Neurotrophins and their receptors: a convergence point for many signalling pathways
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

KEY POINTS * Neurotrophins are most often associated with the promotion of neuronal growth and survival, but their influence on brain function is significantly broader — they are also
involved in plastic and pathological processes. * Clues to the multiple functions of neurotrophins come from the study of mutant animals. In particular, as knocking out any neurotrophin gene
leads to a lethal phenotype, the analysis of heterozygous mice has pointed to roles for the neurotrophins in locomotor and feeding behaviours. * The fact that the actions of the
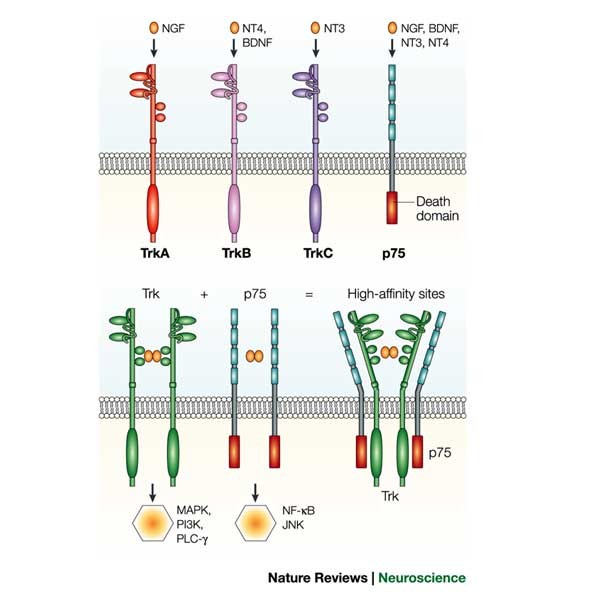
neurotrophins depend on two receptor classes — the Trk receptors and p75 — significantly increases the degrees of freedom for neurotrophin signalling in terms of specificity, affinity and
downstream signalling pathways. * Neurotrophins have significant direct effects on synaptic transmission, plasticity and their possible behavioural correlates. However, the downstream
mechanisms that mediate these effects are not completely understood. Several signalling pathways have been put forward as candidates, and recently ion channels have joined the list of
potential effectors of the synaptic actions of neurotrophins. * Transactivation of neurotrophin receptors by G protein-coupled receptors has emerged as a new theme in the biology of
neurotrophin function. Although the precise role of this transactivation is unknown, one possibility is that it adds a safety factor that might protect neurons from death in the absence of
neurotrophins. * Neurotrophin receptors, particularly p75, might have an important role in the control of axonal regeneration, as they act as co-receptors for Nogo, a protein that is known
to inhibit axonal growth. In addition, the neurotrophins can modulate the response of growth cones to guidance molecules such as semaphorins. * There is some genetic evidence that points to
a specific contribution of the neurotrophins to psychiatric disease. Specifically, polymorphisms of brain-derived neurotrophic factor have been linked to depression, bipolar disorders and
schizophrenia. ABSTRACT The neurotrophins are a family of proteins that are essential for the development of the vertebrate nervous system. Each neurotrophin can signal through two different
types of cell surface receptor — the Trk receptor tyrosine kinases and the p75 neurotrophin receptor. Given the wide range of activities that are now associated with neurotrophins, it is
probable that additional regulatory events and signalling systems are involved. Here, I review recent findings that neurotrophins, in addition to promoting survival and differentiation,
exert various effects through surprising interactions with other receptors and ion channels. Access through your institution Buy or subscribe This is a preview of subscription content,
access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $189.00 per year only $15.75 per issue Learn
more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS
OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MASTER REGULATORS OF NEUROGENESIS: THE DYNAMIC
ROLES OF EPHRIN RECEPTORS ACROSS DIVERSE CELLULAR NICHES Article Open access 06 November 2024 EVOLUTION OF CENTRAL NEURAL CIRCUITS: STATE OF THE ART AND PERSPECTIVES Article 26 October 2022
NEUREXINS: MOLECULAR CODES FOR SHAPING NEURONAL SYNAPSES Article 08 January 2021 REFERENCES * McAllister, A., Katz, L. & Lo, D. Neurotrophins and synaptic plasticity. _Annu. Rev.
Neurosci._ 22, 295–318 (1999). CAS PubMed Google Scholar * Poo, M. -M. Neurotrophins as synaptic modulators. _Nature Rev. Neurosci._ 2, 24–31 (2001). CAS Google Scholar * Huang, E.
& Reichardt, L. Neurotrophins: roles in neuronal development and function. _Annu. Rev. Neurosci._ 24, 677–736 (2001). CAS PubMed PubMed Central Google Scholar * Chao, M. V. &
Hempstead, B. L. p75 and trk: a two-receptor system. _Trends Neurosci._ 18, 321–326 (1995). CAS PubMed Google Scholar * Kaplan, D. R. & Miller, F. D. Neurotrophin signal transduction
in the nervous system. _Curr. Opin. Neurobiol._ 10, 381–391 (2000). CAS PubMed Google Scholar * Dechant, G. & Barde, Y. -A. The neurotrophin receptor p75NTR: novel functions and
implications for diseases of the nervous system. _Nature Neurosci._ 5, 1131–1136 (2002). CAS PubMed Google Scholar * Gall, C. & Isackson, P. Limbic seizures increase neuronal
production of messenger RNA for nerve growth factor. _Science_ 245, 758–761 (1989). CAS PubMed Google Scholar * Blochl, A. & Thoenen, H. Characterization of nerve growth factor
release from hippocampal neurons: evidence for a constitutive and an unconventional sodium-dependent regulated pathway. _Eur. J. Neurosci._ 7, 1220–1228 (1995). CAS PubMed Google Scholar
* Wang, X. H. & Poo, M. -M. Potentiation of developing synapses by postsynaptic release of NT-4. _Neuron_ 19, 825–835 (1997). CAS PubMed Google Scholar * Gall, C. Regulation of brain
neurotrophin expression by physiological activity. _Trends Pharmacol. Sci._ 13, 401–403 (1992). CAS PubMed Google Scholar * Schoups, A., Elliott, R., Friedman, W. & Black, I. NGF and
BDNF are differentially modulated by visual experience in the developing geniculocortical pathway. _Brain Res. Dev._ 86, 326–334 (1995). CAS Google Scholar * Lein, E., Hohn, A. &
Shatz, C. Dynamic regulation of BDNF and NT-3 expression during visual system development. _J. Comp. Neurol._ 420, 1–18 (2000). CAS PubMed Google Scholar * Kohara, K., Kitamura, A.,
Morishima, M. & Tsumoto, T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. _Science_ 291, 2419–2423 (2001). CAS PubMed Google Scholar *
Balkowiec, A. & Katz, D. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. _J. Neurosci._ 22, 10399–10407
(2002). CAS PubMed PubMed Central Google Scholar * Chen, K. et al. Disruption of a single allele of the nerve growth factor gene results in atrophy of basal forebrain cholinergic neurons
and memory deficits. _J. Neurosci._ 17, 7288–7296 (1997). CAS PubMed PubMed Central Google Scholar * Lyons, W. E. et al. Brain-derived neurotrophic factor-deficient mice develop
aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. _Proc. Natl Acad. Sci. USA_ 96, 15239–15244 (1999). CAS PubMed PubMed Central Google Scholar *
Kernie, S., Liebl, D. & Parada, L. BDNF regulates eating behavior and locomotor activity in mice. _EMBO J._ 19, 1290–1300 (2000). CAS PubMed PubMed Central Google Scholar * Rios, M.
et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. _Mol. Endocrinol._ 15, 1748–1757 (2001). PROFOUND EFFECTS ON
FEEDING AND AGGRESSIVE BEHAVIOURS HAVE BEEN OBSERVED IN THREE DIFFERENT LINES OF MICE WITH REDUCED LEVELS OF BDNF. THESE RESULTS INDICATE THAT A PARTIAL DEPLETION OF BDNF CAN HAVE A KEY ROLE
IN REGULATING BEHAVIOURAL RESPONSES, IN THIS CASE, THROUGH SEROTONERGIC ABNORMALITIES. CAS PubMed Google Scholar * Korte, M. et al. Hippocampal long-term potentiation is impaired in mice
lacking brain-derived neurotrophic factor. _Proc. Natl Acad. Sci. USA_ 92, 8856–8860 (1995). CAS PubMed PubMed Central Google Scholar * Patterson, S. et al. Recombinant BDNF rescues
deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. _Neuron_ 16, 1137–1145 (1996). CAS PubMed Google Scholar * Heymach, J. V. & Shooter, E. M. The
biosynthesis of neurotrophin heterodimers by transfected mammalian cells. _J. Biol. Chem._ 270, 12297–12304 (1995). CAS PubMed Google Scholar * Mowla, S. J. et al. Biosynthesis and
post-translational processing of the precursor to brain-derived neurotrophic factor. _J. Biol. Chem._ 276, 12660–12666 (2001). CAS PubMed Google Scholar * Dechant, G. et al. Expression
and binding characteristics of the BDNF receptor chick trkB. _Development_ 119, 545–558 (1993). CAS PubMed Google Scholar * Mahadeo, D., Kaplan, L., Chao, M. V. & Hempstead, B. L.
High affinity nerve growth factor binding displays a faster rate of association than p140(trk) binding — implications for multisubunit polypeptide receptors. _J. Biol. Chem._ 269, 6884–6891
(1994). CAS PubMed Google Scholar * Schropel, A., von Schack, D., Dechant, G. & Barde, Y. -A. Early expression of the nerve growth factor receptor ctrkA in chick sympathetic and
sensory ganglia. _Mol. Cell. Neurosci._ 6, 544–556 (1995). CAS PubMed Google Scholar * Arevalo, J. et al. TrkA immunoglobulin-like ligand binding domains inhibit spontaneous activation of
the receptor. _Mol. Cell Biol._ 20, 5908–5916 (2000). CAS PubMed PubMed Central Google Scholar * Esposito, D. et al. The cytoplasmic and transmembrane domains of the p75 and TrkA
receptors regulate high affinity binding to nerve growth factor. _J. Biol. Chem._ 276, 32687–32695 (2001). CAS PubMed Google Scholar * Hempstead, B. L., Martin-Zanca, D., Kaplan, D. R.,
Parada, L. F. & Chao, M. V. High-affinity NGF binding requires co-expression of the trk proto-oncogene and the low-affinity NGF receptor. _Nature_ 350, 678–683 (1991). CAS PubMed
Google Scholar * Benedetti, M., Levi, A. & Chao, M. V. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. _Proc.
Natl Acad. Sci. USA_ 90, 7859–7863 (1993). CAS PubMed PubMed Central Google Scholar * Bibel, M., Hoppe, E. & Barde, Y. Biochemical and functional interactions between the
neurotrophin receptors trk and p75NTR. _EMBO J._ 18, 616–622 (1999). CAS PubMed PubMed Central Google Scholar * Baloh, R., Enomoto, H., Johnson, E. & Milbrandt, J. The GDNF family
ligands and receptors — implications for neural development. _Curr. Opin. Neurobiol._ 10, 103–110 (2000). CAS PubMed Google Scholar * Ginty, D. & Segal, R. Retrograde neurotrophin
signaling: Trk-ing along the axon. _Curr. Opin. Neurobiol._ 12, 268–274 (2002). CAS PubMed Google Scholar * Grimes, M., Beattie, E. & Mobley, W. A signaling organelle containing the
nerve growth factor-activated receptor tyrosine kinase, TrkA. _Proc. Natl Acad. Sci. USA_ 94, 9909–9914 (1997). CAS PubMed PubMed Central Google Scholar * Patapoutian, A. &
Reichardt, L. Trk receptors: mediators of neurotrophin action. _Curr. Opin. Neurobiol._ 11, 272–280 (2001). CAS PubMed Google Scholar * Hempstead, B. The many faces of p75NTR. _Curr.
Opin. Neurobiol._ 12, 260–267 (2002). CAS PubMed Google Scholar * Roux, P. & Barker, P. Neurotrophin signaling through the p75 neurotrophin receptor. _Prog. Neurobiol._ 67, 203–233
(2002). CAS PubMed Google Scholar * Lonze, B. & Ginty, D. Function and regulation of CREB family transcription factors in the nervous system. _Neuron_ 35, 605–623 (2002). CAS PubMed
Google Scholar * York, R. et al. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. _Nature_ 392, 622–626 (1998). CAS PubMed Google Scholar * Majdan, M.
& Miller, F. Neuronal life and death decisions: functional antagonism between the Trk and p75 neurotrophin receptors. _Int. J. Dev. Neurosci._ 17, 153–161 (1999). CAS PubMed Google
Scholar * Dowling, P. et al. Upregulated p75NTR neurotrophin receptor on glial cells in MS plaques. _Neurology_ 53, 1676–1682 (1999). CAS PubMed Google Scholar * Roux, P., Colicos, M.,
Barker, P. & Kennedy, T. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. _J. Neurosci._ 19, 6887–6896 (1999). CAS PubMed PubMed Central Google
Scholar * Beattie, M. et al. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. _Neuron_ 36, 375–386 (2002). CAS PubMed PubMed Central Google Scholar *
Harrington, A. W., Kim, J. Y. & Yoon, S. O. Activation of Rac GTPase by p75 is necessary for c-_jun_ N-terminal kinase-mediated apoptosis. _J. Neurosci._ 22, 156–166 (2002). CAS PubMed
PubMed Central Google Scholar * Khursigara, G. et al. A pro-survival function for the p75 receptor death domain mediated via the caspase recruitment domain receptor interacting protein
2. _J. Neurosci._ 21, 5854–5863 (2001). CAS PubMed PubMed Central Google Scholar * DeFreitas, M., McQuillen, P. & Shatz, C. A novel p75NTR signaling pathway promotes survival, not
death, of immunopurified neocortical subplate neurons. _J. Neurosci._ 21, 5121–5129 (2001). CAS PubMed PubMed Central Google Scholar * Lee, R., Kermani, P., Teng, K. & Hempstead, B.
Regulation of cell survival by secreted proneurotrophins. _Science_ 294, 1945–1948 (2001). THE PRECURSOR FORMS OF NEUROTROPHINS HAVE BEEN IMPLICATED IN THE FOLDING AND PROCESSING OF THE
MATURE PROTEINS. THIS PAPER INDICATES THAT THE PRO-SEQUENCE OF NGF PREFERENTIALLY BINDS TO THE P75 RECEPTOR. CAS PubMed Google Scholar * Chao, M. V. Growth factor signaling: where is the
specificity? _Cell_ 68, 995–997 (1992). CAS PubMed Google Scholar * Lohof, A. M., Ip, N. & Poo, M. -M. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and
BDNF. _Nature_ 363, 350–353 (1993). CAS PubMed Google Scholar * Kang, H. & Schuman, E. M. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult
hippocampus. _Science_ 267, 1658–1662 (1995). CAS PubMed Google Scholar * Levine, E. S., Dreyfus, C. F., Black, I. B. & Plummer, M. R. Brain-derived neurotrophic factor rapidly
enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. _Proc. Natl Acad. Sci. USA_ 92, 8074–8077 (1995). CAS PubMed PubMed Central Google
Scholar * Xie, C. et al. Deficient long-term memory and long-lasting long-term potentiation in mice with a targeted deletion of neurotrophin-4. _Proc. Natl Acad. Sci. USA_ 97, 8116–8121
(2000). CAS PubMed PubMed Central Google Scholar * Korte, M. et al. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic
factor mutant mice. _Proc. Natl Acad. Sci. USA_ 93, 12547–12552 (1996). CAS PubMed PubMed Central Google Scholar * Schuman, E. Neurotrophin regulation of synaptic transmission. _Curr.
Opin. Neurobiol._ 9, 105–109 (1999). CAS PubMed Google Scholar * Schinder, A. & Poo, M. -M. The neurotrophin hypothesis for synaptic plasticity. _Trends Neurosci._ 23, 639–645 (2000).
CAS PubMed Google Scholar * Yang, F. et al. PI-3 kinase and IP3 are both necessary and sufficient to mediate NT3-induced synaptic potentiation. _Nature Neurosci._ 4, 19–28 (2001). CAS
PubMed Google Scholar * Minichiello, L. et al. Essential role for TrkB receptors in hippocampus-mediated learning. _Neuron_ 24, 401–414 (1999). CAS PubMed Google Scholar * Minichiello,
L. et al. Mechanism of TrkB-mediated hippocampal long-term potentiation. _Neuron_ 36, 121–137 (2002). CAS PubMed Google Scholar * Peterson, D. A., Dickinson-Anson, H. A., Leppert, J. T.,
Lee, K. -F. & Gage, F. H. Central neuronal loss and behavioral impairment in mice lacking neurotrophin receptor p75. _J. Comp. Neurol._ 404, 1–20 (1999). CAS PubMed Google Scholar *
von Schack, D. et al. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. _Nature Neurosci._ 4, 977–978 (2001). CAS PubMed
Google Scholar * Dalva, M. et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. _Cell_ 103, 945–956 (2000). CAS PubMed Google Scholar * Grunwald,
I. et al. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. _Neuron_ 32, 1027–1040 (2001). CAS PubMed Google Scholar * Henderson, J. et al. The
receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. _Neuron_ 32, 1041–1056 (2001). CAS PubMed Google Scholar * Takasu, M., Dalva, M., Zigmond, R. & Greenberg,
M. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. _Science_ 295, 491–495 (2002). CAS PubMed Google Scholar * Montell, C., Birnbaumer, L.
& Flockerzi, V. The TRP channels, a remarkably functional family. _Cell_ 108, 595–598 (2002). CAS PubMed Google Scholar * Li, H., Xu, X. & Montell, C. Activation of a
TRPC3-dependent cation current through the neurotrophin BDNF. _Neuron_ 24, 261–273 (1999). BDNF BINDING TO TRKB PRODUCED A RAPID INFLUX OF CATIONS THROUGH TRPC3 THAT WAS DEPENDENT ON
ACTIVATION OF PHOSPHOLIPASE C. AN INTERACTION BETWEEN TRKB RECEPTORS AND TRPC3 ION CHANNELS WAS OBSERVED, INDICATING THAT ION CHANNELS MIGHT BE CLOSELY ASSOCIATED WITH RECEPTOR TYROSINE
KINASES. CAS PubMed Google Scholar * Caterina, M. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. _Nature_ 389, 816–824 (1997). CAS PubMed Google
Scholar * Shu, X. & Mendell, L. Neurotrophins and hyperalgesia. _Proc. Natl Acad. Sci. USA_ 96, 7693–7696 (1999). CAS PubMed PubMed Central Google Scholar * Chuang, H. -h. et al.
Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. _Nature_ 411, 957–962 (2001). THIS STUDY SHOWS THAT THE CAPSAICIN (TRPV1) RECEPTOR
IS ACTIVATED THROUGH NGF BINDING TO TRKA RECEPTORS. THE INTERACTION BETWEEN A RECEPTOR TYROSINE KINASE AND A PAIN-RELATED CHANNEL PROVIDES A MECHANISM FOR THE ABILITY OF SENSORY NEURONS TO
RESPOND TO NGF-MEDIATED HEAT SENSITIVITY AND ALSO POINTS TO A MECHANISM FOR THE HEIGHTENED HYPERALGESIA THAT IS OBSERVED AFTER ADMINISTRATION OF NEUROTROPHINS IN CLINICAL TRIALS OF
NEURODEGENERATIVE DISEASES. CAS PubMed Google Scholar * Johagen, M. et al. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer's disease.
_Dement. Geriat. Cogn._ 9, 246–257 (1998). Google Scholar * Thoenen, H. & Sendtner, M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic
approaches. _Nature Neurosci._ 5, S1046–S1050 (2002). Google Scholar * Lin, S. et al. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and
hippocampal postsynaptic densities. _Brain Res. Mol. Brain Res._ 55, 20–27 (1998). CAS PubMed Google Scholar * Tucker, K. & Fadool, D. Neurotrophin modulation of voltage-gated
potassium channels in rat through TrkB receptors is time and sensory experience dependent. _J. Physiol. (Lond.)_ 542, 413–429 (2002). CAS Google Scholar * Figurov, A., Pozzo-Miller, L.,
Olafsson, T., Wang, B. & Lu, B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. _Nature_ 381, 706–709 (1996). CAS PubMed
Google Scholar * Gottschalk, W., Pozzo-Miller, L., Figurov, A. & Lu, B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the
developing hippocampus. _J. Neurosci._ 18, 6830–6839 (1998). CAS PubMed PubMed Central Google Scholar * Xu, B. et al. The role of brain-derived neurotrophic factors in the mature
hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. _J. Neurosci._ 20, 6888–6897 (2000). CAS PubMed PubMed Central Google Scholar *
Kovalchuk, Y., Hanse, E., Kafitz, K. & Konnerth, A. Postsynaptic induction of BDNF-mediated long-term potentiation. _Science_ 295, 1729–1734 (2002). CAS PubMed Google Scholar *
Balkowiec, A., Kunze, D. & Katz, D. Brain-derived neurotrophic factor acutely inhibits AMPA-mediated currents in developing sensory relay neurons. _J. Neurosci._ 20, 1904–1911 (2000).
CAS PubMed PubMed Central Google Scholar * Carroll, R., Beattie, E., von Zastrow, M. & Malenka, R. Role of AMPA receptor endocytosis in synaptic plasticity. _Nature Rev. Neurosci._
2, 315–324 (2001). CAS Google Scholar * Blum, R., Kafitz, K. & Konnerth, A. Neurotrophin-evoked depolarization requires the sodium channel NaV1.9. _Nature_ 419, 687–693 (2002). CAS
PubMed Google Scholar * Kafitz, K., Rose, C., Thoenen, H. & Konnerth, A. Neurotrophin-evoked rapid excitation through TrkB receptors. _Nature_ 401, 918–921 (1999). A REMARKABLY RAPID
RESPONSE OF A SODIUM CHANNEL BY BDNF TREATMENT IS DOCUMENTED IN REFERENCES 79 AND 80. CAS PubMed Google Scholar * Choi, D. -Y., Toledo-Aral, J., Segal, R. & Halegoua, S. Sustained
signaling by phospholipase C-γ mediates nerve growth factor-triggered gene expression. _Mol. Cell Biol._ 21, 2695–2705 (2001). CAS PubMed PubMed Central Google Scholar * Toledo-Aral, J.,
Brehm, P., Halegoua, S. & Mandel, G. A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. _Neuron_ 14, 607–611 (1995).
CAS PubMed Google Scholar * Arevalo, J. et al. A novel mutation within the extracellular domain of TrkA causes constitutive receptor activation. _Oncogene_ 20, 1229–1234 (2001). CAS
PubMed Google Scholar * Daub, H., Weiss, F. U., Wallasch, C. & Ullrich, A. Role of transactivation of the EGF receptor in signalling by G-protein coupled receptors. _Nature_ 379,
557–560 (1996). CAS PubMed Google Scholar * Luttrell, L., Daaka, Y. & Lefkowitz, R. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. _Curr. Opin. Cell Biol._ 11,
177–183 (1999). CAS PubMed Google Scholar * Lee, F. & Chao, M. Activation of Trk neurotrophin receptors in the absence of neurotrophins. _Proc. Natl Acad. Sci. USA_ 98, 3555–3560
(2001). CAS PubMed PubMed Central Google Scholar * Lee, F. S., Ragagopal, R., Kim, A. H., Chang, P. & Chao, M. V. Activation of Trk neurotrophin receptor signaling by pituitary
adenylate cyclase-activating polypeptides. _J. Biol. Chem._ 277, 9096–9102 (2002). CAS PubMed Google Scholar * Takei, N. et al. Pituitary adenylate cyclase-activating polypeptide promotes
the survival of basal forebrain cholinergic neurons _in vitro_ and _in vivo_: comparison with effects of nerve growth factor. _Eur. J. Neurosci._ 12, 2273–2280 (2000). CAS PubMed Google
Scholar * Williams, L. R. et al. Continuous infusion of NGF prevents basal forebrain neuronal death after fimbria fornix transection. _Proc. Natl Acad. Sci. USA_ 83, 9231–9235 (1986). CAS
PubMed PubMed Central Google Scholar * Kotecha, S. et al. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. _Neuron_ 35,
1111–1122 (2002). CAS PubMed Google Scholar * Otto, C. et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating
polypeptide type I receptor-deficient mice. _J. Neurosci._ 21, 5520–5527 (2001). CAS PubMed PubMed Central Google Scholar * Hashimoto, H., Shintani, N. & Baba, A. Higher brain
functions of PACAP and a homologous _Drosophila_ memory gene _amnesiac_: insights from knockouts and mutants. _Biochem. Biophys. Res. Commun._ 297, 427–432 (2002). CAS PubMed Google
Scholar * Ledent, C. et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. _Nature_ 388, 674–678 (1997). CAS PubMed Google Scholar *
Airaksinen, M. & Saarma, M. The GDNF family: signalling, biological functions and therapeutic value. _Nature Rev. Neurosci._ 3, 383–394 (2002). CAS Google Scholar * Tsui-Pierchala, B.,
Milbrandt, J. & Johnson, E. NGF utilizes c-Ret via a novel GFL-independent, inter-RTK signaling mechanism to maintain the trophic status of mature sympathetic neurons. _Neuron_ 33,
261–273 (2002). CAS PubMed Google Scholar * Duman, R., Heninger, G. & Nestler, E. A molecular and cellular theory of depression. _Arch. Gen. Psychiatry_ 54, 597–606 (1997). CAS
PubMed Google Scholar * Cai, D., Shen, Y., DeBellard, M., Tang, S. & Filbin, M. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a
cAMP-dependent mechanism. _Neuron_ 22, 89–101 (1999). CAS PubMed Google Scholar * Luo, Y., Raible, D. & Raper, J. Collapsin, a protein in brain that induces the collapse and paralysis
of neuronal growth cones. _Cell_ 75, 217–227 (1993). CAS PubMed Google Scholar * Tuttle, R. & O'Leary, D. Neurotrophins rapidly modulate growth cone response in the axon
guidance molecule, collapsin-1. _Mol. Cell. Neurosci._ 11, 1–8 (1998). CAS PubMed Google Scholar * Dontchev, V. & Letourneau, P. Nerve growth factor and semaphorin 3A signaling
pathways interact in regulating sensory neuronal growth cone motility. _J. Neurosci._ 22, 6659–6669 (2002). CAS PubMed PubMed Central Google Scholar * Fournier, A., GrandPre, T. &
Strittmatter, S. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. _Nature_ 409, 341–346 (2001). CAS PubMed Google Scholar * Wang, K. et al.
Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. _Nature_ 417, 941–944 (2002). CAS PubMed Google Scholar * Liu, B., Fournier, A., GrandPre,
T. & Strittmatter, S. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. _Science_ 297, 1190–1193 (2002). CAS PubMed Google Scholar * Domeniconi, M. et
al. Myelin-associated glycoprotein interacts with the Nogo-66 receptor to inhibit neurite outgrowth. _Neuron_ 35, 283–290 (2002). CAS PubMed Google Scholar * Wang, K., Kim, J.,
Sivasankaran, R., Segal, R. & He, Z. p75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. _Nature_ 420, 74–78 (2002). CAS PubMed Google Scholar * Wong, S. et
al. A p75NTR and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. _Nature Neurosci._ 5, 1302–1308 (2002). THESE PAPERS MERGE NEUROTROPHIN RECEPTOR
SIGNALLING TO INHIBITION OF REGENERATION IN THE CNS THROUGH THE ACTIONS OF THREE UNRELATED PROTEINS — NOGO, P75 AND MAG. CAS PubMed Google Scholar * Yamashita, T., Higuchi, H. &
Tohyama, M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. _J. Cell Biol._ 157, 565–570 (2002). AN IMPORTANT LINK IS MADE BETWEEN THE ABILITY OF MAG TO
BLOCK AXONAL GROWTH AND RHO ACTIVITY THROUGH THE P75 RECEPTOR. THE RESULTS LED TO THE EXPERIMENTS SHOWING THAT THE P75 AND NOGO RECEPTORS ACT IN A COMPLEX. CAS PubMed PubMed Central
Google Scholar * Yamashita, T., Tucker, K. & Barde, Y. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. _Neuron_ 24, 585–593 (1999). CAS PubMed
Google Scholar * Walsh, G., Krol, K., Crutcher, K. & Kawaja, M. Enhanced neurotrophin-induced axon growth in myelinated portions of the CNS in mice lacking the p75 neurotrophin
receptor. _J. Neurosci._ 19, 4155–4168 (1999). CAS PubMed PubMed Central Google Scholar * Dobrowsky, R. T. & Carter, B. D. p75 neurotrophin receptor signaling: mechanisms for
neurotrophic modulation of cell stress? _J. Neurosci. Res._ 61, 237–243 (2000). CAS PubMed Google Scholar * Cosgaya, J. & Shooter, E. Binding of nerve growth factor to its p75
receptor in stressed cells induces selective IκB-β degradation and NF-κB nuclear translocation. _J. Neurochem._ 79, 391–399 (2001). CAS PubMed Google Scholar * Sklar, P. et al.
Family-based asssociation study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. _Mol. Psychiatry_ 7, 579–593 (2002). CAS PubMed Google Scholar * Neves-Pereira,
M. et al. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. _Am. J. Hum. Genet._ 71, 651–655 (2002). CAS
PubMed PubMed Central Google Scholar * Sen, S. et al. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression.
_Neuropsychopharmacology_ 28, 397–401 (2003). CAS PubMed Google Scholar * Egan, M. et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and
hippocampal function. _Cell_ 112, 257–269 (2003). THIS STUDY IS THE FIRST TO SHOW THAT A HUMAN POLYMORPHISM IN BDNF IS ASSOCIATED WITH MEMORY DEFICITS. A SINGLE AMINO ACID VARIATION IN THE
PRO-DOMAIN OF BDNF ACCOUNTS FOR THE ABILITY OF BDNF TO UNDERGO PROPER SECRETION. CAS PubMed Google Scholar * Ventriglia, M. et al. Association between the BDNF 196 A/G polymorphism and
sporadic Alzheimer's disease. _Mol. Psychiatry_ 7, 136–137 (2002). CAS PubMed Google Scholar * Smith, M., Makino, S., Kvetnansky, R. & Post, R. Stress alters the expression of
brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. _J. Neurosci._ 15, 1768–1777 (1995). CAS PubMed PubMed Central Google Scholar * Ueyama, T. et al.
Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. _Neurosci. Res._ 28, 103–110 (1997). CAS PubMed Google Scholar * Shirayama, Y., Chen,
A., Nakagawa, S., Russell, D. & Duman, R. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. _J. Neurosci._ 22, 3251–3261 (2002).
ADMINISTRATION OF EXOGENOUS BDNF EXERTED PROFOUND POSITIVE EFFECTS IN FORCED SWIM AND LEARNED HELPLESSNESS ASSAYS, INDICATING THAT BDNF SIGNALLING MIGHT BE RELATED TO DEPRESSION. CAS PubMed
PubMed Central Google Scholar * Elliott, T. & Shadbolt, N. Competition for neurotrophic factors: mathematical analysis. _Neural Comput._ 10, 1939–1981 (1998). CAS PubMed Google
Scholar * Crowley, C. et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. _Cell_ 76, 1001–1012
(1994). CAS PubMed Google Scholar * Bartoletti, A. et al. Heterozygous knock-out mice for brain-derived neurotrophic factor show a pathway-specific impairment of long-term potentiation
but normal critical period for monocular deprivation. _J. Neurosci._ 22, 10072–10077 (2002). CAS PubMed PubMed Central Google Scholar * Dluzen, D. et al. Evaluation of nigrostriatal
dopaminergic function in adult +/+ and +/− BDNF mutant mice. _Exp. Neurol._ 170, 121–128 (2001). CAS PubMed Google Scholar * Carroll, P., Lewin, G., Koltzenburg, M., Toyka, K. &
Thoenen, H. A role for BDNF in mechanosensation. _Nature Neurosci._ 1, 42–46 (1998). CAS PubMed Google Scholar * Ernfors, P., Lee, K. F. & Jaenisch, R. Mice lacking brain-derived
neurotrophic factor develop with sensory deficits. _Nature_ 368, 147–150 (1994). CAS PubMed Google Scholar * Bianchi, L. et al. Degeneration of vestibular neurons in late embryogenesis of
both heterozygous and homozygous _BDNF_ null mutant mice. _Development_ 122, 1965–1973 (1996). CAS PubMed Google Scholar * Elmer, E. et al. Suppressed kindling epileptogenesis and
perturbed _BDNF_ and _TrkB_ gene regulation in _NT-3_ mutant mice. _Exp. Neurol._ 145, 93–103 (1997). CAS PubMed Google Scholar * Donovan, M., Hahn, R., Tessarollo, L. & Hempstead, B.
Neurotrophin-3 is required for mammalian cardiac development: identification of an essential nonneuronal neurotrophin function. _Nature Genet._ 14, 210–213 (1996). CAS PubMed Google
Scholar * Airaksinen, M. et al. Specific subtypes of cutaneous mechanoreceptors require neurotrophin-3 following peripheral target innervation. _Neuron_ 16, 287–295 (1996). CAS PubMed
Google Scholar * Ernfors, P., Lee, K. -F., Kucera, J. & Jaenisch, R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive
afferents. _Cell_ 77, 503–512 (1994). CAS PubMed Google Scholar * DiStefano, P., Chelsea, D., Schick, C. & McKelvy, J. Involvement of a metalloprotease in low-affinity nerve growth
factor receptor truncation: inhibition of truncation _in vitro_ and _in vivo_. _J. Neurosci._ 13, 2405–2414 (1993). CAS PubMed PubMed Central Google Scholar * Schecterson, L., Kanning,
K., Hudson, M. & Bothwell, M. The neurotrophin receptor p75 is cleaved by regulated intramembranous proteolysis. _Soc. Neurosci. Abstr._ 27, 822.10 (2002). INTRAMEMBRANOUS CLEAVAGE OF
NOTCH, THE AMYLOID PRECURSOR PROTEIN AND ERBB4 RECEPTORS GENERATES INTRACELLULAR CYTOPLASMIC FRAGMENTS THAT PRODUCE MARKED CHANGES IN SIGNALLING AND TRANSCRIPTIONAL ACTIVITIES. THE CLEAVAGE
OF P75 BY A Γ-SECRETASE REVEALS A NEW MECHANISM FOR TRANSMITTING NEUROTROPHIN SIGNALS FROM THE CELL SURFACE TO INTRACELLULAR LOCATIONS. Google Scholar * Brown, M., Ye, J., Rawson, R. &
Goldstein, J. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. _Cell_ 100, 391–398 (2000). CAS PubMed Google Scholar * Fahnestock, M.,
Michalski, B., Xu, B. & Coughlin, M. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer's disease. _Mol.
Cell. Neurosci._ 18, 210–220 (2001). CAS PubMed Google Scholar * Cosgaya, J. M., Chan, J. R. & Shooter, E. M. The neurotrophin receptor p75NTR as a positive modulator of myelination.
_Science_ 298, 1245–1248 (2002). CAS PubMed Google Scholar * Wu, C., Lai, C. F. & Mobley, W. C. Nerve growth factor activates persistent Rap1 signaling in endosomes. _J. Neurosci._
21, 5406–5416 (2001). CAS PubMed PubMed Central Google Scholar * Lee, F., Rajagopal, R., Kim, A., Chang, P. & Chao, M. Activation of Trk neurotrophin receptor signaling by pituitary
adenylate cyclase-activating polypeptides. _J. Biol. Chem._ 277, 9096–9102 (2002). CAS PubMed Google Scholar * Chen, M. et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor
and an antigen for monclonal antibody IN-1. _Nature_ 403, 434–439 (2000). CAS PubMed Google Scholar * Ernfors, P., Henschen, A., Olson, L. & Persson, H. Expression of nerve growth
factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. _Neuron_ 2, 1605–1613 (1989). CAS PubMed Google Scholar * Koliatsos, V.,
Crawford, T. & Price, D. Axotomy induces nerve growth factor receptor immunoreactivity in spinal motor neurons. _Brain Res._ 549, 297–304 (1991). CAS PubMed Google Scholar * Hayes,
R., Wiley, R. & Armstrong, D. Induction of nerve growth factor receptor (p75NGFr) mRNA within hypoglossal motoneurons following axonal injury. _Brain Res. Mol. Brain Res._ 15, 291–297
(1992). CAS PubMed Google Scholar * Martinez-Murillo, R., Fernandez, A., Bentura, M. & Rodrigo, J. Subcellular localization of low-affinity nerve growth factor receptor-immunoreactive
protein in adult rat Purkinje cells following traumatic injury. _Exp. Brain Res._ 119, 47–57 (1998). CAS PubMed Google Scholar * Friedman, W. Neurotrophins induce death of hippocampal
neurons via the p75 receptor. _J. Neurosci._ 20, 6340–6346 (2000). CAS PubMed PubMed Central Google Scholar * Kokaia, Z., Andsberg, G., Martinez-Serrano, A. & Lindvall, O. Focal
cerebral ischemia in rats induces expression of p75 neurotrophin receptor in resistant striatal cholinergic neurons. _Neuroscience_ 84, 1113–1125 (1998). CAS PubMed Google Scholar * Park,
J., Lee, J., Sato, T. & Koh, J. Co-induction of p75NTR and p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the rat. _J.
Neurosci._ 20, 9096–9103 (2000). CAS PubMed PubMed Central Google Scholar * Mufson, E. & Kordower, J. Cortical neurons express nerve growth factor receptors in advanced age and
Alzheimer's disease. _Proc. Natl Acad. Sci. USA_ 89, 569–573 (1992). CAS PubMed PubMed Central Google Scholar * Lemke, G. & Chao, M. V. Axons regulate Schwann cell expression of
major myelin and NGF receptor genes. _Development_ 102, 499–504 (1988). CAS PubMed Google Scholar * Taniuchi, M., Clark, H., Schweitzer, J. & Johnson, E. Expression of nerve growth
factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. _J. Neurosci._ 8, 664–681 (1988). CAS
PubMed PubMed Central Google Scholar * Chang, A., Nishiyama, A., Peterson, J., Prineas, J. & Trapp, B. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple
sclerosis lesions. _J. Neurosci._ 20, 6404–6412 (2000). CAS PubMed PubMed Central Google Scholar * Calza, L., Giardino, L., Pozza, M., Micera, A. & Aloe, L. Time-course changes of
nerve growth factor, corticotropin-releasing hormone, and nitric oxide synthase isoforms and their possible role in the development of inflammatory response in experimental allergic
encephalomyelitis. _Proc. Natl Acad. Sci. USA_ 94, 3368–3373 (1997). CAS PubMed PubMed Central Google Scholar * Nataf, S. et al. Low affinity NGF receptor expression in the central
nervous system during experimental allergic encephalomyelitis. _J. Neurosci. Res._ 52, 83–92 (1998). CAS PubMed Google Scholar * Hu, X. -Y. et al. Increased p75NTR expression in
hippocampal neurons containing hyperphosphorylated τ in Alzheimer patients. _Exp. Neurol._ 178, 104–111 (2002). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The
assistance of Albert Kim is gratefully acknowledged. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Skirball Institute of Biomolecular Medicine, New York University School of Medicine, New
York, 10016, New York, USA Moses V. Chao Authors * Moses V. Chao View author publications You can also search for this author inPubMed Google Scholar RELATED LINKS RELATED LINKS DATABASES
SWISS-PROT BDNF CREB GDNF JNK Kv1.3 MAG Nav1.9 NF-κB NGF Nogo PACAP Semaphorin 3A Shc TrkA TrkB TrkC Tumour necrosis factor OMIM Alzheimer disease GLOSSARY * LONG-TERM POTENTIATION (LTP). An
enduring increase in the amplitude of excitatory postsynaptic potentials as a result of high-frequency (tetanic) stimulation of afferent pathways. It is measured both as the amplitude of
excitatory postsynaptic potentials and as the magnitude of the postsynaptic-cell population spike. LTP is most often studied in the hippocampus and is often considered to be the cellular
basis of learning and memory in vertebrates. * APOPTOSIS The process of programmed cell death, characterized by distinctive morphological changes in the nucleus and cytoplasm, chromatin
cleavage at regularly spaced sites, and the endonucleolytic cleavage of genomic DNA. * LIGHT/DARK EXPLORATION TEST This test depends on the natural tendency of rodents to explore the
environment in the absence of a threat and to retreat to an enclosed area when fearful. The animals are placed in an apparatus that has a dark and an illuminated compartment. Reduced
exploration of the bright compartment and a reduced number of transitions between compartments are commonly interpreted as measures of anxiety. * FURIN An endopeptidase with specificity for
the consensus sequence Arg-X-Lys/Arg-Arg. * KINDLING An experimental model of epilepsy in which an increased susceptibility to seizures arises after daily focal stimulation of specific brain
areas (for example, the amygdala) — stimulation that does not reach the threshold to elicit a seizure by itself. * CONDITIONAL MUTATION A mutation that can be selectively targeted to
specific organs (or cell types within an organ) or induced at a specific developmental stage. * POLYMORPHISM The simultaneous existence in the same population of two or more genotypes in
frequencies that cannot be explained by recurrent mutations. * LEARNED HELPLESSNESS A commonly used model of depression in which animals are exposed to inescapable shock and subsequently
tested for deficits in learning a shock-avoidance task. Learned helplessness is a rare example in which, rather than working from the psychiatric disorder to the model, the behavioural
effect was originally discovered in experimental animals (dogs) and later invoked to explain depression. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Chao, M. Neurotrophins and their receptors: A convergence point for many signalling pathways. _Nat Rev Neurosci_ 4, 299–309 (2003). https://doi.org/10.1038/nrn1078 Download citation * Issue
Date: 01 April 2003 * DOI: https://doi.org/10.1038/nrn1078 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative