A metallic anti-biofouling surface with a hierarchical topography containing nanostructures on curved micro-riblets
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Metallic surface finishes have been used in the anti-biofouling, but it is very difficult to produce surfaces with hierarchically ordered structures. In the present study, anti-biofouling
metallic surfaces with nanostructures superimposed on curved micro-riblets were produced via top-down fabrication. According to the attachment theory, these surfaces feature few attachment
points for organisms, the nanostructures prevent the attachment of bacteria and algal zoospores, while the micro-riblets prohibit the settlement of macrofoulers. Anodic oxidation was
performed to induce superhydrophilicity. It forms a hydration layer on the surface, which physically blocks foulant adsorption along with the anti-biofouling topography. We characterized the
surfaces via scanning electron and atomic force microscopy, contact-angle measurement, and wear-resistance testing. The contact angle of the hierarchical structures was less than 1°.
Laboratory settlement assays verified that bacterial attachment was dramatically reduced by the nanostructures and/or the hydration layer, attributable to superhydrophilicity. The
micro-riblets prohibited the settlement of macrofoulers. Over 77 days of static immersion in the sea during summer, the metallic surface showed significantly less biofouling compared to a
surface painted with an anticorrosive coating.
Biofouling is the unwanted accumulation of biological materials on underwater structures. Buildup of such materials causes severe malfunction of heat exchangers, ships’ hulls, and offshore
plants.1 Biofouling increases the hydrodynamic volume and friction of submerged hulls, thus increasing power consumption.2 For heat exchangers, the flow rate decreases when organisms
accumulate in the water channel. In the absence of anti-biofouling treatment, the channel becomes completely blocked, which leads to catastrophic failure.3,4 Although various chemical
coatings containing biocides help to prevent marine fouling, their use has been restricted by the European Union and the International Maritime Organization because of major negative effects
on marine ecosystems.5 The prohibition of toxic chemicals in antifouling coatings has accelerated the search for environmentally friendly alternatives. One such alternative is a
fouling-release coating that modifies the surface topography and chemistry to prevent microbial settlement and attachment.
Comprehensive and methodical studies regarding engineered anti-biofouling topographies have been conducted by numerous researchers. It is commonly accepted (and experimentally proven) that
an appropriate micro- or nanosurface topography modified either hydrophobically or hydrophilically reduces biofouling by various life forms via the following mechanisms.
Surface modification (hydrophobic, hydrophilic treatment) can induce antifouling. A hydrophobic surface reduces polar and hydrogen-bonding interactions with biofoulants and increases the
surface-to-foulant separation distance increasing the energy required and the kinetic barriers that organisms must overcome when attaching to the surface; a hydrophilic surface strongly bind
water molecules and form a hydration layer that physically blocks foulant adsorption. Yoon et al prepared (separate) superhydrophobic and superhydrophilic surfaces by annealing
stainless-steel plates with carbon nanotube-polytetrafluoroethylene and titanium dioxide (TiO2), respectively.6 Bacterial growth on both surfaces was examined under static and dynamic
conditions in a flow channel. Under static conditions, bacterial growth was higher on the superhydrophobic surface than on the superhydrophilic surface. The hydration layer of the
superhydrophilic surface reduced bacterial growth.
If the nanostructures are smaller than the biofouler, bioadhesion and subsequent biofilm development can be halted. Seyfi et al. developed antibacterial superhydrophobic coatings based on
polydimethylsiloxane (PDMS)/silver phosphate nanocomposites.7 Antimicrobial properties were enhanced by the low surface free energy and appropriate roughness; the surface protrusions were
smaller than bacteria.
When the fouling involves larger (marine) microorganisms, topographic surfaces having a single length scale are unlikely to be as effective as conventional antifouling coatings with biocides
because the organisms that engage in biofouling are diverse. Several hierarchical ordered topographies have been considered because bacteria, algae, and barnacles exhibit different settling
characteristics.8,9 Inspired by shark skin, Schumacher et al. created hierarchical topographies, in which larger microgratings were superimposed on smaller micro-riblets. These reduced
settlement of a fouling plant (Ulva) and an animal of interest (Balanus amphitrite). The vertical walls of the ridges, which lacked smaller micro-riblets, were favored attachment sites for
Ulva zoospores. Thus, ridge walls lacking anti-biofouling patterns should be avoided.8
The importance of ordered structures was also emphasized in the work of Diaz et al.10 According to their work, the ordered structures hinder the formation of ordered aggregates of bacteria
while well-defined aggregates of bacteria were formed on the randomly oriented structures. In other words, the ordered structures can pose better functionality over random structures if they
are engineered for specific functions. This was also confirmed by the work of Jung et al.11 In their anti-icing experiment, the ordered nanostructures showed better anti-icing performance
over randomly oriented nanostructures with similar dimensions to the ordered one. Thus, a hydrophobic or hydrophilic surface with a hierarchically ordered topography over the entire surface
can improve anti-biofouling.
Any antifouling system must be mechanically and chemically stable in the long term; the marine environment is harsh, featuring constant exposure to salt water, sunlight, and abrasion. It has
been challenging to prepare durable functional surfaces. Polymer-based antifouling coatings have been extensively studied; they are non-toxic, simple to fabricate, and well-suited to
large-scale marine applications such as ships’ hulls and submerged buildings. Although polymer mechanical properties and surface chemistry can be enhanced by combination with organic,
inorganic, or metallic fillers, most polymers can be vulnerable to long-term exposure to solar UV light.12 Such exposure causes photochemical damage near the surface; the composites then
degrade. UV exposure also reduces the polymer molecular weight and thus renders it brittle, compromising the physical and mechanical properties. Apart from UV damage, some polymers such as
polyethylene glycol (PEG) swell in the marine environment, compromising the mechanical properties. Also, adhesion of the coating to the metallic/composite substrate must be considered.13 To
ensure durability, metals are very useful because of their versatile chemical, physical and mechanical properties. However, metals have seldom been used to fabricate engineered surfaces
because creation of functional materials featuring ordered micro-/nanostructures is demanding in terms of both energy and time, especially if traditional processes are employed, which
require a great deal of energy; metals exhibit high melting points and poor machinability.14,15 Also (and very importantly), metal fabrication is generally not scalable. The formation of
ordered nanostructures on the tops of curved structures is even more challenging.16 Even though such structures can be a good candidate for the ridge-less hierarchical structure emphasized
by Schumacher et al., the formation of ordered nanostructures on the tops of curved structures is challenging.
Several efforts have been made to derive hierarchical metallic surfaces via laser processing, synthesis, and etching.17 The hierarchical topographies fabricated by the laser-etching process
were reported by numerous researchers since the method is a simple and maskless process. For example, an anti-icing aluminum alloy surface with hierarchical topography fabricated by a
picosecond laser was presented.18 The micro-scale line structures can be uniform since it was formed by predetermined line-by-line laser irradiation path. However, the nanostructures were
oriented randomly since they were formed during non-uniform vaporization of the melt and rapid solidification. The one-step hydrothermal synthesis19 and the HCl etching followed by H2O
treatment20 were also reported for the simple fabrication of metal surfaces with micro-nano hierarchical structures. Although the above-mentioned processes are scalable, simple, it is not
easy to obtain highly ordered nanostructures by the processes, which are an important factor in anti-biofouling topography.
In this study, we developed anti-biofouling metallic surfaces with ordered nanostructures superimposed on curved micro-riblets as shown in Fig. 1 In this study, we developed anti-biofouling
metallic surfaces with nanostructures superimposed on curved micro-riblets. According to the attachment theory, the structures with smaller spacings than those of the organisms do not favor
attachment because the structures make fewer contacts with the cells. In this manner, our nanostructures can reduce bacterial and algal zoospore attachment, and the micro-riblets prohibit
larval fouling. We subjected the metal surface to anodic oxidation (to induce superhydrophilicity). The surface strongly binds water molecules that form a hydration layer, which physically
blocks foulant adsorption. Given such strong surface hydration, it is difficult for biologicals (e.g., protein molecules, bacteria, and marine organisms) to foul the surfaces. Nanostructures
were formed over the entire surface, including the micro-riblet peaks and valleys, and the bottom. Nanostructures were formed over the entire surface, including the micro-riblet peaks and
valleys, and the bottom. We take a top-down approach. Laser interference lithography and conventional lithography were used for initial fabrication of the nanostructures and micro-riblets.
To superimpose the nanostructures on the tops of the micro-riblets, the elastic modulus of the PDMS nanomold was modified to enhance conformal contact between the micro-riblets and the
nanostructures during imprinting. Then, master hierarchical structures were replicated via pulsed reverse current (PRC) nickel electrochemical deposition; this enhances durability.21
Usefully, the replication process employs a reusable metallic master (Fig. S1); the process is thus practical. Once a reusable metallic master is prepared, the metallically engineered
hierarchical surface can be replicated repeatedly (in one step) for as long as the passivation layer endures. Note that we eliminate the need for a seed layer; this is expensive and renders
it difficult to fabricate large-area samples. Although nickel is easily oxidized in air, anodic oxidation22 was performed to prepare a uniform nickel oxide layer and induce
superhydrophilicity.23 We used scanning electron microscopy (SEM), atomic force microscopy (AFM), contact-angle measurement, and wear-resistance tests to characterize the surface. The
measured static contact angle of the hierarchical structures was less than 1°. Settlement assays were performed using marine bacteria (Flavobacterium sp.) and the larvae of a sessile animal
(Bugula neritina). We also performed static immersion tests. After 77 days of immersion at sea, we compared our surface to a surface coated with anticorrosive paint. Both the laboratory
assays and the static immersion test confirmed that our surface exhibited excellent anti-biofouling properties.
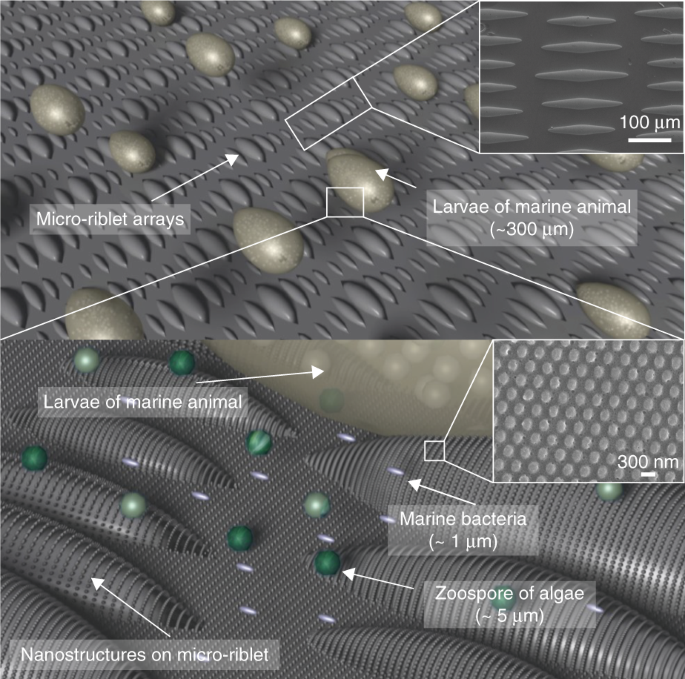
A schematic of metallic hierarchical structures with nanostructures on micro-riblets that prevent the settlement of various marine organisms including bacteria, algae, and marine animals.
Given the complexity of the marine environment, it is difficult to identify a single antifouling topography that meets all requirements; composite antifouling coatings that combine multiple
antifouling principles are required. We used two mechanisms to combat the attachment of various oceanic species. One theory that seeks to explain adhesion of cells to a substrate is the
attachment point theory.24 When the structural spacing is larger than the sizes of the organisms, cells contact multiple potential attachment points. In such a case, cells often fit into
grooves of the structures, and are thus protected from removal by water flow. On the other hand, structures with smaller spacings than those of the organisms do not favor attachment because
the structures make fewer contacts with the cells. Thus, our nanostructures can reduce bacterial and algal zoospore numbers, and the micro-riblets prohibit larval fouling. Another mechanism
of antifouling is the presence of a hydration layer. A superhydrophilic surface strongly binds water molecules that form a hydration layer, which physically blocks foulant adsorption. If
surface hydration is strong, biologicals (e.g., protein molecules, bacteria, and marine organisms) find it difficult to displace the strongly bonded surface water molecules and thus cannot
foul the surface. Our antifouling strategy features a surface with few attachment points for organisms and a hydration layer.
The dimensions of the micro-riblet structure were biomimetically determined by mimicking the skin of a shortfin mako shark (Isurus oxyrinchus) to simultaneously induce drag-reduction and
anti-biofouling properties. The drag reduction of these structures was previously studied,25 which possibly aid additional antifouling because reduced drag might result in less fouling since
an increased flow speed gives microorganisms less residential time to cause fouling. Considering the desired drag reduction, blade or saw-toothed structures were potential candidates but
were ultimately excluded, due to their geometric instability and incompatibility with nanostructures superposition.26 The longitudinal and transverse lengths of the largest riblet pattern
were 220 and 30 μm, and those of the smallest pattern were 130 and 25 μm. The largest gap in the micro-riblet array was about 100 µm, and the smallest was about 35 µm. The pitch of
nanostructures was determined as 300 nm, as this period showed antimicrobial characteristics and low adhesion in the previous work.27 The length of bacteria is typically in the range of 1–3
μm and the size of larvae is in the range of 320 μm. Thus, the presented micro-riblets and nanostructures have fewer attachment points against the target organisms compared to the smooth
surface. With fewer attachment points with organisms, the nanostructures can prevent the attachment of bacteria and algal zoospores, while the micro-riblets can prohibit the settlement of
macrofoulers.
In terms of surface modification, we decided to induce superhydrophilicity to form a hydration layer. With strong surface hydration, it is hard for biological media (e.g., protein molecules,
bacteria, marine organisms, etc.) to foul on the surfaces. Nickel oxide is hydrophilic.23 As the initial contact angle of bare nickel coated with nickel oxide was 56.7°, the apparent
contact angle decrease as the roughness increase according to the Wenzel equation.28 As we superimposed the nanostructures on the micro-riblets, the hierarchical structures exhibited
superhydrophilicity.
While our future works include a systematic variation of micro-and nano-patterns to investigate the effect of how variations would affect the overall results, there are several studies
showing how dimensional variations in nanostructures affects the bactericidal property or anti-adhesion of bacteria.
The effect of topographical geometry on mechano-bactericidal efficacy can be found in the following two studies. In the finding of Dickson et al.,29 optimal nanopillar spacing was between
130 and 380 nm against Escherichia coli proliferation. The larger periodicity (e.g., 600 nm) exhibited less bactericidal. Wu et al. investigated the bactericidal property of various Au
nanostructures including nanopillars (diameter: 50 nm), nanorings (diameter: 100–200 nm), nanonuggets (diameter: 100–200 nm). Regardless of their shapes and diameter, all three surfaces have
a killing efficiency of more than 99%. However, by decreasing the height of nanostructures to 50 nm, the surface lost bactericidal property.30
The dimensional variation of nanostructures for anti-adhesion of bacteria, the concept we used in the study, was also reported.31 Three nanostructured-Si surfaces were prepared with periods
of 200, 400, and 800 nm. The pillar height of all surfaces was 500 nm. The contact area fraction with bacteria for each of the nanosurfaces was 0.16, 0.02, and 0.11, respectively.
Staphylococci experience weaker adhesion forces for all three nanosurfaces compared to a smooth surface, however, there was no significant difference in the adhesion force between the
nanosurfaces, despite the difference in the contact area fractions. The important finding of their work lies in the bacterial detachment from a nanosurface in slight shear conditions (e.g.,
rinsing or dipping).
In terms of geometrical durability, instead of using sharp nanostructures for mechano-bactericidal efficacy, relatively “blunt” nanostructures with anti-adhesion of bacteria may be more
suitable for use in harsh external conditions. It is expected such nanostructures experience repeated shear conditions by the wave, resulting in the detachment of marine bacteria. A
systematic variation of the presented micro-and nano-patterns for optimal anti-biofouling is the subject of ongoing research.
To ensure that nanostructures form uniformly on the tops and bottoms of curved micro-riblets, imprinting must be optimized. The key parameters to control during imprinting of nanostructures
onto micro-riblets are the elastic modulus of the PDMS nanomold and the imprinting pressure. The elastic modulus depends on the concentration of the cross-linker within the PDMS.25 A
relatively low elastic modulus provides better conformal contact between the nanomold and the micro-riblets than a high elastic modulus; the mold is less stiff than in the former case. We
used a cross-linker concentration of 5% (w/w) because PDMS curing was difficult at lower concentrations. We then explored the effect of imprinting pressure on the dimensions of the riblets
and nanostructures (Fig. 2a). The imprinting pressure affected micro-riblet length; for example, a relatively low pressure (0.424 N/cm2) was associated with a thicker residual layer and a
shorter micro-riblet length (Fig. 2b). We found that an imprinting pressure of 5 N/cm2 deformed the nanostructures, or prohibited nanostructure formation. The resin did not remain on the
tops of the micro-riblets, instead flowing to the bottoms, because the pressure was concentrated on micropattern protrusions (Fig. 2c). The optimal imprinting pressure was about 3.7 N/cm2,
at which pressure nanostructures formed uniformly on all surfaces including the tops of the micro-riblets, the bottoms, and the interface (the micro-riblet valleys). Figure 3a shows an image
of the micro-riblets that served as the substrate for imprinting. Figure 3b shows the nanostructures in the PDMS mold. Figure 3c shows the metallic hierarchical topographies fabricated
using metallic micro-riblets and the PDMS mold. The nanostructures formed uniformly over the entire surface, including the tops and valleys of the micro-riblets. The nanostructure height in
the PDMS nanomold was 250 nm. The heights of the nanostructures on the bottom surface and on the tops of the micro-riblets after imprinting were about 160 nm and 130 nm, respectively, as
confirmed in Fig. 3d, e. The difference in height between the mold and the replica reflects volumetric shrinkage and differences in cavity filling. During UV imprinting, volumetric shrinkage
upon the polymerization of UV resin is inevitable. In addition, the imprint pressure affects cavity filling, and thus the imprinted pattern volume. In general, a higher imprinting pressure
ensures better cavity filling. However, we could not apply an imprinting pressure above 3.724 N/cm2; cavity filling was thus incomplete. In terms of the top and bottom nanostructure heights,
the local imprinting pressure was higher at the tops of the micro-riblets compared to the bottoms; resin may have flowed into the bottoms. Thus, the imprinted nanostructure volume at the
tops of the micro-riblets was less than that at the bottoms, explaining the lower height. Cavity filling is enhanced by higher pressure; however, a relatively low imprinting
pressure should be imparted to transcribe the nanostructures to the micro-riblets during imprinting processes such as that used in this study. Enhancement of transcribability is a subject of
ongoing research.
a Length of the longest micro-riblet as a function of imprinting pressure. A PDMS mold with a lower elastic modulus provides better conformal contact between the mold and the micro-riblets
than a mold with a higher elastic modulus. Scanning electron micrographs of the imprints obtained using PDMS molds with the 5% (w/w) cross-linker. b 0.424 N/cm2 and c 5 N/cm2.
Scanning electron micrographs of a metallic micro-riblets, b nanostructures from the PDMS mold, and c the metallic hierarchical topographies fabricated using metallic micro-riblets and the
PDMS mold. In these topographies, the longitudinal and transverse lengths of the largest (center) riblet pattern were 220 and 30 μm, and those of the smallest pattern were 130 and 25 μm. d
An atomic force microscopy 3D image of nanostructures on the bottom. e Surface profiles of nanostructures on the tops of the micro-riblets and on the bottoms respectively. The lattice
constant and height of the bottom nanostructures were 330 and 160 nm, respectively. The nanostructures on the micro-riblets were 130 nm in height.
Any antifouling system must be mechanically and chemically stable in the long term; the marine environment is harsh, featuring constant exposure to salt water, sunlight, and abrasion. In
addition, coatings on fast (over 15 knots) water vehicles experience high levels of frictional stress. It is challenging to combine functional excellence with durability. To ensure
durability, metals or metal oxides are very useful because of their versatile chemical, physical and mechanical properties. However, metals have seldom been used to fabricate engineered
surfaces because creation of functional materials featuring ordered micro-/nano-structures is demanding in terms of both energy and time, especially if traditional processes are employed,
which require a great deal of energy; metals exhibit high melting points and poor machinability.14,15 Also (and very importantly), metal fabrication is generally not scalable. Here, we
fabricated a metallic surface with hierarchical structures using PRC nickel electrochemical deposition, which ensures durability.21 The thickness of the metallic surface is about 100 μm.
Such a thin metal can be wrapped around a roll of diameter 50 mm without deforming the micro- or nano-structures, as demonstrated previously.32 The radius of curvature is extremely large
compared to that of the flat surface of a ship or offshore platform; the radii of curvature of such structures are rather small. Thus, we believe that the thin metallic surface can be
conformally integrated into curved surfaces with the exception of surfaces that are markedly curved (e.g., the front or rear of a hull). We used nickel since it has high hardness,
anti-corrosion,33 antifouling characteristics34 that may make it suitable for use under harsh external conditions of the sea. Nickel is one possible candidate for the presented fabrication
process; nickel alloy such as nickel–copper35 or nickel– phosphorus36 can be a promising candidate for enhanced anti-corrosion and antifouling properties.
Figure 4 shows the contact-angle measurements before and after sandpaper abrasion. Nickel served as the substrate. Although nickel is readily oxidized in air, we performed anodic oxidation
to create a uniform nickel oxide layer of 2–4 nm on the nickel surface.22 The Wenzel equation28 indicates that the initial contact angle (the Young contact angle) and roughness determine
surface wettability. If the initial contact angle is