Distributed implantation of a flexible microelectrode array for neural recording
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Flexible multichannel electrode arrays (fMEAs) with multiple filaments can be flexibly implanted in various patterns. It is necessary to develop a method for implanting the fMEA in
different locations and at various depths based on the recording demands. This study proposed a strategy for reducing the microelectrode volume with integrated packaging. An implantation
system was developed specifically for semiautomatic distributed implantation. The feasibility and convenience of the fMEA and implantation platform were verified in rodents. The acute and
chronic recording results provied the effectiveness of the packaging and implantation methods. These methods could provide a novel strategy for developing fMEAs with more filaments and
recording sites to measure functional interactions across multiple brain regions. SIMILAR CONTENT BEING VIEWED BY OTHERS MINIMALLY-INVASIVE INSERTION STRATEGY AND IN VIVO EVALUATION OF
MULTI-SHANK FLEXIBLE INTRACORTICAL PROBES Article Open access 23 September 2021 FLEXIBLE MULTICHANNEL ELECTRODES FOR ACUTE RECORDING IN NONHUMAN PRIMATES Article Open access 20 July 2023
FULLY FLEXIBLE IMPLANTABLE NEURAL PROBES FOR ELECTROPHYSIOLOGY RECORDING AND CONTROLLED NEUROCHEMICAL MODULATION Article Open access 27 June 2024 INTRODUCTION Microelectrode arrays made of
silicon or other stiff materials have to be implanted with the original topology1,2,3,4,5. A flexible multichannel electrode array (fMEA) with the same topology can be placed in the cortex
at various positions within a certain range. The distance between the filaments and the depth of each filament can be adjusted based on the requirements6,7. Because of its flexibility, the
fMEA can be implanted in various brain regions. We term this process distributed implantation. Direct implantation is infeasible due to the lack of required stiffness. Other materials or
methods must be used to assist with fMEA implantation8,9,10,11,12,13. The injection is a promising implantation method. However, current injection technology requires complex preparation and
a complicated packaging process after implantation, and the brain is vulnerable to overpressure14,15,16,17,18,19. Reinforced materials are popular for assisting with fMEA implantation.
Degradable materials such as polyethylene glycol (PEG) or fibroin can be coated on the outside of the flexible filament to temporarily reinforce the stiffness20,21,22. After implantation,
the coating material degrades and is absorbed by body tissue. However, in many cases, the stiffness of the reinforcing material is insufficient, which increases the difficulty of
implantation. The reinforcing material needs to degrade and be absorbed, which may take time and result in toxicity23,24. Another popular method involves inserting an fMEA into the cortex
with stiff shuttling probes or microneedles7,8,9,10,25,26. The fMEA filament is fixed on a shuttle probe6,26,27. However, there is usually more than one leg or filament on the fMEA7,11. In
that case, complicated manual operations are required to individually align and fix the flexible shank on the shuttle probe26. Another option is to simultaneously align several filaments to
the same number of shuttle probes and glue them together7. However, as the number of shanks increases, the current operational methods become incapable of implanting them effectively and
safely. At present, neither the reinforced material nor the pre-glued shuttle probe satisfies the demands of fMEA distributed implantation. Elon Musk reported an implant known as Robert,
which can automatically implant individual fMEA filaments6. However, Robert is too complex and expensive to be widely used. An fMEA is composed of two parts: the implant part and the
connector part. The recording sites are located at the front end of the implant part. The connector part, which is often soldered to a multichannel plug, remains outside and is mounted on
the head. A bundle of wire lines connects the fMEA to the socket of a multichannel recording system. A microelectromechanical system (MEMS) process is commonly used to fabricate the fMEA. To
minimize the volume of the implant part, the line width and line space of this part can be reduced to one micrometer or the nanometer scale8,9,26,28,29. However, the line spacing and
dimension of the connector part must be increased to fit a plug connector11,28. A typical miniature plug connector, such as the Omnetics connector, a common interface for implant electrodes,
has a minimum pin spacing of 640 μm. A 32 pin double-row Omnetics connector is 3 mm wide and 10 mm long. As the number of recording sites on the fMEA increases, the connector part can
increase to an unreasonably large size28. A potential solution for improving fMEAs was developed in this paper. To allow for simple and practicable implantation, homemade implanting tools,
combined with the guide hole at the front end of the implant filaments, can semiautomatically catch and implant individual fMEA threads. The position and depth of each thread in the fMEA can
be controlled with this tool. This reduces the manual operation while improving the implantation accuracy and speed. To reduce the fMEA volume necessary for plug-socket fan-out methods, we
propose a direct interconnection method. The specially designed pads at the connector part of the fMEA, combined with the modified gold ball bonding process, connect the fMEA and the
amplifier. This method eliminates the need for pairs of plug-socket connectors. The proposed implantation and interconnection methods provide a solution for developing fMEAs with multiple
filaments and recording sites. RESULTS FABRICATION AND PACKAGING OF THE FMEA The Polyimide (PI) 2600 series was chosen as the flexible substrate due to its excellent biocompatibility and
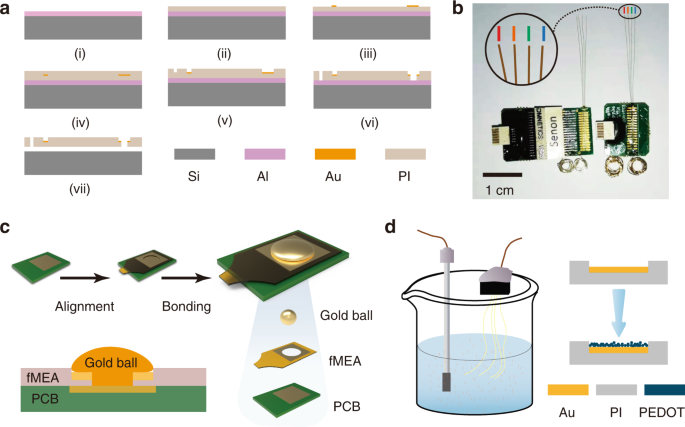
flexibility. The device has a PI–metal–PI sandwich structure. The fabrication process of the fMEA is shown in Fig. 1a. The fMEA had four filaments and eight recording sites on each filament,
as shown in Fig. 1b and Fig. S1. The filament was ~2.5 cm in length. Thus, it was long enough to be implanted in any area of the rat brain using the semiautomatic implantation platform. The
width of the filament was only 70 μm to minimize the volume of the implant part. The recording sites were 10 μm long and arranged in a line along the filament. The distance between the
recording sites was 100 μm. A loop at the front end of each filament was fabricated as a handle to assist with implantation. The loop was surrounded and marked by a gold ring to facilitate
observation. The square bonding pad had a hollow round hole in the center to facilitate small-scale integrated packaging. To ensure that the filament had enough mechanical strength to
withstand clamping, dragging, and pulling during the operation, polyimide with a tensile strength of 350 MPa was chosen. Flexible microelectrodes with a thickness of 6 μm were fabricated,
and the bending stiffness of the thin-film filament was 1.07 × 10−11 Nm2 (Supplementary Methods). By adjusting the spin-coating parameters, an fMEA with a thickness of 2.4 μm or less can be
produced. Neural signal acquisition, amplification, digitization, and multiplexing were executed by the amplifier module to ensure signal quality and reduce the number of external leads. We
connected the fMEA to the module with a modified gold ball bonding process, as shown in Fig. 1c. The volume of the module was less than half of the volume of a traditional package
microelectrode with an Omnetics connector, as shown in Fig. 1b. The proposed fMEA with the amplifier weighs 0.580 g, while the traditional package microelectrode weighs 1.328 g. The
small-size integrated packaging solution based on gold ball bonding is easy to operate and can be scaled up to hundreds or more channels. IMPEDANCE TEST AND MICROTOPOGRAPHY OBSERVATION
Poly(3,4-ethylenedioxythiophene) (PEDOT) is commonly used to modify recording sites to improve impedance and stability30,31,32,33. PEDOT was grown on the recording sites by galvanostatic
polymerization with an aqueous solution containing 0.02 M EDOT monomer and 0.1 M TsONa (sodium p-toluene sulfonate) electrolyte, as shown in Fig. 1d. The impedance data were collected from
more than 160 channels, as shown in Fig. 2a. Electrochemical impedance spectroscopy was measured in the PBS solution before and after electroplating, as shown in Fig. 2b. The interface
impedance decreased by an order of magnitude after electroplating. Confocal laser scanning microscope (CLSM) observations showed that the recording interface changed from yellow to darker in
color after electroplating, as shown in Fig. 2c. The surface morphology and microstructure of the recording pads observed with scanning electron microscopy (SEM) are shown in Fig. 2c. The
gold pad was flat, while the PEDOT pad was rough. The average roughness of the gold surface was ~78 nm, while the corresponding value of the PEDOT surface was ~233 nm, as measured with CLSM,
and the results are shown in Fig. 2d. The roughness of the PEDOT surface, combined with the surface activity, reduced the electrochemical impedance. The impedance stability of the fMEA is
shown in Fig. 2e. The impedance increased from the initial value of 68.8 ± 13.5 kΩ (mean ± s.e.m.) to 94.2 ± 35.9 kΩ after one week of immersion. Over the next three weeks, the average
impedance value was stable. The average impedance increased to ~126.3 ± 24.8 kΩ in the fourth week. The impedance evolution in vitro was consistent with a previous report34. The increased
impedance may be caused by electrode surface absorption. PLATFORM CONSTRUCTION AND IMPLANTATION TEST We built a semiautomated implantation platform for the distributed implantation of the
flexible microelectrodes. The implantation platform was composed of three subsystems, as shown in Fig. 3a and Fig. S2, including the triaxial positioning system, the filament pick-up and
insertion module, and the observation system. The filament pick-up and insertion module and the observation system were mounted on the triaxial positioning system and can be moved and
positioned precisely. The triaxial positioning system was computer-controlled. The resolution of all axes was 1 μm. The travel lengths of the X, Y, and Z axes were 300, 300, and 100 mm,
respectively. As shown in Fig. 3b, the filament pick-up and insertion system was composed of a rigid microneedle and a holder. The microneedle tip was shaped like a T to hook the filament
before implantation and to remove it after implantation. The microneedle moved vertically, powered by a motor (motor_vertical), to adjust its relative position with the holder during
implantation. The holder was _L_-shaped to allow the microneedle to control the implanted filament. The holder was driven by a motor (motor_horizontal) and rotated slightly to lean on or
away from the microneedle. The observation system contained two microscopes that were placed on both sides of the filament pick-up and insertion system, aimed at the microneedle tip at an
~45° angle. The implant position can be flexibly selected with the observation system to effectively avoid cerebral facial blood vessels. The implantation operations can be easily
accomplished with the semiautomated implantation platform, as shown in Fig. 3c. The filament was picked up and moved until it came into contact with the cortex surface. Then, the filament
was implanted vertically at a rate of ~50 μm per second. As the filament approached the target area, the implantation rate was reduced to 10 μm per second. In 22 implantation operations, all
picking up, transferring, and location motions were performed with micron-level accuracy. Twenty filaments were successfully implanted. There were two cases of implantation failure due to
failure to separate the filament from the implant microneedle. The average pick-up and location time of each filament was less than 1 min, and the success rate reached 90%.
ELECTROPHYSIOLOGICAL RECORDING Neurostudio was used to acquire neural signals at a sampling rate of 30 kHz, and MATLAB was used for data processing. Five microelectrodes (m1–m5) were
implanted in five rats. Microelectrodes m1 and m2 were implanted in the hippocampus CA1 region, while microelectrodes (m3–m5) were implanted in both the CPu (caudate putamen) and PrL
(prelimbic cortex) regions. Unfortunately, microelectrode m5 had to be discarded because the rat died of hypothermia after surgery. The neural signals of the rats were recorded after
microelectrode implantation, as shown in Fig. 4 and Fig. S3. The spiking yield was 43.75 ± 0.09% (mean ± s.e.m.) across the four effective surgeries, which is comparable to the results
reported by Neuralink (45.60 ± 0.03%)6. More than one neuron can be sorted from one channel. The extracellular signals were acquired, and three typical channels are shown in Fig. 4a. The
signal-to-noise ratio (SNR) reached as high as 19 dB. We recorded the extracellular signals for 7 weeks to assess the long-term stability of the microelectrode in vivo, as shown in Fig. 4b.
The amplitude was reduced after 7 weeks. However, the spikes were still clearly distinguishable. DISCUSSION Flexible microelectrodes are expected to contribute to brain research due to their
low mechanical stiffness, which promotes tissue compatibility and improves the stability of long-term recordings35,36,37,38. Unlike traditional rigid microelectrodes, flexible
microelectrodes can be flexibly arranged to achieve on-demand implantation in the whole brain, which is suitable for various electrophysiological experiments, such as the simultaneous
collection of neural signals from multiple brain regions. A semiautomated flexible microelectrode implantation system was developed to implant fMEAs. The fMEA does not require a laborious
manual arrangement, and the implantation process is simple and quick. Each filament was implanted separately, and the implant position was flexibly selected to avoid cerebral facial blood
vessels. This implantation method can be repeated and scaled up to implant flexible microelectrodes with hundreds or thousands of channels. When compared with the highly integrated surgical
robot built by Neuralink, our semiautomated system has several shortcomings in terms of functionality, but it provides a simple yet reliable platform for flexible filament implantation in
general research. However, the bulky packaging volume of conventional Omnetics connectors is unacceptable as the number of flexible microelectrode channels increases. This becomes a
non-negligible problem when packaging high-throughput flexible microelectrodes with small sizes. The proposed gold ball bonding method directly integrated the fMEA and the chip, minimizing
the size and weight of the device. The chip was integrated with the fMEA, allowing the entire system to function with only a few power lines and data lines. The small-size integrated
packaging solution is easy to scale up to more channels. MATERIALS AND METHODS PREPARATION OF THE FMEA As shown in Fig. 1a, the key fabrication steps are as follows: (i) A 4 inch, 250
μm-thick single-polished silicon wafer was selected as the substrate and cleaned using piranha solution. A 100 nm-thick aluminum layer was deposited on the substrate by sputtering to serve
as the release layer. (ii) The bottom PI layer was spin-coated and cured. (iii) A 150 nm-thick patterned gold layer was grown by vacuum evaporation and patterned by a lift-off process. (iv)
After the top PI layer was spin-coated and cured, thermal annealing was conducted at 350 °C for 1 h in a nitrogen atmosphere to improve the stability of the microelectrode. (v) The wafer was
patterned by photolithography. The top PI layer was then etched by reactive ion etching (RIE) to expose the recording sites at the front end and the bonding pads at the rear end. (vi) The
shape of the fMEA was patterned and etched with the same methods as in the previous step. (vii) The flexible microelectrodes were released from the silicon substrate by aluminum etching and
cleaned with deionized water. CONNECTING THE FMEA TO THE AMPLIFIER MODULE The fMEA and the amplifier module were connected directly without a plug and socket. The module was custom-made
based on a 32-channel application-specific integrated circuit (ASIC)29. We connected the fMEA to the module with a modified gold ball bonding process, as shown in Fig. 1c. The connection
pads at the rear of the fMEA were hollow in the center. The position and arrangement of the fMEA pads were the same as those of the pads on the amplifier module. After the alignment, the
pads of the fMEA were aligned with the corresponding pads on the circuit. The fMEA margin was glued to the circuit for temporary fixation. The ball size and the press were adjusted before
bonding to ensure that the gold ball and the pads under it were tightly fixed. The optimal gold ball size in this study was 100 μm in diameter. The press was 2 N. Medium ultrasonic power was
adopted. The gold ball was welded on the circuit pad through the hollow hole (60 μm in diameter) of the fMEA, and the peripheral region of the ball was tightly pressed to the gold pad of
the fMEA. With this method, the fMEA and the module were connected. Epoxy or wax was used to protect and reinforce the bonding area. The module-integrated fMEA is shown in Fig. 1b.
ELECTRODEPOSITION OF PEDOT PEDOT was grown on the recording sites by galvanostatic polymerization with an aqueous solution. The electroplating solution was prepared by ultrasonically
dispersing 0.02 M EDOT in a 0.1 M sodium p-toluene sulfonate electrolyte. A 4 mm square platinum foil electrode was used as both the counter electrode and reference electrode to construct a
two-electrode system, as shown in Fig. 1d. The electrochemical deposition was performed with the two-electrode system. An electrochemical workstation was used to supply a constant current of
6.5 nA for 15 s to all recording sites. IMPEDANCE MEASUREMENT The impedance test was performed in the phosphate-buffered saline (PBS) solution discussed in the electrochemical deposition
section. The impedance measurements were performed by applying a sinusoidal AC voltage of 10 mV at 1 kHz. The electrochemical impedance spectroscopy measurements were obtained by applying a
10 mV sinusoidal AC voltage. The frequency ranged from 100 to 10,000 Hz. To assess the impedance stability, the fMEA was immersed in a 36 °C PBS solution, and the impedance was measured at 1
kHz once every 24 h for 4 weeks. ANIMAL SURGERY Adult Sprague–Dawley female rats (200–220 g) were used in this paper. All procedures were approved by the Animal Advisory Committee at East
China Normal University and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The rats were anesthetized with
isoflurane (1–5%) and placed in the stereotactic apparatus. A 37 °C constant heating blanket was spread under the animal. Erythromycin ointment was applied to the eye to prevent dehydration.
The skull was exposed with a small incision to determine the target location. A 3 mm diameter hole was created with a craniotomy. The dura was resected, and brain tissue was maintained
under artificial cerebrospinal fluid to prevent desiccation. MICROELECTRODE IMPLANTATION The stereotactic apparatus with a craniotomy rat was transferred and fixed on the implantation
system. Before implantation, a stool with the packaged fMEA was placed on the rat’s neck, and the fMEA was connected to the Neurostudio system to record the extracellular signal. The tips of
the four filaments stretched ~1 mm beyond the table facet. The implantation process can be divided into three steps. The key operations are shown in Fig. 3c and described as follows. The
filament pick-up and insertion module were set to their initial state. The holder was kept away from the microneedle and at least 2 mm below it. By manipulating the triaxial positioning
system, the microneedle tip was moved to the vicinity of the loop of the first filament. Under microscopy observation, the microneedle was aligned with and inserted into the loop.
Motor_vertical was used to move the microneedle down to ~0.2 mm below the holder. The holder was rotated slightly to lean on the microneedle with motor_horizontal. The filament was hooked
onto the tip of the microneedle and clamped between the holder and the microneedle rod. The filament was picked up and moved with the needle. By manipulating the triaxial positioning system,
the filament was moved to a position directly above the operating area on the rat’s head. A location without blood vessels was chosen, and the position of the microneedle was adjusted until
the tip touched the cortex surface; this position was set as zero depth. The filament was implanted by manipulating the _Z_-axis of the positioning system at a rate of ~50 μm per second.
When the electrode approached the target area, the rate was decreased to 10 μm per second. The extracellular signal and position coordinates were monitored during implantation. After the
filament reached the target location, the holder was rotated to loosen the filament. Using the triaxial positioning system, the microneedle was withdrawn ~200 μm to separate it from the loop
at the front end of the filament. The filament was released from the pick-up and insertion module and remained in the brain. After this operation, the pick-up and insertion module was reset
to its initial state. All filaments were implanted by repeating the above steps. All filaments of microelectrodes m1 and m2 were successfully implanted into the hippocampal CA1 region.
Microelectrodes m3–m5 were successfully implanted into both the CPu and PrL regions. Two filaments (marked with red and orange in Fig. 1b) of the microelectrode were implanted into the CPu
region, while the other two filaments (marked with green and blue in Fig. 1b) were implanted into the PrL region. Microelectrode m5 was discarded because the rat died of hypothermia after
surgery. ACUTE AND CHRONIC ELECTROPHYSIOLOGICAL ACQUISITION After all of the filaments were implanted, the filaments were fixed to the rat skull with dental acrylic. The connector part of
the fMEA was carefully transferred from the stool to the rat head and fixed with dental acrylic. Acute neural signals were recorded by Neurostudio on the day of surgery. The rat was then
released. After it recovered from anesthesia, it was raised in a separate cage. One week after implantation, the neural activity was collected while the rat was awake or sleeping in a box.
Seven weeks after surgery, extracellular signals were collected in the free-moving state. REFERENCES * Campbell, P. K. et al. A silicon-based, three-dimensional neural interface:
manufacturing processes for an intracortical electrode array. _IEEE Trans. Biomed. Eng._ 38, 758–768 (1991). Article Google Scholar * Wise, K. D. et al. Microelectrodes, microelectronics,
and implantable neural microsystems. _Proc. IEEE_ 96, 1184–1202 (2008). Article Google Scholar * Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural
activity. _Nature_ 551, 232–236 (2017). Article Google Scholar * Zhao, S. et al. A novel linear microprobe array for the fabrication of neural microelectrodes. _Sci. China Technol. Sci._
58, 346–351 (2015). Article Google Scholar * Fofonoff, T. et al. A highly flexible manufacturing technique for microelectrode array fabrication. in _Proc. Second Joint 24th Annual
Conference and the Annual Fall Meeting of the Biomedical Engineering Society_, 3, 2107–2108 (Engineering in Medicine and Biology, 2002). * Musk, E. & Neuralink An integrated
brain-machine interface platform with thousands of channels. _J. Med. Internet Res._ 21, e16194 (2019). Article Google Scholar * Zhao, Z. et al. Parallel, minimally-invasive implantation
of ultra-flexible neural electrode arrays. _J. Neural Eng._ 16, 035001 (2019). Article Google Scholar * Zhao, Z. et al. Nanoelectronic coating enabled versatile multifunctional neural
probes. _Nano Lett._ 17, 4588–4595 (2017). Article Google Scholar * Wei, X. et al. Nanofabricated ultraflexible electrode arrays for high-density intracortical recording. _Adv. Sci._ 5,
1700625 (2018). Article Google Scholar * Du, Z. J. et al. Ultrasoft microwire neural electrodes improve chronic tissue integration. _Acta Biomater._ 53, 46–58 (2017). Article Google
Scholar * Guan, S. et al. Elastocapillary self-assembled neurotassels for stable neural activity recordings. _Sci. Adv._ 5, eaav2842 (2019). Article Google Scholar * Felix, S. H. et al.
Insertion of flexible neural probes using rigid stiffeners attached with biodissolvable adhesive. _J. Vis. Exp._ 79, e50609 (2013). Google Scholar * He, F. et al. Ultraflexible neural
electrodes for long-lasting intracortical recording. _iScience_ 23, 101387 (2020). Article Google Scholar * Yang, X. et al. Bioinspired neuron-like electronics. _Nat. Mater._ 18, 510–517
(2019). Article Google Scholar * Vitale, F. et al. Fluidic microactuation of flexible electrodes for neural recording. _Nano Lett._ 18, 326–335 (2018). Article Google Scholar * Zhou, T.
et al. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. _Proc. Natl Acad. Sci. USA_ 114, 5894–5899 (2017). Article Google Scholar
* Liu, J. et al. Syringe-injectable electronics. _Nat. Nanotechnol._ 10, 629–636 (2015). Article Google Scholar * Schuhmann, J. et al. Syringe-injectable mesh electronics for stable
chronic rodent electrophysiology. _J. Vis. Exp._ 21, 58003 (2018). Google Scholar * Fu, T. et al. Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology.
_Proc. Natl Acad. Sci. USA_ 114, E10046–E10055 (2017). Google Scholar * Seo, K. J. et al. Transparent, flexible, penetrating microelectrode arrays with capabilities of single-unit
electrophysiology. _Adv. Biosyst._ 3, 1800276 (2019). Article Google Scholar * Kim, D. H. et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. _Nat.
Mater._ 9, 511–517 (2010). Article Google Scholar * Tien, L. W. et al. Silk as a multifunctional biomaterial substrate for reduced glial scarring around brain-penetrating electrodes.
_Adv. Funct. Mater._ 23, 3185–3193 (2013). Article Google Scholar * Lee, Y., Kong, C., Chang, J. W. & Jun, S. B. Carbon-fiber based microelectrode aarray embedded with a biodegradable
silk support for in vivo neural recording. _J. Korean Med. Sci._ 34, e24 (2019). Article Google Scholar * Kil, D. et al. Dextran as a resorbable coating material for flexible neural
probes. _Micromachines_ 10, 61 (2019). Article Google Scholar * Lu, L. et al. Soft and MRI compatible neural electrodes from carbon nanotube fibers. _Nano Lett._ 19, 1577–1586 (2019).
Article Google Scholar * Luan, L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. _Sci. Adv._ 3, e1601966 (2017). Article Google Scholar *
Zhao, Z. et al. Flexible deep brain neural probes based on a parylene tube structure. _J. Micromech. Microeng._ 28, 015012 (2017). Article Google Scholar * Scholvin, J. et al. Close-packed
silicon microelectrodes for scalable spatially oversampled neural recording. _IEEE Trans. Biomed. Eng._ 63, 120–130 (2016). Article Google Scholar * Rios, G. et al. Nanofabricated neural
probes for dense 3-D recordings of brain activity. _Nano Lett._ 16, 6857–6862 (2016). Article Google Scholar * Xiao, Y., Cui, X. & Martin, D. C. Electrochemical polymerization and
properties of PEDOT/S-EDOT on neural microelectrode arrays. _J. Electroanal. Chem._ 573, 43–48 (2004). Google Scholar * Cui, X. Y. & Martin, D. C. Electrochemical deposition and
characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. _Sens. Actuator B Chem._ 89, 92–102 (2003). Article Google Scholar * Ludwig, K. A. et al. Chronic
neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. _J. Neural Eng._ 3, 59–70 (2006). Article Google
Scholar * Cui, X. T. & Zhou, D. D. Poly (3,4-ethylenedioxythiophene) for chronic neural stimulation. _IEEE Trans. Neural Syst. Rehabil. Eng._ 15, 502–508 (2007). Article Google Scholar
* Kozai, T. D. Y. et al. Chronic in vivo evaluation of PEDOT/CNT for stable neural recordings. _IEEE Trans. Biomed. Eng._ 63, 111–119 (2016). Article Google Scholar * Feiner, R. &
Dvir, T. Tissue–electronics interfaces: from implantable devices to engineered tissues. _Nat. Rev. Mater._ 3, 17076 (2018). Article Google Scholar * Engler, A. J., Sen, S., Sweeney, H. L.
& Discher, D. E. Matrix elasticity directs stem cell lineage specification. _Cell_ 126, 677–689 (2006). Article Google Scholar * McCreery, D., Pikov, V. & Troyk, P. R. Neuronal
loss due to prolonged controlled-current stimulation with chronically implanted microelectrodes in the cat cerebral cortex. _J. Neural Eng._ 7, 036005 (2010). Article Google Scholar *
Szarowski, D. H. et al. Brain responses to micro-machined silicon devices. _Brain Res._ 983, 23–35 (2003). Article Google Scholar Download references ACKNOWLEDGEMENTS The project was
supported by the National Key Technologies Research and Development Program of China (2017YFA0205903, 2017YFA0701100); the National Natural Science Foundation of China (61634006, 62071447);
the Strategic Priority Research Program of Chinese Academy of Sciences Pilot Project (XDB32030102, XDB32040203, XDA16021305); and the Key Research Programs of Frontier Sciences, CAS
(QYZDY-SSW-JSC004). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory of Integrated Optoelectronics, Institute of Semiconductors, Chinese Academy of Sciences, 100083,
Beijing, China Chunrong Wei, Yang Wang, Weihua Pei, Zhiduo Liu, Gege Ming, Xiaowei Yang, Li Zheng & Yijun Wang * University of Chinese Academy of Sciences, 100049, Beijing, China
Chunrong Wei, Weihua Pei, Zhiduo Liu, Gege Ming, Pingping Wu, Li Zheng & Yijun Wang * School of Future Technologies, University of Chinese Academy of Sciences, 100049, Beijing, China
Chunrong Wei, Gege Ming, Pingping Wu & Li Zheng * School of Microelectronics, University of Sciences and Technology of China, 230000, Hefei, China Yang Wang * Institute of Automation,
Chinese Academy of Sciences, 100190, Beijing, China Xinyong Han * Key Laboratory of Brain Functional Genomics, East China Normal University, 200062, Shanghai, China Longnian Lin * Brain
Machine Fusion Intelligence Institute, 215131, Suzhou, China Ruru Chen * Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, 100190, Beijing, China Pingping Wu *
Chinese Institute for Brain Research, 102206, Beijing, China Yijun Wang Authors * Chunrong Wei View author publications You can also search for this author inPubMed Google Scholar * Yang
Wang View author publications You can also search for this author inPubMed Google Scholar * Weihua Pei View author publications You can also search for this author inPubMed Google Scholar *
Xinyong Han View author publications You can also search for this author inPubMed Google Scholar * Longnian Lin View author publications You can also search for this author inPubMed Google
Scholar * Zhiduo Liu View author publications You can also search for this author inPubMed Google Scholar * Gege Ming View author publications You can also search for this author inPubMed
Google Scholar * Ruru Chen View author publications You can also search for this author inPubMed Google Scholar * Pingping Wu View author publications You can also search for this author
inPubMed Google Scholar * Xiaowei Yang View author publications You can also search for this author inPubMed Google Scholar * Li Zheng View author publications You can also search for this
author inPubMed Google Scholar * Yijun Wang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.W., W.P., and Y.W. conceived the project.
C.W., W.P., Y.W., X.Y., and X.H. designed and performed the experiments. C.W., G.M., and L.Z. performed the data analysis. C.W., L.L., R.C., P.W., and Y.W. contributed to the discussion.
C.W. and Z.L. prepared the figures. C.W. and W.P. co-wrote the paper. All authors approved the final paper. CORRESPONDING AUTHOR Correspondence to Weihua Pei. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wei, C., Wang, Y., Pei, W. _et al._ Distributed implantation of a flexible
microelectrode array for neural recording. _Microsyst Nanoeng_ 8, 50 (2022). https://doi.org/10.1038/s41378-022-00366-2 Download citation * Received: 08 November 2021 * Revised: 14 January
2022 * Accepted: 02 February 2022 * Published: 12 May 2022 * DOI: https://doi.org/10.1038/s41378-022-00366-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative