
Increasing APPsα levels may be of therapeutic value. Pharmacotherapeutic and gene-therapeutic approaches could complement amyloid-targeting strategies.
Amyloid precursor protein (APP) gives rise to the amyloid-β peptide and thus has a key role in the pathogenesis of Alzheimer disease. By contrast, the physiological functions of APP and the
closely related APP-like proteins (APLPs) remain less well understood. Studying these physiological functions has been challenging and has required a careful long-term strategy, including
the analysis of different App-knockout and Aplp-knockout mice. In this Review, we summarize these findings, focusing on the in vivo roles of APP family members and their processing products
for CNS development, synapse formation and function, brain injury and neuroprotection, as well as ageing. In addition, we discuss the implications of APP physiology for therapeutic
approaches.
The authors thank C. Bold, A. Mehr and M. Richter for help with figure preparation. The authors' work is supported by the Deutsche Forschungsgemeinschaft (FOR1332 on “Physiological functions
of the APP gene family”) and the Alzheimer Research Prize of the Breuer Foundation to U.C.M.
Thomas Deller and Martin Korte: These authors contributed equally to this work.
Ruprecht-Karls University Heidelberg, Institute of Pharmacy and Molecular Biotechnology, Bioinformatics and Functional Genomics, Im Neuenheimer Feld 364, D-69120, Heidelberg, Germany
Goethe University Frankfurt, Institute of Clinical Neuroanatomy, Neuroscience Center, Theodor-Stern-Kai 7, D-60590, Frankfurt am Main, Germany
TU Braunschweig, Zoological Institute, Cellular Neurobiology, Spielmannstrasse 7, D-38106, Raunschweig, Germany
Helmholtz Centre for Infection Research, Neuroinflammation and Neurodegeneration Group, Inhoffenstrasse 7, 38124, Braunschweig, Germany
T.D. and M.K. declare no competing interests. U.C.M. is listed as a co-inventor on a patent application aiming to use APPsα for Alzheimer disease therapy.
A presynaptic glycoprotein located in the membrane of synaptic vesicles that is regularly used as a marker for synapses.
A form of early endosomes responsible for recycling membrane proteins, including receptors, back to the plasma membrane. They connect the endocytic pathway to the exocytic pathway.
Cleavage of an internal peptide bond in a polypeptide or protein.
Cleavage of peptide bonds from the end of a polypeptide chain.
Presynaptic specializations without a neuronal postsynaptic site. They contain presynaptic marker proteins, including proteins of the active zone and synaptic vesicles.
A form of regulated, necrotic cell death that involves death receptors and the activation of receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and RIPK3.
The last plate formed in the anlage of the mammalian cortex; it matures into cortical layers II–VI.
Zone arising from the cortical preplate during corticogenesis. It contains Cajal–Retzius cells, which regulate radial neuronal migration, and it matures into cortical layer I.
Cellular protrusions formed during cell migration at the leading edge of motile cells or motile cell processes. They contain microfilaments that pull the cell forwards.
Transgenic Alzheimer disease (AD) model that exhibits plaque pathology; the mice overexpress amyloid precursor protein (APP) with a humanized amyloid-β region with the human Swedish double
mutation and the AD-associated ΔE9 variant of presenilin 1.
(PPF). A form of short-term synaptic plasticity in which a second excitatory postsynaptic potential is increased when that stimulus closely follows a prior stimulation.
Amyloid precursor protein (APP) fragment generated by combined cleavage of η-secretase and α-secretase.
Resulting from the overactivation of glutamate receptors, and potentially toxic to certain neurons.
The smallest domains or polypeptide stretches that exhibit the same functional properties as larger proteins.
Anyone you share the following link with will be able to read this content: