The myxoid liposarcoma fus-ddit3 fusion oncoprotein deregulates nf-κb target genes by interaction with nfkbiz
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT _FUS_ (also called _TLS_), _EWSR1_ and _TAF15_ (also called _TAF2N_) are related genes involved in tumor type-specific fusion oncogenes in human malignancies. The _FUS-DDIT3_ fusion
oncogene results from a t(12;16)(q13;p11) chromosome translocation and has a causative role in the initiation of myxoid/round cell liposarcomas (MLS/RCLS). The FUS-DDIT3 protein induces
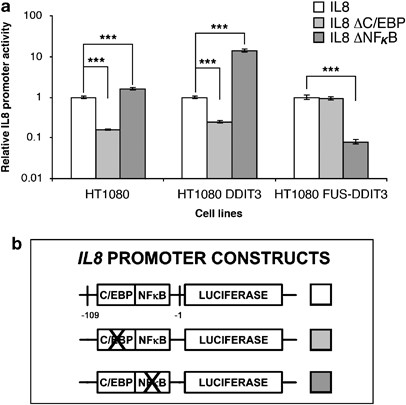
increased expression of the CAAT/enhancer-binding protein (C/EBP) and nuclear factor-κB (NF-κB)-controlled gene _IL8_, and the N-terminal FUS part is required for this activation. Chromatin
immunoprecipitation analysis showed that FUS-DDIT3 binds the _IL8_ promoter. Expression studies of the _IL8_ promoter harboring a C/EBP–NF-κB composite site pinpointed the importance of
NF-κB for _IL8_ expression in FUS-DDIT3-expressing cells. We therefore probed for possible interaction of FUS-DDIT3 with members of the NF-κB family. The nuclear factor NFKBIZ colocalizes
with FUS-DDIT3 in nuclear structures, and immunoprecipitation experiments showed that FUS-DDIT3 binds the C-terminal of NFKBIZ. We also report that additional NF-κB-controlled genes are
upregulated at the mRNA level in FUS-DDIT3-expressing cell lines and they can be induced by NFKBIZ. Taken together, the results indicate that FUS-DDIT3 deregulates some NF-κB-controlled
genes through interactions with NFKBIZ. Similar mechanisms may be a part of the transformation process in other tumor types carrying _FUS_, _EWSR1_ and _TAF15_ containing fusion oncogenes.
Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this
journal Receive 50 print issues and online access $259.00 per year only $5.18 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now
Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS FUSION PROTEIN-DRIVEN IGF-IR/PI3K/AKT SIGNALS DEREGULATE HIPPO PATHWAY PROMOTING ONCOGENIC COOPERATION OF YAP1 AND FUS-DDIT3 IN MYXOID
LIPOSARCOMA Article Open access 22 April 2022 A NOVEL ROLE OF MNT AS A NEGATIVE REGULATOR OF REL AND THE NF-ΚB PATHWAY Article Open access 08 January 2021 AN ALYREF-MYCN COACTIVATOR COMPLEX
DRIVES NEUROBLASTOMA TUMORIGENESIS THROUGH EFFECTS ON USP3 AND MYCN STABILITY Article Open access 25 March 2021 REFERENCES * Åman P, Panagopoulos I, Lassen C, Fioretos T, Mencinger M,
Toresson H _et al_. (1996). Expression patterns of the human sarcoma-associated genes FUS and EWS and the genomic structure of FUS. _Genomics_ 37: 1–8. Article Google Scholar * Åman P, Ron
D, Mandahl N, Fioretos T, Heim S, Arheden K _et al_. (1992). Rearrangement of the transcription factor gene CHOP in myxoid liposarcomas with t(12;16)(q13;p11). _Genes Chromosomes Cancer_ 5:
278–285. Article Google Scholar * Basile A, Sica A, d’Aniello E, Breviario F, Garrido G, Castellano M _et al_. (1997). Characterization of the promoter for the human long pentraxin PTX3.
Role of NF-kappaB in tumor necrosis factor-alpha and interleukin-1beta regulation. _J Biol Chem_ 272: 8172–8178. Article CAS Google Scholar * Biegel JA, Conard K, Brooks JJ . (1993).
Translocation (11;22)(p13;q12): primary change in intra-abdominal desmoplastic small round cell tumor. _Genes Chromosomes Cancer_ 7: 119–121. Article CAS Google Scholar * Bratt T .
(2000). Lipocalins and cancer. _Biochim Biophys Acta_ 1482: 318–326. Article CAS Google Scholar * Brinckerhoff CE, Rutter JL, Benbow U . (2000). Interstitial collagenases as markers of
tumor progression. _Clin Cancer Res_ 6: 4823–4830. CAS PubMed Google Scholar * Caretti G, Salsi V, Vecchi C, Imbriano C, Mantovani R . (2003). Dynamic recruitment of NF-Y and histone
acetyltransferases on cell-cycle promoters. _J Biol Chem_ 278: 30435–30440. Article CAS Google Scholar * Chen Z, Ruffner DE . (1998). Amplification of closed circular DNA _in vitro_.
_Nucleic Acids Res_ 26: 1126–1127. Article CAS Google Scholar * Crozat A, Åman P, Mandahl N, Ron D . (1993). Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma.
_Nature_ 363: 640–644. Article CAS Google Scholar * Diffin F, Porter H, Mott MG, Berry PJ, Brown KW . (1994). Rapid and specific diagnosis of t(11;22) translocation in paediatric
Ewing's sarcoma and primitive neuroectodermal tumours using RNA-PCR. _J Clin Pathol_ 47: 562–564. Article CAS Google Scholar * Engström K, Willen H, Kåbjörn-Gustafsson C, Andersson
C, Olsson M, Göransson M _et al_. (2006). The myxoid/round cell liposarcoma fusion oncogene FUS-DDIT3 and the normal DDIT3 induce a liposarcoma phenotype in transfected human fibrosarcoma
cells. _Am J Pathol_ 168: 1642–1653. Article Google Scholar * Göransson M, Elias E, Ståhlberg A, Olofsson A, Andersson C, Åman P . (2005). Myxoid liposarcoma FUS-DDIT3 fusion oncogene
induces C/EBP beta-mediated interleukin 6 expression. _Int J Cancer_ 115: 556–560. Article Google Scholar * Göransson M, Wedin M, Åman P . (2002). Temperature-dependent localization of
TLS-CHOP to splicing factor compartments. _Exp Cell Res_ 278: 125–132. Article Google Scholar * Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M . (1999). NF-kappaB
function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. _Mol Cell Biol_ 19: 2690–2698. Article CAS Google Scholar * Kitamura H, Kanehira K, Okita
K, Morimatsu M, Saito M . (2000). MAIL, a novel nuclear I kappa B protein that potentiates LPS-induced IL-6 production. _FEBS Lett_ 485: 53–56. Article CAS Google Scholar * Kovar H .
(2005). Context matters: the hen or egg problem in Ewing's sarcoma. _Semin Cancer Biol_ 15: 189–196. Article CAS Google Scholar * Kovar H, Zoubek A, Pfleiderer C, Jug G, Auinger A,
Aryee D _et al_. (1994). The EWS gene rearrangement in Ewing tumors: key to the disease. _Klin Padiatr_ 206: 196–200. Article CAS Google Scholar * Law WJ, Cann KL, Hicks GG . (2006). TLS,
EWS and TAF15: a model for transcriptional integration of gene expression. _Brief Funct Genomic Proteomic_ 5: 8–14. Article CAS Google Scholar * Libermann TA, Baltimore D . (1990).
Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. _Mol Cell Biol_ 10: 2327–2334. Article CAS Google Scholar * Littlewood TD, Hancock DC, Danielian
PS, Parker MG, Evan GI . (1995). A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. _Nucleic Acids Res_ 23: 1686–1690.
Article CAS Google Scholar * Matsuo S, Yamazaki S, Takeshige K, Muta T . (2007). Crucial roles of binding sites for NF-kappaB and C/EBPs in IkappaB-zeta-mediated transcriptional
activation. _Biochem J_ 405: 605–615. Article CAS Google Scholar * Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T _et al_. (1993). Transcription factors NF-IL6
and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. _Proc Natl Acad Sci USA_ 90: 10193–10197. Article CAS Google Scholar
* Morohoshi F, Ootsuka Y, Arai K, Ichikawa H, Mitani S, Munakata N _et al_. (1998). Genomic structure of the human RBP56/hTAFII68 and FUS/TLS genes. _Gene_ 221: 191–198. Article CAS Google
Scholar * Motoyama M, Yamazaki S, Eto-Kimura A, Takeshige K, Muta T . (2005). Positive and negative regulation of nuclear factor-kappaB-mediated transcription by IkappaB-zeta, an inducible
nuclear protein. _J Biol Chem_ 280: 7444–7451. Article CAS Google Scholar * Mukaida N, Mahe Y, Matsushima K . (1990). Cooperative interaction of nuclear factor-kappa B- and
cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. _J Biol Chem_ 265: 21128–21133. CAS PubMed Google
Scholar * Nakopoulou L, Giannopoulou I, Gakiopoulou H, Liapis H, Tzonou A, Davaris PS . (1999). Matrix metalloproteinase-1 and -3 in breast cancer: correlation with progesterone receptors
and other clinicopathologic features. _Hum Pathol_ 30: 436–442. Article CAS Google Scholar * Ohno T, Rao VN, Reddy ES . (1993). EWS/Fli-1 chimeric protein is a transcriptional activator.
_Cancer Res_ 53: 5859–5863. CAS PubMed Google Scholar * Oikawa K, Ishida T, Imamura T, Yoshida K, Takanashi M, Hattori H _et al_. (2006). Generation of the novel monoclonal antibody
against TLS/EWS-CHOP chimeric oncoproteins that is applicable to one of the most sensitive assays for myxoid and round cell liposarcomas. _Am J Surg Pathol_ 30: 351–356. Article Google
Scholar * Olofsson A, Willen H, Göransson M, Engström K, Meis-Kindblom JM, Stenman G _et al_. (2004). Abnormal expression of cell cycle regulators in FUS-CHOP carrying liposarcomas. _Int J
Oncol_ 25: 1349–1355. CAS PubMed Google Scholar * Panagopoulos I, Höglund M, Mertens F, Mandahl N, Mitelman F, Åman P . (1996). Fusion of the EWS and CHOP genes in myxoid liposarcoma.
_Oncogene_ 12: 489–494. CAS PubMed Google Scholar * Panagopoulos I, Mandahl N, Ron D, Höglund M, Nilbert M, Mertens F _et al_. (1994). Characterization of the CHOP breakpoints and fusion
transcripts in myxoid liposarcomas with the 12;16 translocation. _Cancer Res_ 54: 6500–6503. CAS PubMed Google Scholar * Perez-Losada J, Pintado B, Gutierrez-Adan A, Flores T,
Banares-Gonzalez B, del Campo JC _et al_. (2000a). The chimeric FUS/TLS-CHOP fusion protein specifically induces liposarcomas in transgenic mice. _Oncogene_ 19: 2413–2422. Article CAS
Google Scholar * Perez-Losada J, Sanchez-Martin M, Rodriguez-Garcia MA, Perez-Mancera PA, Pintado B, Flores T _et al_. (2000b). Liposarcoma initiated by FUS/TLS-CHOP: the FUS/TLS domain
plays a critical role in the pathogenesis of liposarcoma. _Oncogene_ 19: 6015–6022. Article CAS Google Scholar * Rabbitts TH, Forster A, Larson R, Nathan P . (1993). Fusion of the
dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. _Nat Genet_ 4: 175–180. Article CAS Google Scholar * Riggi N,
Cironi L, Provero P, Suva ML, Stehle JC, Baumer K _et al_. (2006). Expression of the FUS-CHOP fusion protein in primary mesenchymal progenitor cells gives rise to a model of myxoid
liposarcoma. _Cancer Res_ 66: 7016–7023. Article CAS Google Scholar * Ron D, Habener JF . (1992). CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription
factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. _Genes Dev_ 6: 439–453. Article CAS Google Scholar * Sanchez Garcia I, Rabbitts TH . (1994).
Transcriptional activation by TAL1 and FUS-CHOP proteins expressed in acute malignancies as a result of chromosomal abnormalities. _Proc Natl Acad Sci USA_ 91: 7869–7873. Article CAS
Google Scholar * Schwarzbach MH, Koesters R, Germann A, Mechtersheimer G, Geisbill J, Winkler S _et al_. (2004). Comparable transforming capacities and differential gene expression patterns
of variant FUS/CHOP fusion transcripts derived from soft tissue liposarcomas. _Oncogene_ 23: 6798–6805. Article CAS Google Scholar * Ståhlberg A, Håkansson J, Xian X, Semb H, Kubista M .
(2004). Properties of the reverse transcription reaction in mRNA quantification. _Clin Chem_ 50: 509–515. Article Google Scholar * Sun SC, Xiao G . (2003). Deregulation of NF-kappaB and
its upstream kinases in cancer. _Cancer Metastasis Rev_ 22: 405–422. Article CAS Google Scholar * Thelin-Järnum S, Göransson M, Burguete AS, Olofsson A, Åman P . (2002). The myxoid
liposarcoma specific TLS-CHOP fusion protein localizes to nuclear structures distinct from PML nuclear bodies. _Int J Cancer_ 97: 446–450. Article Google Scholar * Thelin-Järnum S, Lassen
C, Panagopoulos I, Mandahl N, Åman P . (1999). Identification of genes differentially expressed in TLS-CHOP carrying myxoid liposarcomas. _Int J Cancer_ 83: 30–33. Article Google Scholar *
Totzke G, Essmann F, Pohlmann S, Lindenblatt C, Janicke RU, Schulze-Osthoff K . (2006). A novel member of the IkappaB family, human IkappaB-zeta, inhibits transactivation of p65 and its DNA
binding. _J Biol Chem_ 281: 12645–12654. Article CAS Google Scholar * Trinh DV, Zhu N, Farhang G, Kim BJ, Huxford T . (2008). The nuclear I kappaB protein I kappaB zeta specifically
binds NF-kappaB p50 homodimers and forms a ternary complex on kappaB DNA. _J Mol Biol_ 379: 122–135. Article CAS Google Scholar * Uranishi H, Tetsuka T, Yamashita M, Asamitsu K, Shimizu
M, Itoh M _et al_. (2001). Involvement of the pro-oncoprotein TLS (translocated in liposarcoma) in nuclear factor-kappa B p65-mediated transcription as a coactivator. _J Biol Chem_ 276:
13395–13401. Article CAS Google Scholar * Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A _et al_. (2002). Accurate normalization of real-time quantitative RT-PCR
data by geometric averaging of multiple internal control genes. _Genome Biol_ 3: RESEARCH0034. Article Google Scholar * Vincenti MP, Coon CI, Brinckerhoff CE . (1998). Nuclear factor
kappaB/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin-1beta-stimulated synovial fibroblasts. _Arthritis Rheum_ 41: 1987–1994. Article CAS Google
Scholar * Yamazaki S, Muta T, Takeshige K . (2001). A novel IkappaB protein, IkappaB-zeta, induced by proinflammatory stimuli, negatively regulates nuclear factor-kappaB in the nuclei. _J
Biol Chem_ 276: 27657–27662. Article CAS Google Scholar * Zinszner H, Albalat R, Ron D . (1994). A novel effector domain from the RNA-binding protein TLS or EWS is required for oncogenic
transformation by CHOP. _Genes Dev_ 8: 2513–2526. Article CAS Google Scholar * Zinszner H, Immanuel D, Yin Y, Liang FX, Ron D . (1997). A topogenic role for the oncogenic N-terminus of
TLS: nucleolar localization when transcription is inhibited. _Oncogene_ 14: 451–461. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Ulric Pedersen for image
processing. This work was supported by grants from the Inga-Britt and Arne Lundberg Research Foundation, the Swedish Cancer Society, Assar Gabrielssons Research Foundation and the Johan
Jansson Foundation for Cancer Research. RM was supported by an AIRC grant. AS is supported by a postdoctoral fellowship award from the Swedish Research Council. AUTHOR INFORMATION AUTHORS
AND AFFILIATIONS * Department of Pathology, Lundberg Laboratory for Cancer Research (LLCR), Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden M Göransson, M K Andersson, C
Andersson, A Olofsson & P Åman * Dipartimento di Scienze Biomolecolari e Biotecnologie, Università degli Studi di Milano, Milano, Italy C Forni & R Mantovani * Department of Clinical
Neuroscience and Rehabilitation, Center for Brain Repair and Rehabilitation (CBR), Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden A Ståhlberg Authors * M Göransson View
author publications You can also search for this author inPubMed Google Scholar * M K Andersson View author publications You can also search for this author inPubMed Google Scholar * C
Forni View author publications You can also search for this author inPubMed Google Scholar * A Ståhlberg View author publications You can also search for this author inPubMed Google Scholar
* C Andersson View author publications You can also search for this author inPubMed Google Scholar * A Olofsson View author publications You can also search for this author inPubMed Google
Scholar * R Mantovani View author publications You can also search for this author inPubMed Google Scholar * P Åman View author publications You can also search for this author inPubMed
Google Scholar CORRESPONDING AUTHOR Correspondence to P Åman. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Göransson, M., Andersson, M., Forni, C. _et
al._ The myxoid liposarcoma FUS-DDIT3 fusion oncoprotein deregulates NF-κB target genes by interaction with NFKBIZ. _Oncogene_ 28, 270–278 (2009). https://doi.org/10.1038/onc.2008.378
Download citation * Received: 21 August 2008 * Accepted: 01 September 2008 * Published: 13 October 2008 * Issue Date: 15 January 2009 * DOI: https://doi.org/10.1038/onc.2008.378 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * MLS/RCLS * FUS * DDIT3 * NF-κB * NFKBIZ * sarcoma