The muscle–intervertebral disc interaction mediated by l-baiba modulates extracellular matrix homeostasis and panoptosis in nucleus pulposus cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Upon engaging in physical activity, skeletal muscle synthesizes myokines, which not only facilitate crosstalk with various organs, including the brain, adipose tissue, bone, liver,
gut, pancreas, and skin but also promote intramuscular signaling. Crosstalk is vital for maintaining various physiological processes. However, the specific interactions between skeletal
muscle and intervertebral discs remain largely unexplored. β-Aminoisobutyric acid (BAIBA), an exercise-induced myokine and a metabolite of branched-chain amino acids in skeletal muscle, has
emerged as a key player in this context. Our study demonstrated that exercise significantly elevates BAIBA levels in skeletal muscle, plasma, and nucleus pulposus (NP) tissues. Moreover,
exercise enhances extracellular matrix (ECM) synthesis in NP tissues and upregulates L-BAIBA synthase in skeletal muscle. Both in vivo and in vitro evidence revealed that L-BAIBA impedes
PANoptosis and ECM degradation in NP cells by activating the AMPK/NF-κB signaling pathway. These findings suggest that exercise, coupled with the resulting increase in L-BAIBA, may serve as
an effective intervention to decelerate the progression of intervertebral disc degeneration (IDD). Consequently, L-BAIBA, which originates from skeletal muscle, is a promising new
therapeutic approach for IDD. SIMILAR CONTENT BEING VIEWED BY OTHERS EXERCISE-INDUCED FNDC5/IRISIN PROTECTS NUCLEUS PULPOSUS CELLS AGAINST SENESCENCE AND APOPTOSIS BY ACTIVATING AUTOPHAGY
Article Open access 26 July 2022 MFG-E8 ALLEVIATES INTERVERTEBRAL DISC DEGENERATION BY SUPPRESSING PYROPTOSIS AND EXTRACELLULAR MATRIX DEGRADATION IN NUCLEUS PULPOSUS CELLS VIA
NRF2/TXNIP/NLRP3 AXIS Article Open access 19 April 2022 ATF1 AND MIR-27B-3P DRIVE INTERVERTEBRAL DISC DEGENERATION THROUGH THE PPARG/NF-ΚB SIGNALING AXIS Article Open access 14 May 2025
INTRODUCTION Intervertebral disc degeneration (IDD) is a primary contributor to low back pain (LBP), a condition with significant physical and mental impacts on patients. Clinically, IDD
manifests with symptoms such as limb radiating pain, sensory abnormalities, and muscle strength loss, which collectively exacerbate patient distress1. The intervertebral disc (IVD) is
composed of the nucleus pulposus (NP), fibrous annulus (AF), and cartilaginous endplates, with the physiological function of the NP being particularly critical2. Although the exact
pathogenesis of IDD is not fully understood, research indicates that factors such as inflammatory infiltration, oxidative stress, and programmed cell death are pivotal in its
progression3,4,5. These elements contribute to the reduction of extracellular matrix (ECM) components such as aggrecan (ACAN) and type II collagen (COL2A), as well as to the upregulation of
ECM-degrading enzymes such as disintegrin-like and metalloprotease with thrombospondin type-1 motif enzymes (ADAMTSs) and matrix metalloproteinases (MMPs). Current clinical interventions
primarily offer symptomatic relief without preventing the progression of IDD6. Thus, understanding the pathophysiology of IDD and identifying effective therapeutic targets remain imperative.
Exercise is heralded for its myriad health benefits, and international guidelines for LBP treatment suggest that appropriate exercise regimens can alleviate symptoms of LBP and restore
impaired motor function7. Exercise-induced spinal loading augments fluid circulation to intervertebral discs, thereby improving nutrient metabolite exchange with the discs8. An increasing
number of studies have advanced our understanding of the physiological mechanisms by which exercise exerts positive effects on intervertebral discs. For example, a study revealed that
running enhanced lumbar IVD magnetic resonance T2 time with a stride speed of 2 m/s (akin to jogging or brisk walking), substantiating the efficacy of running in augmenting NP water
hydration9. Furthermore, running has been shown to increase the number of NP cells and stimulate collagen and aggrecan synthesis in IVDs in rat models10,11,12. Conversely, conflicting
results have surfaced from a randomized controlled study that reported a lack of favorable effects of exercise on the intervertebral disc13. Similarly, no significant alterations in the
levels of proteoglycans or collagen in the NP were observed after 15 weeks of running in beagles14. Thus, the exact relationship between exercise and IDD warrants further exploration. The
health-promoting effects of exercise extend beyond physical fitness, with skeletal muscle acting as a vital secretory organ. Skeletal muscle releases myokines that not only regulate its own
metabolism but also have systemic effects on various organs, including bones, the liver, and the brain, through the circulatory system15,16. Branched-chain amino acids (BCAAs), which are
essential amino acids predominantly metabolized in skeletal muscle, constitute half of the muscle’s total amino acid intake. β-Aminoisobutyric acid (BAIBA), a small molecule derived from
BCAAs, has emerged as a novel endogenous protective myokine17. BAIBA is instrumental in several biological processes, notably reducing inflammation, inhibiting oxidative stress, and
preventing cell death18,19,20, all of which are crucial factors in the progression of IDD. Additional myokines, such as irisin, IL-6, and IGF-1, have also been implicated in IDD
pathogenesis21,22,23. Nevertheless, the specific relationship between BAIBA and IDD has yet to be elucidated. Apoptosis, pyroptosis, and necroptosis, all of which are forms of programmed
cell death (PCD), are implicated in the progression of IDD24,25,26. The term “PANoptosis,” conceptualized by Kanneganti et al. in 2019, refers to the phenomenon in which cells undergo
apoptosis, pyroptosis, and necroptosis simultaneously. This intricate form of cell death was initially observed in macrophages responding to the influenza A virus27,28. PANoptosis combines
the salient characteristics of pyroptosis, apoptosis, and necroptosis but remains a distinct entity from each process in isolation. Diverse triggers ranging from infectious pathogens to
shifts in the intracellular environment, such as pronounced cytokine release consequent to cell death, provoke PANoptosis29. For example, elevated tumor necrosis factor-alpha (TNFα) during
SARS-CoV-2 infection has been shown to induce PANoptosis in bone marrow-derived macrophages30. Moreover, the upregulation of proinflammatory mediators such as TNFα plays a crucial role in
IDD progression31. These findings suggest a potential association between PANoptosis and the pathogenesis of IDD. In our study, we assessed the impact of exercise and the muscle-secreted
myokine L-BAIBA on intervertebral disc degeneration. The data demonstrated that exercise mitigates the progression of IDD and that L-BAIBA significantly contributes to the promotion of
extracellular matrix synthesis and the inhibition of PANoptosis in nucleus pulposus cells. These findings suggest that L-BAIBA may represent a novel therapeutic approach for the management
of IDD. MATERIALS AND METHODS COLLECTION OF CLINICAL SPECIMENS The NP tissue used in this study was obtained from patients requiring internal fixation for spinal fusion at Sun Yat-sen
Memorial Hospital of Sun Yat-sen University. The patients’ diseases included lumbar disc herniation, lumbar spinal stenosis, and lumbar spondylolisthesis. NP tissue from the central region
of the IVD was collected during the operation, washed with saline to remove the adherent blood, immediately immersed in 4% paraformaldehyde for 48 h, dehydrated and paraffin-embedded for
sectioning. The experimental design and protocol of this part of the study were reviewed and approved by the Ethics Committee of Sun Yat-sen Memorial Hospital (SYSKY-2023-956-01). In this
study, magnetic resonance imaging (MRI) images of patients were obtained from an imaging system, and the IDD grade was assessed according to the Pfirrmann classification. CELL CULTURE Male
Sprague‒Dawley (SD) rats were killed by intraperitoneal injection of an overdose of pentobarbital. The caudal vertebrae of the rats were cut at the Co7/Co8 intervertebral space, and the
caudal skin and paravertebral musculature were carefully separated until the structure of the fibrous annulus could be clearly observed. The IVD was incised with a sharp blade, and the
jelly-like nucleus pulposus was removed with forceps and placed in tubes containing 10% FBS in DMEM. After the NP tissue was centrifuged at 1000 rpm for 5 min, the medium was discarded, 0.2%
protease was added, and the mixture was digested for 45 min at 37 °C. At the end of digestion, DMEM containing 10% FBS was added, the mixture was centrifuged at 1000 rpm for 5 min, and the
supernatant was discarded. The tissues were digested with 2.5% type II collagenase in a 37 °C incubator for 15 min. After digestion, the NP tissues were washed with PBS. Finally, the
digested NP tissue was cultured in DMEM containing 1% penicillin/streptomycin and 10% FBS at 37 °C. CELL VIABILITY ASSAY Rat NP cells were inoculated evenly into 96-well plates, and when the
cells were completely attached to the dishes, different concentrations (1, 10, 50 and 100 μM) of L-BAIBA (Sigma–Aldrich, United States) and 50 ng/ml TNFα were added, and the cells were
incubated for 24, 48 and 72 h. After that, the original medium was discarded, 100 µl of DMEM containing CCK-8 reagent (Hanbio, China) was added, and the cells were incubated at 37 °C for 1
h. Cell viability was calculated by measuring the absorbance at 450 nm with an optical densitometer. HIGH-DENSITY CULTURE AND ALCIAN BLUE STAINING NP cells were digested and diluted to a
density of 10,000 cells/10 μl, and then, 10 μl of cell suspension was added to the center of the 24-well plate and incubated in a 37 °C incubator for 1 h. One milliliter of DMEM containing
1% insulin transferrin selenium +2% FBS was added. Then, the cells were treated with L-BAIBA, TNFα and Compound C (Selleck, United States). After 5 days, the cells were fixed with 4%
paraformaldehyde for 10 min, treated with acidification solution for 5 min, and finally stained with alcian blue (Solarbio, China). ANIMAL MODELS The SD rats used in this study were
purchased from the Animal Experiment Centre of Sun Yat-sen University, and all the animal experiments were conducted under the supervision of the Institutional Animal Care and Use Committee
(IACUC) of Sun Yat-sen University and approved by the Institutional Research Ethics Committee of Sun Yat-sen University (SYSU-IACUC-2023-001583). SD rats were randomly divided into three
groups: the sham surgery (sham), annulus fibrosus puncture (AFP) and AFP + L-BAIBA groups. After anesthesia, the rats were placed flat on an operating table in the prone position. AFP was
performed using a 20 G needle, which was inserted into the Co7/8 intervertebral disc vertically, rotated 360° clockwise, and held for 30 s. A Hamilton syringe with a 34 G needle was used to
inject 2 µl of L-BAIBA (10 µg/ml) into the IVD in the AFP + L-BAIBA group once a week for 4 weeks. In addition, IVDs were punctured with 34 G needles in the AFP and sham groups. At the end
of the experiment, MRI scans of the caudal vertebrae were performed on all the rats. After MRI, the rats were euthanized by the administration of 3% sodium pentobarbital (0.4 ml/100 g),
after which the plasma, intravertebral discs and gastrocnemius muscles were collected. RUNNING SCHEMES The rats in the exercise group underwent a one-week adaptive training program, starting
with 10 min of running per day, increasing by 10 min per day up to 60 min, and then started formal training: 60 min per day for 5 weeks at a speed of 16.7 m/min. The running schemes were
selected with reference to previous studies10,12. The control rats were only allowed to move around in the cage. TRANSCRIPTOMIC ANALYSIS OF RAT NP CELLS NP cells were divided into two
groups: the TNFα group and the TNFα + L-BAIBA group. Total RNA was extracted from rat NP cells using TRIzol reagent, and the HaploX Genomics Center was commissioned to perform mRNA
transcriptome sequencing. The raw data were subjected to expression difference analysis and functional enrichment analysis after data quality control and reference genome comparison. The
threshold was set as follows: | log2 (fold change)| >1 and _p_ < 0.05. MOLECULAR DOCKING The X-ray crystal structures of AMPKα1 (PDB: 6C9J), AMPKα2 (PDB: 2H6D), AMPKβ1 (PDB: 6C9F),
AMPKβ2 (PDB: 6B2E) and AMPKγ1 (PDB: 4RER) were retrieved from the Protein Data Bank. The predicted structures of AMPKγ2 and AMPKγ3 were generated by AlphaFold. The protonation state of
L-BAIBA (PubChem ID: 439434) was set at pH = 7.4, and L-BAIBA was expanded to 3D structures using Open Babel. AutoDock Tools (ADT3) were applied to prepare and parametrize the receptor
protein and ligands. The docking grid documents were generated using AutoGrid in sitemap, and AutoDock Vina (1.2.0) was used for docking simulation. The optimal pose was selected to analyze
the interaction. Finally, the protein‒ligand interaction figure was generated in PyMOL. STATISTICAL ANALYSIS The data were collected from at least three independent experiments and are
expressed as the means ± standard deviations. Unpaired two-tailed Student’s _t_ tests were used to compare differences between two groups, whereas one-way ANOVA was used to evaluate
differences between multiple groups. A value of _p_ < 0.05 was considered statistically significant. Detailed descriptions of the other materials and methods are available in the
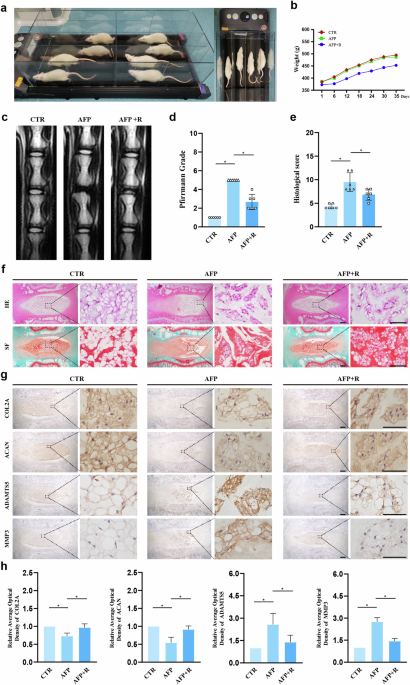
Supporting Information. RESULTS TREADMILL EXERCISE RETARDS AFP-INDUCED IDD IN RATS To elucidate the potential ameliorative effects of treadmill exercise on AFP-induced IDD, we conducted a
regimen of treadmill exercise in rats, as depicted in Fig. 1a. After five weeks, we observed a trend toward reduced body weight in the AFP + Running (AFP + R) group compared with the Control
(CTR) and AFP groups, as illustrated in Fig. 1b. However, the reduction did not appear to be statistically significant (CTR vs. AFP + R, _p_ = 0.23; AFP vs. AFP + R, _p_ = 0.32). MRI
analysis demonstrated that the T2-weighted imaging signal intensity of the IVDs in the CTR group displayed a normal white high signal (Fig. 1c). In contrast, the IVDs in the AFP group
presented a black low signal, whereas the IVDs in the AFP + R group presented a gray intermediate signal, which was an intermediary between those in the CTR and AFP groups. Further
assessment using the MRI-based Pfirrmann grading system revealed a lower score in the AFP + R group than in the AFP group, indicating mitigated IDD severity (Fig. 1d). The histological
analysis revealed that after puncturing, the disc presented a reduced number of NP cells, blurred boundaries between the NP and the AF, and inward protrusions of the AF in a serpentine
pattern according to hematoxylin and eosin (HE) and safranin O and fast green (SF) staining (Fig. 1e, f). These pathological manifestations were significantly ameliorated in the AFP + R
group. Additionally, IHC analysis of ECM-synthesizing enzymes (COL2A, ACAN) and catabolic enzymes (ADAMTS5, MMP3) revealed elevations of COL2A and ACAN expression levels by 24.1% and 36.9%,
respectively, with concurrent reductions of 46.2% in ADAMTS5 and 47.8% in MMP3 expression in the AFP + R group compared with the AFP group, suggesting an anabolic shift in extracellular
matrix homeostasis (Fig. 1g, h). EXERCISE PROMOTES L-BAIBA PRODUCTION AND SECRETION IN THE SKELETAL MUSCLE OF RATS Consistent with previous studies indicating that exercise and contracted
skeletal muscle enhance BAIBA production and secretion20,32, we observed a significant increase in skeletal muscle BAIBA levels after exercise using LC‒MS to measure BAIBA levels (Fig. 2a),
which was accompanied by increased plasma levels of BAIBA (CTR: 2.06 ± 0.22 μM/L; AFP: 2.00 ± 0.21 μM/L; AFP + R: 4.41 ± 0.46 μM/L, as depicted in Fig. 2b). Moreover, in contrast with the
CTR and AFP groups, the AFP + R group presented modest increases in BAIBA levels in the NP tissue (Fig. 2c). As shown in Supplementary Fig. 1, BAIBA levels did not differ in human NP tissue
with different degrees of degeneration. Examination of gastrocnemius muscle mass revealed no significant alterations across the groups (Fig. 2d, e), and HE staining confirmed no substantial
change in muscle cross-sectional area due to exercise (Fig. 2f, g). PGC-1α is known to regulate muscle adaptation to exercise and promote BAIBA secretion33; therefore, we evaluated PGC-1α
mRNA levels in the gastrocnemius muscle by RT‒qPCR. As expected, PGC-1α expression was upregulated following exercise (Fig. 2h), which was confirmed through IHC and IF analyses (Fig. 2i–k).
To elucidate the enantiomers of BAIBA present, we measured the expression levels of enzymes associated with L-BAIBA synthesis (ABAT) and D-BAIBA synthesis (DPYD, DPYS, UPB1, AGXT2) (Fig.
2l). Notably, only the ABAT mRNA level significantly increased in the gastrocnemius muscle postexercise (Fig. 2m). The results of IHC and IF also confirmed an increase in ABAT expression in
the AFP + R group, whereas UPB1 expression remained relatively unchanged across all groups (Fig. 2n–q). Thus, our results imply that the increase in L-BAIBA postexercise may contribute
significantly to the retardation of IDD development. L-BAIBA IMPROVES ECM SYNTHESIS IN RAT NP CELLS UNDER TNFΑ TREATMENT To evaluate the therapeutic efficacy of L-BAIBA (Fig. 3a) in IDD, we
initially performed a CCK8 assay to assess the impact of L-BAIBA on the viability of NP cells. The administration of various concentrations of L-BAIBA (0, 10, 50, or 100 μM) over different
time periods (24, 48, or 72 h) did not significantly affect cell viability, as depicted in Fig. 3b. Significantly, cell viability decreased to 65% following 72 h of exposure to TNFα but
subsequently improved to 74% with the addition of 100 μM L-BAIBA (Fig. 3c). Alcian blue staining, which is indicative of the degree of ECM secretion, demonstrated that L-BAIBA mitigated
TNFα-induced ECM degradation, particularly at a concentration of 100 μM (Fig. 3d). Furthermore, the protein levels of COL2A and ACAN increased by 57% and 44%, respectively, compared with
those in the TNFα group following treatment with 100 μM L-BAIBA. Moreover, the protein levels of ADAMTS5 and MMP3 decreased by 58% and 52%, respectively, under identical conditions (Fig. 3e,
f). Therefore, a 100 μM concentration of L-BAIBA was selected for further experiments. The RT‒qPCR results further confirmed the role of L-BAIBA in increasing COL2A and ACAN mRNA expression
while suppressing ADAMTS5 and MMP3 mRNA expression (Fig. 3g). Immunofluorescence assays further confirmed that L-BAIBA upregulated COL2A expression and downregulated MMP3 expression under
TNFα-stimulated conditions (Fig. 3h, i). L-BAIBA INHIBITS TNFΑ-INDUCED PANOPTOSIS IN RAT NP CELLS Programmed cell death, which includes apoptosis, pyroptosis, and necroptosis, plays a
critical role in reducing the number of NP cells and degrading the ECM24. Elevated levels of TNFα, a pivotal molecular driver of disc degeneration, exacerbate this process31. To investigate
the correlation between PANoptosis and IDD, we first collected human NP tissues with varying degrees of degeneration (Fig. 4a). We then assessed the expression levels of apoptosis (C-CAS3),
pyroptosis (C-GSDMD), and necroptosis (p-MLKL) markers by IHC. The results revealed a 45% increase in C-CAS3+ cells, a 53% increase in C-GSDMD+ cells, and a 64% increase in p-MLKL+ cells in
NP tissues with severe degeneration compared with those with mild degeneration (Fig. 4b, c). Furthermore, WB analysis revealed that TNFα at a concentration of 50 ng/ml effectively induced
PANoptosis in NP cells (Fig. 4d, e and Supplementary Fig. 2). To explore the impact of L-BAIBA on NP cell PANoptosis, we conducted WB analysis. The results demonstrated a 77% reduction in
C-CAS7 and a 62% reduction in C-CAS3 protein expression in the L-BAIBA group compared with the TNFα group. For pyroptosis, the protein expression levels of NLRP3, C-GSDMD, and C-CAS1
decreased by 49%, 62%, and 61%, respectively. Additionally, L-BAIBA significantly inhibited the phosphorylation of MLKL and RIPK3, key initiators of necroptosis (Fig. 4f, g and Supplementary
Fig. 3). L-BAIBA SUPPRESSES IDD IN A RAT MODEL To further assess the protective effects of L-BAIBA in vivo, we administered 2 µl of L-BAIBA (10 µg/ml) using a Hamilton syringe. The detailed
methodology and experimental groupings are depicted in Fig. 5a. MRI analysis revealed that the disc space diminished and that the signal intensity of the NP turned into a black low signal
following AFP, whereas L-BAIBA partially mitigated these adverse effects (Fig. 5b). Pfirrmann score analysis revealed lower scores in the L-BAIBA group than in the AFP group, suggesting a
protective role of L-BAIBA (Fig. 5c). Histological assays incorporating HE, SF, and Alcian blue staining revealed that L-BAIBA ameliorated the AFP-induced reductions in NP cell number and
ECM (Fig. 5d). As shown in Fig. 5e, the histological score in the L-BAIBA group was 3.7 points lower than that in the AFP group. We also analyzed the protein expression levels of ECM
synthesis markers (COL2A and ACAN) and degradation markers (ADAMTS5 and MMP3) through IHC. Compared with those in the CTR group, the expression levels of COL2A and ACAN decreased in the AFP
group but were restored by L-BAIBA treatment. Conversely, the expression levels of ADAMTS5 and MMP3 increased in the AFP group but decreased in the L-BAIBA group (Fig. 5f, g). To explore the
relationship between L-BAIBA and PANoptosis in this model, we assessed PANoptosis-relevant biomarker expression by IHC and IF (Fig. 6a). IHC analysis revealed that AFP increased the
expression levels of apoptosis, pyroptosis, and necroptosis markers, which were significantly reduced following L-BAIBA treatment (Fig. 6b, c). Immunofluorescence analysis further confirmed
that the expression levels of C-CAS3, C-GSDMD, and p-MLKL significantly decreased after L-BAIBA treatment (Fig. 6d). AMPKΑ1 PHOSPHORYLATION IS INCREASED BY L-BAIBA To elucidate the mechanism
by which L-BAIBA regulates ECM metabolism and PANoptosis in NP cells, we conducted transcriptome sequencing under treatment with 100 μM L-BAIBA and 50 ng/ml TNFα. Differential gene
expression was analyzed with thresholds of |fold change | > 2 and _p_ < 0.05, resulting in the identification of 211 upregulated and 155 downregulated genes (Fig. 7a, b). KEGG pathway
enrichment analysis of the upregulated genes highlighted the AMPK pathway, with AMPKα1 as a pivotal constituent (Fig. 7c, d). Gene set enrichment analysis (GSEA) confirmed L-BAIBA-induced
AMPK pathway activation (Fig. 7e). To validate these findings, we performed WB to assess the phosphorylation level of AMPKα1 following L-BAIBA treatment. TNFα markedly reduced the
p-AMPKα1/AMPKα1 ratio, an effect that was reversed by L-BAIBA (Fig. 7f). Molecular docking assays were performed to investigate the interaction between L-BAIBA and AMPK. The three- and
two-dimensional structures of the molecular docking data are presented in Fig. 7g and Supplementary Fig. 4. The docking results indicated that L-BAIBA formed hydrogen bonds with various
amino acid residues across different AMPK subunits (AMPKα1, AMPKα2, AMPKβ1, AMPKβ2, AMPKγ1, AMPKγ2, and AMPKγ3). Notably, L-BAIBA was found to interact with the TYR residue of AMPKα1. Among
the complexes, those formed by L-BAIBA with AMPKα1, AMPKα2, and AMPKγ1 were the most stable, exhibiting binding energies of −4 kcal/mol, −4.2 kcal/mol, and −4.2 kcal/mol, respectively. BY
ACTIVATING AMPK, L-BAIBA SUPPRESSES THE NF-ΚB SIGNALING PATHWAY KEGG functional enrichment of transcriptionally sequenced downregulated genes (fold change < −2, _p_ < 0.05) revealed
considerable aggregation within the NF-κB signaling pathway (Fig. 8a, b). Additionally, GSEA was performed on the gene set related to the NF-κB signaling pathway. GSEA of all genes in the
TNFα and TNFα + L-BAIBA groups revealed enrichment of the NF-κB pathway, although the difference did not reach statistical significance (_p_ = 0.06). In contrast, GSEA of differentially
expressed genes (_p_ < 0.05) between the same groups revealed significant enrichment in the NF-κB pathway (_p_ = 0.0002) (Fig. 8c, d). As shown in Fig. 8e, L-BAIBA decreased the
TNFa-induced p-p65/p65 and p-IKBα/IKBα ratios, suggesting that L-BAIBA inhibits the activation of the NF-κB signaling pathway. Furthermore, previous studies have indicated that AMPK
activation inhibits NF-κB signaling34. To investigate this phenomenon in NP cells, we used WB to examine NF-κB pathway markers. We observed that the AMPK inhibitor Compound C (CC) not only
increased the phosphorylation of P65 and IKBα but also increased the nuclear expression of p65 (Fig. 8f). Immunofluorescence assays further confirmed that L-BAIBA delayed the TNFa-induced
translocation of P65 into the nuclei of NP cells (Fig. 8g). L-BAIBA SUPPRESSES TNFΑ-INDUCED ECM DEGRADATION, APOPTOSIS AND PYROPTOSIS BY ACTIVATING AMPKΑ1 To determine whether L-BAIBA
regulates ECM metabolism and PANoptosis through AMPKα1, we transfected NP cells with siRNA to suppress AMPKα1 transcription. The silencing efficiency was evaluated by WB, which revealed the
maximal efficiency of siRNA-1 (Fig. 9a, b); therefore, siRNA-1 was selected for subsequent experiments. WB analysis revealed that AMPKα1 suppression counteracted the L-BAIBA-induced
reduction in ADAMTS5 and MMP3 expression (Fig. 9c, d) and reversed the L-BAIBA-induced upregulation of COL2A and ACAN (Fig. 9e, f). Alcian blue staining was then employed to evaluate ECM
synthesis, which revealed that L-BAIBA-enhanced ECM synthesis was diminished by CC (Fig. 9g). Moreover, we investigated the expression of PANoptosis markers following AMPKα1 knockdown. The
TNFa-induced increases in C-CAS3 and C-CAS7 expression were mitigated by L-BAIBA and then restored by si-AMPKα1 (Fig. 9h, i). Similar trends were observed for NLRP3, C-GSDMD, and C-CAS1
(Fig. 9h, j). Notably, the ratios of p-MLKL/MLKL and p-RIPK3/RIPK3, which were initially inhibited by L-BAIBA, were unaffected by AMPKα1 downregulation, suggesting that the influence of
L-BAIBA on necroptosis in NP cells was independent of AMPKα1 (Fig. 9h, k and Supplementary Fig. 5). DISCUSSION Numerous studies have highlighted the beneficial impacts of physical exercise
on intervertebral discs35,36. Nonetheless, it warrants emphasis that not every exercise modality confers equivalent benefits. High-impact athletics such as gymnastics, wrestling, and rugby
can lead to lumbar disc injuries, whereas weightlifting has been associated with reduced proteoglycan production in IVDs37. Interestingly, swimmers and baseball participants present a
greater prevalence of IDD than do runners or nonathletes, potentially because of the recurring torsional forces endemic to these sports38. Conversely, running is generally considered
beneficial or at least nondetrimental to IVDs39,40. In our study, we developed a running model for SD rats involving 16.7 m/min for 60 min daily. Following a five-week regimen of consistent
running, we observed a protective effect against AFP-induced IDD. This protection was evidenced by an increase in the number of NP cells, increased extracellular matrix synthesis, and
reduced expression of ECM-degrading enzymes, aligning with findings from previous studies10,11. Myokines, which are amino acids or peptides secreted by skeletal muscle, have garnered
significant research interest. Hundreds of myokines have been identified, with a growing focus on their roles in muscle interactions with other organs, including adipose tissue, bone, liver,
intestine, pancreas, blood vessels, brain, and skin41. Irisin, a notable myokine, inhibits NP cell senescence and promotes the expression of ECM anabolic genes while reducing ECM catabolic
gene expression21,42. A meta-analysis revealed elevated serum levels of IL-6, another myokine, in degenerated IVD tissues compared with normal tissues, with subsequent studies elucidating
the regulatory mechanisms of IL-6 in IDD22,43. Additionally, Deng et al. demonstrated that the overexpression of the myokine IGF-1 in rabbit intervertebral discs increased ACAN and COL2A
mRNA expression44. These studies suggest the potential for a myokine-mediated interplay between skeletal muscle and intervertebral disc tissues. In the present study, we observed a
significant increase in BAIBA levels in skeletal muscle and plasma after a five-week running regimen in SD rats, reinforcing the idea that exercise stimulates BAIBA synthesis and
secretion20,45. Concomitantly, an increase in BAIBA levels was observed within IVDs, bolstering the notion of a musculoskeletal-disc axis. Given that small molecules such as glucose (180.16
g/mol) can diffuse into IVDs to nourish NP cells8, L-BAIBA, with its lower molecular weight (103.12 g/mol), could similarly diffuse into NP cells. This supposition was validated by liquid
chromatography‒mass spectrometry, confirming increased BAIBA concentrations within the NP tissues postexercise in SD rats. Pertinently, the identification of the amino acid transporter
proteins SLC1A5 and SLC38A2 in human NP cells suggests a potential pathway for BAIBA cellular import46. Aerobic exercise is known to increase PGC-1α expression in skeletal muscle47.
Correspondingly, our results revealed a significant increase in PGC-1α expression in skeletal muscle postexercise in rats. Notably, in vitro cultures demonstrated that myocytes
overexpressing PGC-1α presented a marked increase in BAIBA in the medium. Parallel findings were observed in vivo, in which plasma BAIBA concentrations were 11-fold higher in PGC-1α
transgenic mice than in PGC-1α knockout mice33. The enhancements in mitochondrial function associated with aerobic activity, including increases in mitochondrial density and the number and
activity of mitochondrial enzymes, are closely linked to BAIBA anabolism48,49. Our research thus focused on the enzymatic pathways facilitating L-BAIBA and D-BAIBA production, specifically
ABAT (an enzyme related to L-BAIBA synthesis) and AGXT2, UPB1, DPYS, and DPYD (enzymes related to D-BAIBA synthesis). Postaerobic conditioning, we observed a significant increase in ABAT
expression in the gastrocnemius muscle of SD rats. This finding aligns with Kitase et al.‘s discovery that muscle contraction enhances the synthesis of L-BAIBA but not D-BAIBA32.
Adenylate-activated protein kinase (AMPK) is a heterotrimer composed of a catalytic α subunit (α1 and α2) and two regulatory subunits, β (β1 and β2) and γ (γ1, γ2, and γ3). AMPK plays
crucial roles in various cellular processes, including cell growth, senescence, apoptosis, autophagy, and mitochondrial biosynthesis, by phosphorylating downstream substrates50. Several
studies have implicated AMPK in the pathogenesis of IDD51,52,53. Furthermore, BAIBA has been shown to activate AMPK19,54. Our transcriptome sequencing and Western blot analysis results
indicated that L-BAIBA promotes the phosphorylation of AMPKa1. Moreover, molecular docking revealed that L-BAIBA has a strong binding affinity for the α-subunit of AMPK, suggesting that
L-BAIBA may regulate intervertebral disc degeneration through the activation of AMPK. Previous studies have shown that AMPK suppresses NF-κB-mediated inflammatory responses34,55. In our
study, AMPK inhibited the nuclear translocation of p65 in NP cells, suggesting that the AMPK/NF-κB axis plays a significant role in the regulatory effect of L-BAIBA on IDD. PANoptosis, a
form of inflammatory programmed cell death, is characterized by pyroptosis, apoptosis, and necroptosis. Previous studies have shown that TNFα can induce apoptosis, pyroptosis, or necroptosis
in NP cells56,57,58. In our study, we detected concurrent increases in biomarkers indicative of all three programmed cell death modalities in TNFα-challenged NP cells. Furthermore, KEGG
analysis of our transcriptomic data revealed the downregulation of the TNFa pathway by L-BAIBA, suggesting a suppressive influence on PANoptosis. The NF-κB pathway, which is activated by
inflammatory factors, has a well-established association with apoptosis and pyroptosis in NP cells59,60. Correspondingly, our data revealed that AMPK was responsive to the inhibitory effects
of L-BAIBA on apoptosis and pyroptosis but not necroptosis, indicating that L-BAIBA suppresses pyroptosis and apoptosis in NP cells through the AMPK/NF-κB axis. In conclusion, our research
revealed that exercise stimulates the expression of ABAT and PGC-1α within skeletal muscle, subsequently increasing the synthesis and secretion of L-BAIBA. L-BAIBA, in turn, exerts a pivotal
inhibitory effect on PANoptosis and promotes extracellular matrix synthesis, predominantly via the activation of the AMPK/NF-κB axis (as depicted in Fig. 10). Based on these significant
results, L-BAIBA is a promising therapeutic agent for the management of intervertebral disc degeneration. DATA AVAILABILITY The data presented in the study are deposited in the Sequence Read
Archive (SRA) repository, accession number PRJNA1018342. REFERENCES * Knezevic, N. N., Candido, K. D., Vlaeyen, J. W. S., Van Zundert, J. & Cohen, S. P. Low back pain. _Lancet_ 398,
78–92 (2021). Article PubMed Google Scholar * Yan, J. et al. Cholesterol induces pyroptosis and matrix degradation via mSREBP1-driven endoplasmic reticulum stress in intervertebral disc
degeneration. _Front. Cell Dev. Biol._ 9, 803132 (2022). Article PubMed PubMed Central Google Scholar * Wang, Y. et al. Oxidative stress in intervertebral disc degeneration: molecular
mechanisms, pathogenesis and treatment. _Cell Prolif._ 56, e13448 (2023). Article CAS PubMed PubMed Central Google Scholar * Lyu, F. J. et al. Painful intervertebral disc degeneration
and inflammation: from laboratory evidence to clinical interventions. _Bone Res._ 9, 7 (2021). Article CAS PubMed PubMed Central Google Scholar * Qin, T. et al. MicroRNA-155 suppressed
cholesterol-induced matrix degradation, pyroptosis and apoptosis by targeting RORα in nucleus pulposus cells. _Cell Signal_ 107, 110678 (2023). Article CAS PubMed Google Scholar * Binch,
A. L. A., Fitzgerald, J. C., Growney, E. A. & Barry, F. Cell-based strategies for IVD repair: clinical progress and translational obstacles. _Nat. Rev. Rheumatol._ 17, 158–175 (2021).
Article PubMed Google Scholar * Chou, R., Atlas, S. J., Stanos, S. P. & Rosenquist, R. W. Nonsurgical interventional therapies for low back pain: a review of the evidence for an
American Pain Society clinical practice guideline. _Spine_ 34, 1078–1093 (2009). Article PubMed Google Scholar * Ferguson, S. J., Ito, K. & Nolte, L. P. Fluid flow and convective
transport of solutes within the intervertebral disc. _J. Biomech._ 37, 213–221 (2004). Article PubMed Google Scholar * Belavý, D. L. et al. Running exercise strengthens the intervertebral
disc. _Sci. Rep. UK_ 7, 45975 (2017). Article Google Scholar * Brisby, H. et al. The effect of running exercise on intervertebral disc extracellular matrix production in a rat model.
_Spine_ 35, 1429–1436 (2010). Article PubMed Google Scholar * Luan, S. et al. Running exercise alleviates pain and promotes cell proliferation in a rat model of intervertebral disc
degeneration. _Int J. Mol. Sci._ 16, 2130–2144 (2015). Article CAS PubMed PubMed Central Google Scholar * Sasaki, N. et al. Physical exercise affects cell proliferation in lumbar
intervertebral disc regions in rats. _Spine_ 37, 1440–1447 (2012). Article PubMed Google Scholar * Owen, P. J. et al. Exercise for the intervertebral disc: a 6-month randomised controlled
trial in chronic low back pain. _Eur. Spine J._ 29, 1887–1899 (2020). Article PubMed Google Scholar * Säämänen, A. M. et al. Effect of running exercise on proteoglycans and collagen
content in the intervertebral disc of young dogs. _Int. J. Sports Med._ 14, 48–51 (1993). Article PubMed Google Scholar * Pedersen, B. K. Muscles and their myokines. _J. Exp. Biol._ 214,
337–346 (2011). Article CAS PubMed Google Scholar * Severinsen, M. C. K. & Pedersen, B. K. Muscle-organ crosstalk: the emerging roles of myokines. _Endocr. Rev._ 41, 594–609 (2020).
Article PubMed PubMed Central Google Scholar * Yi, X. et al. Signaling metabolite β-aminoisobutyric acid as a metabolic regulator, biomarker, and potential exercise pill. _Front.
Endocrinol._ 14, 1192458 (2023). Article Google Scholar * Jung, T. W., Park, H. S., Choi, G. H., Kim, D. & Lee, T. β-aminoisobutyric acid attenuates LPS-induced inflammation and
insulin resistance in adipocytes through AMPK-mediated pathway. _J. Biomed. Sci._ 25, 27 (2018). Article PubMed PubMed Central Google Scholar * Zheng, X. et al. β-Aminoisobutyric acid
supplementation attenuated salt-sensitive hypertension in Dahl salt-sensitive rats through prevention of insufficient fumarase. _Amino Acids_ 54, 169–180 (2022). Article CAS PubMed Google
Scholar * Yu, Y. et al. Exercise-generated β-aminoisobutyric acid (BAIBA) reduces cardiomyocyte metabolic stress and apoptosis caused by mitochondrial dysfunction through the miR-208b/AMPK
pathway. _Front. Cardiovasc. Med._ 9, 803510 (2022). Article CAS PubMed PubMed Central Google Scholar * Vadalà, G. et al. Effect of Irisin on human nucleus pulposus cells: new insights
into the biological cross-talk between muscle and intervertebral disk. _Spine_ 48, 468–475 (2023). Article PubMed Google Scholar * Deng, X., Zhao, F., Kang, B. & Zhang, X. Elevated
interleukin-6 expression levels are associated with intervertebral disc degeneration. _Exp. Ther. Med._ 11, 1425–1432 (2016). Article CAS PubMed PubMed Central Google Scholar * Li, B.
et al. Reduced expression of insulin-like growth factor 1 receptor leads to accelerated intervertebral disc degeneration in mice. _Int. J. Immunopathol. Pharm._ 26, 337–347 (2013). Article
CAS Google Scholar * Yuan, J., & Ofengeim, D. A guide to cell death pathways. _Nat. Rev. Mol. Cell Biol._, https://doi.org/10.1038/s41580-023-00689-6. (2023). * Fan, H. et al.
Necroptosis of nucleus pulposus cells involved in intervertebral disc degeneration through MyD88 signaling. _Front. Endocrinol._ 13, 994307 (2022). Article Google Scholar * Deng, Z. et al.
BRD9 inhibition attenuates matrix degradation and pyroptosis in nucleus pulposus by modulating the NOX1/ROS/NF-κB axis. _Inflammation_ 46, 1002–1021 (2023). Article CAS PubMed Google
Scholar * Kuriakose, T. et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. _Sci. Immunol._ 1, aag2045 (2016).
Article PubMed PubMed Central Google Scholar * Malireddi, R. K. S., Kesavardhana, S. & Kanneganti, T. D. ZBP1 and TAK1: master regulators of NLRP3 inflammasome/pyroptosis, apoptosis,
and necroptosis (PAN-optosis). _Front. Cell Infect. Microbiol._ 9, 406 (2019). Article CAS PubMed PubMed Central Google Scholar * Wang, Y. & Kanneganti, T. D. From pyroptosis,
apoptosis and necroptosis to PANoptosis: a mechanistic compendium of programmed cell death pathways. _Comput. Struct. Biotechnol. J._ 19, 4641–4657 (2021). Article CAS PubMed PubMed
Central Google Scholar * Karki, R. et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes.
_Cell_ 184, 149–168.e17 (2021). Article CAS PubMed Google Scholar * Pan, H. et al. The mechanisms and functions of TNF-α in intervertebral disc degeneration. _Exp. Gerontol._ 174, 112119
(2023). Article CAS PubMed Google Scholar * Kitase, Y. et al. β-aminoisobutyric acid, l-BAIBA, is a muscle-derived osteocyte survival factor. _Cell Rep._ 22, 1531–1544 (2018). Article
CAS PubMed PubMed Central Google Scholar * Roberts, L. D. et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with
cardiometabolic risk factors. _Cell Metab._ 19, 96–108 (2014). Article CAS PubMed PubMed Central Google Scholar * Salminen, A., Hyttinen, J. M. & Kaarniranta, K. AMP-activated
protein kinase inhibits NF-κB signaling and inflammation: impac on healthspan and lifespan. _J. Mol. Med._ 89, 667–676 (2011). Article CAS PubMed Google Scholar * Belavý, D. L.,
Albracht, K., Bruggemann, G. P., Vergroesen, P. P. & van Dieën, J. H. Can exercise positively influence the intervertebral disc? _Sports Med._ 46, 473–485 (2016). Article PubMed Google
Scholar * Li, B., Yang, Y., Wang, L. & Liu, G. Stem cell therapy and exercise for treatment of intervertebral disc degeneration. _Stem Cells Int._ 2021, 7982333 (2021). Article PubMed
PubMed Central Google Scholar * Wang, D. L., Jiang, S. D. & Dai, L. Y. Biologic response of the intervertebral disc to static and dynamic compression in vitro. _Spine_ 32, 2521–2528
(2007). Article PubMed Google Scholar * Hangai, M. et al. Lumbar intervertebral disk degeneration in athletes. _Am. J. Sports Med._ 37, 149–155 (2009). Article PubMed Google Scholar *
Videman, T. et al. Lifetime exercise and disk degeneration: an MRI study of monozygotic twins. _Med. Sci. Sports Exerc._ 29, 1350–1356 (1997). Article CAS PubMed Google Scholar *
Videman, T. et al. The long-term effects of physical loading and exercise lifestyles on back-related symptoms, disability, and spinal pathology among men. _Spine_ 20, 699–709 (1995). Article
CAS PubMed Google Scholar * Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. _Nat. Rev. Endocrinol._ 8, 457–465 (2012). Article
CAS PubMed Google Scholar * Zhou, W. et al. Exercise-induced FNDC5/irisin protects nucleus pulposus cells against senescence and apoptosis by activating autophagy. _Exp. Mol. Med._ 54,
1038–1048 (2022). Article CAS PubMed PubMed Central Google Scholar * Chen, J. et al. IL-6/YAP1/β-catenin signaling is involved in intervertebral disc degeneration. _J. Cell Physiol._
234, 5964–5971 (2019). Article CAS PubMed Google Scholar * Huang, Z. Q., Zheng, Z. M. & Yan, J. Transgenic expression of human IGF1 in intervertebral degenerative discs. _J. Int.
Med. Res._ 39, 446–455 (2011). Article CAS PubMed Google Scholar * Stautemas, J. et al. Acute aerobic exercise leads to increased plasma levels of R- and S-β-aminoisobutyric acid in
humans. _Front. Physiol._ 10, 1240 (2019). Article PubMed PubMed Central Google Scholar * Kodama, J., Wilkinson, K. J. & Otsuru, S. Nutrient metabolism of the nucleus pulposus: a
literature review. _N. Am. Spine Soc. J._ 13, 100191 (2022). PubMed PubMed Central Google Scholar * Russell, A. P. et al. Endurance training in humans leads to fiber type-specific
increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. _Diabetes_ 52, 2874–2881
(2003). Article CAS PubMed Google Scholar * Egan, B. & Zierath, J. R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. _Cell Metab._ 17, 162–184
(2013). Article CAS PubMed Google Scholar * Tanianskii, D. A., Jarzebska, N., Birkenfeld, A. L., O’Sullivan, J. F. & Rodionov, R. N. Beta-aminoisobutyric acid as a novel regulator of
carbohydrate and lipid metabolism. _Nutrients_ 11, 524 (2019). Article CAS PubMed PubMed Central Google Scholar * Townsend, L. K. & Steinberg, G. R. AMPK and the endocrine control
of metabolism. _Endocr. Rev._ 44, 910–933 (2023). Article PubMed Google Scholar * Xie, C. et al. Apigenin alleviates intervertebral disc degeneration via restoring autophagy flux in
nucleus pulposus cells. _Front. Cell Dev. Biol._ 9, 787278 (2022). Article PubMed PubMed Central Google Scholar * Du, J. et al. CB2R attenuates intervertebral disc degeneration by
delaying nucleus pulposus cell senescence through AMPK/GSK3β pathway. _Aging Dis._ 13, 552–567 (2022). Article PubMed PubMed Central Google Scholar * Zhang, Z. et al. Orientin
downregulating oxidative stress-mediated endoplasmic reticulum stress and mitochondrial dysfunction through AMPK/SIRT1 pathway in rat nucleus pulposus cells in vitro and attenuated
intervertebral disc degeneration in vivo. _Apoptosis_ 27, 1031–1048 (2022). Article CAS PubMed Google Scholar * Zhang, Z. et al. β-aminoisobutyrics acid, a metabolite of BCAA, activates
the AMPK/Nrf-2 pathway to prevent ferroptosis and ameliorates lung ischemia-reperfusion injury. _Mol. Med._ 29, 164 (2023). Article CAS PubMed PubMed Central Google Scholar * Jie, F. et
al. Stigmasterol attenuates inflammatory response of microglia via NF-κB and NLRP3 signaling by AMPK activation. _Biomed. Pharmacother._ 153, 113317 (2022). Article CAS PubMed Google
Scholar * Chen, S. et al. Grem1 accelerates nucleus pulposus cell apoptosis and intervertebral disc degeneration by inhibiting TGF-β-mediated Smad2/3 phosphorylation. _Exp. Mol. Med._ 54,
518–530 (2022). Article CAS PubMed PubMed Central Google Scholar * Cao, C. et al. Inflammatory stimulation mediates nucleus pulposus cell necroptosis through mitochondrial function
disfunction and oxidative stress pathway. _Front. Biosci._ 27, 111 (2022). Article CAS Google Scholar * Huang, Y. et al. Nicotinamide phosphoribosyl transferase controls NLRP3
inflammasome activity through MAPK and NF-κB signaling in nucleus pulposus cells, as suppressed by melatonin. _Inflammation_ 43, 796–809 (2020). Article CAS PubMed Google Scholar * Chen,
W. et al. Rosuvastatin suppresses TNF-α-induced matrix catabolism, pyroptosis and senescence via the HMGB1/NF-κB signaling pathway in nucleus pulposus cells. _Acta Biochim. Biophys. Sin._
55, 795–808 (2023). CAS PubMed PubMed Central Google Scholar * Li, F. et al. Arginase II promotes intervertebral disc degeneration through exacerbating senescence and apoptosis caused by
oxidative stress and inflammation via the NF-κB pathway. _Front. Cell Dev. Biol._ 9, 737809 (2021). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (No.82372437, No.82202653), the Guangdong Basic and Applied Basic Research Foundation (No.2024A1515010719,
No.2024A1515012963), the International-Science Technology Cooperation Program of Guangdong Province (No.2023A0505050136), and the Science and Technology Program of Guangzhou (No.
2024A04J4684). AUTHOR INFORMATION Author notes * These authors contributed equally: Tianyu Qin, Ming Shi, Chao Zhang. AUTHORS AND AFFILIATIONS * Department of Orthopedics, The Eighth
Affiliated Hospital of Sun Yat-sen University, Shenzhen, 528406, China Tianyu Qin, Ming Shi & Song Jin * Department of Spine Surgery, Sun Yat-sen Memorial Hospital of Sun Yat-sen
University, Guangzhou, 510120, China Tianyu Qin, Ming Shi, Chao Zhang, Jiajun Wu, Zhengqi Huang, Xiaohe Zhang, Shuangxing Li, Yuliang Wu, Weitao Han, Bo Gao, Kang Xu & Wei Ye * Guangdong
Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Medical Research Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 510120, China Tianyu
Qin, Ming Shi, Chao Zhang, Jiajun Wu, Zhengqi Huang, Xiaohe Zhang, Shuangxing Li, Yuliang Wu, Weitao Han, Bo Gao, Kang Xu & Wei Ye Authors * Tianyu Qin View author publications You can
also search for this author inPubMed Google Scholar * Ming Shi View author publications You can also search for this author inPubMed Google Scholar * Chao Zhang View author publications You
can also search for this author inPubMed Google Scholar * Jiajun Wu View author publications You can also search for this author inPubMed Google Scholar * Zhengqi Huang View author
publications You can also search for this author inPubMed Google Scholar * Xiaohe Zhang View author publications You can also search for this author inPubMed Google Scholar * Shuangxing Li
View author publications You can also search for this author inPubMed Google Scholar * Yuliang Wu View author publications You can also search for this author inPubMed Google Scholar *
Weitao Han View author publications You can also search for this author inPubMed Google Scholar * Bo Gao View author publications You can also search for this author inPubMed Google Scholar
* Kang Xu View author publications You can also search for this author inPubMed Google Scholar * Song Jin View author publications You can also search for this author inPubMed Google Scholar
* Wei Ye View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Wei Ye, Song Jin and Kang Xu conceived and designed the study. Tianyu Qin, Ming
Shi and Chao Zhang performed the experiments. Tianyu Qin, Jiajun Wu and Zhengqi Huang analyzed the results. Tianyu Qin, Shuangxing Li and Xiaohe Zhang were responsible for data collection.
Yuling Wu, Weitao Han and Bo Gao contributed to the literature search and specimen collection. Wei Ye, Kang Xu and Tianyu Qin wrote and edited the manuscript. CORRESPONDING AUTHORS
Correspondence to Kang Xu, Song Jin or Wei Ye. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICS APPROVAL AND CONSENT TO PARTICIPATE Studies
involving human participants were reviewed and approved by the Ethics Committee of Sun Yat-sen Memorial Hospital [SYSKY-2023-956-01]. The animal study was reviewed and approved by the
Institutional Animal Care and Use Committee of Sun Yat-sen University (SYSU-IACUC-2023-001583). ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Qin, T., Shi, M., Zhang, C. _et al._ The muscle–intervertebral disc
interaction mediated by L-BAIBA modulates extracellular matrix homeostasis and PANoptosis in nucleus pulposus cells. _Exp Mol Med_ 56, 2503–2518 (2024).
https://doi.org/10.1038/s12276-024-01345-5 Download citation * Received: 05 April 2024 * Revised: 13 July 2024 * Accepted: 11 August 2024 * Published: 07 November 2024 * Issue Date: November
2024 * DOI: https://doi.org/10.1038/s12276-024-01345-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative