3,4-dihydroxytoluene, a metabolite of rutin, suppresses the progression of nonalcoholic fatty liver disease in mice by inhibiting p300 histone acetyltransferase activity
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT 3,3′,4′,5,7-Pentahydroxyflavone-3-rhamnoglucoside (rutin) is a flavonoid with a wide range of pharmacological activities. Dietary rutin is hardly absorbed because the microflora in
the large intestine metabolize rutin into a variety of compounds including quercetin and phenol derivatives such as 3,4-dihydroxyphenolacetic acid (DHPAA), 3,4-dihydroxytoluene (DHT),
3,4-hydroxyphenylacetic acid (HPAA) and homovanillic acid (HVA). We examined the potential of rutin and its metabolites as novel histone acetyltransferase (HAT) inhibitors. DHPAA, HPAA and
DHT at the concentration of 25 μM significantly inhibited in vitro HAT activity with DHT having the strongest inhibitory activity. Furthermore, DHT was shown to be a highly efficient
inhibitor of p300 HAT activity, which corresponded with its high degree of inhibition on intracellular lipid accumulation in HepG2 cells. Docking simulation revealed that DHT was bound to
the p300 catalytic pocket, bromodomain. Drug affinity responsive target stability (DARTS) analysis further supported the possibility of direct binding between DHT and p300. In HepG2 cells,
DHT concentration-dependently abrogated p300-histone binding and induced hypoacetylation of histone subunits H3K9, H3K36, H4K8 and H4K16, eventually leading to the downregulation of
lipogenesis-related genes and attenuating lipid accumulation. In _ob/ob_ mice, administration of DHT (10, 20 mg/kg, iv, every other day for 6 weeks) dose-dependently improved the NAFLD
pathogenic features including body weight, liver mass, fat mass, lipid accumulation in the liver, and biochemical blood parameters, accompanied by the decreased mRNA expression of lipogenic
genes in the liver. Our results demonstrate that DHT, a novel p300 histone acetyltransferase inhibitor, may be a potential preventive or therapeutic agent for NAFLD. You have full access to
this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS SELECTIVE INHIBITION OF CBP/P300 HAT BY A-485 RESULTS IN SUPPRESSION OF LIPOGENESIS AND HEPATIC
GLUCONEOGENESIS Article Open access 11 September 2020 SL010110, A LEAD COMPOUND, INHIBITS GLUCONEOGENESIS VIA SIRT2-P300-MEDIATED PEPCK1 DEGRADATION AND IMPROVES GLUCOSE HOMEOSTASIS IN
DIABETIC MICE Article 11 February 2021 H3K9ME3 DEMETHYLATION BY JMJD2B IS REGULATED BY PIRFENIDONE RESULTING IN IMPROVED NASH Article Open access 21 October 2024 INTRODUCTION Nonalcoholic
fatty liver disease (NAFLD), ranging from steatosis to cirrhosis to hepatocarcinoma, is characterized by an abnormal accumulation of triglycerides (TG) in the liver without alcohol
consumption [1]. NAFLD has been well established to not only be confined to liver-related morbidity and mortality but is also a multisystemic disease affecting several extrahepatic organs
and regulatory pathways, such as the kidney, colon, bone, endocrine and cardiovascular systems [2,3,4]. Liver steatosis, marked as the first stage of NAFLD, is closely associated with
obesity, type 2 diabetes mellitus, insulin resistance, and drug-induced liver injury [5]. Recently, several examples of dynamic changes in epigenetic markers due to environmental stimuli,
such as stress and nutritional interventions, have been reported, including histone acetylation as a gene expression marker [6,7,8]. Few studies have provided evidence that the dysregulation
of hepatic function is orchestrated by epigenetic mechanisms in NAFLD [9]. Among the phenomena of epigenetic modulation, histone acetyltransferase (HAT)-mediated acetylation of histone tail
lysine residues is usually associated with the activation of gene transcription [10]. Indeed, altered expression and activity of certain histone acetylation modifying enzymes influence gene
expression in NAFLD [11]. This may lead to the alteration of hepatic metabolism and cellular transformation through the regulation of various signaling pathways, such as hepatic lipid
metabolism, insulin resistance, mitochondrial damage, oxidative stress, and inflammation. These pathways are implicated in the development and progression of NAFLD [12,13,14,15,16].
Recently, beneficial effects of natural dietary compounds on the prevention and treatment of NAFLD have been reported, but little research has been conducted to analyze the molecular
mechanisms related to epigenetic modulation. 3,3′,4′,5,7-Pentahydroxyflavone-3-rhamnoglucoside (rutin) is a flavonoid under the flavonol subgroup that has a wide range of pharmacological
properties, such as antioxidative, antimicrobial, antifungal, and anti-allergic properties [17]. In addition, current research has shown that its multispectrum pharmacological effects
benefit the treatment of various chronic diseases, such as cancer, diabetes mellitus, hypertension, and hypercholesterolemia [18]. Dietary rutin is hardly absorbed because the microflora in
the large intestine metabolize rutin into a variety of compounds, including quercetin and phenol derivatives such as 3,4-dihydroxyphenolacetic acid (DHPAA), 3,4-dihydroxytoluene (DHT),
3,4-hydroxyphenylacetic acid (HPAA), and 4-hydroxy-3-methoxyphenylacetic acid (homovanillic acid, HVA) [19, 20]. Recent studies have shown the suppressive effects of rutin metabolites on
oxidative stress, inflammation, and advanced glycation end product (AGE) formation [20,21,22]; however, to date, no in-depth explanations have been published regarding the molecular
mechanisms of rutin metabolites in NAFLD. This study is the first attempt to demonstrate the efficacy of rutin metabolites in preventing NAFLD and to explore their roles in epigenetic
modulation. In the present study, we investigated the potential of rutin metabolites as HAT inhibitors (HATis) and demonstrated that DHT is a potent HATi that successfully ameliorated NAFLD
in both in vitro and in vivo NAFLD models. DHT suppressed HAT activity, notably p300, lipid accumulation, and lipogenic gene expression induced by a mixture of oleic and palmitic acids in
HepG2 cells. In silico docking simulation and the DARTS assay revealed that DHT directly binds to the bromodomain of p300. In an obese mouse model, administration of DHT reduced body weight,
liver mass, plasma TG, plasma LDL cholesterol, and lipogenic-related gene expression in the liver. MATERIALS AND METHODS CHEMICALS AND REAGENTS Rutin (Cat. No. R5143), DHPAA (Cat. No.
850217), DHT (Cat. No. M34200), HPAA (Cat. No. H49901), and HVA (Cat. No. H1252) in powder form were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in DMSO for the
experiments. Oleic acid, palmitic acid, and oil red O staining solution were also purchased from Sigma-Aldrich. Water-soluble tetrazolium salt (WST-1) used to measure cytotoxicity was
obtained from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). CELL CULTURE HepG2 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in a
humidified atmosphere with 5% CO2 at 37 °C in high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and antibiotics (Coring, NY, USA). A nonfat bovine
serum albumin-conjugated combination of oleic acid (Sigma-Aldrich, St. Louis, MO, USA) and palmitic acid (Sigma) at a ratio of 4:1 (OPA) was used to establish an NAFLD model using HepG2
cells. To evaluate the effect of rutin and its metabolites on hepatic lipid accumulation, HepG2 cells were concurrently treated with the indicated concentrations of OPA for 18 h.
CYTOTOXICITY HepG2 cells were seeded in 24-well plates at a density of 5 × 104 cells/well. Next, at ~70% confluency, cells were treated with the indicated concentrations of rutin metabolites
in each experiment in the presence or absence of OPA. After a 24-h incubation, WST-1 solution (Enzo Life Sciences) was added to the wells, and the cells were incubated for 3 h. Thereafter,
the absorbance values were measured at 450 nm (Molecular Devices, Sunnyvale, CA, USA). LIPID CONTENT HepG2 cells were seeded in 24-well plates at a density of 5 × 104 cells/well. After
reaching ~70% confluency, the cells were treated with OPA in the presence or absence of rutin metabolites. After a 24-h incubation, the cells were fixed with 4% paraformaldehyde for 15 min
at room temperature and were then incubated with 60% isopropanol for 5 min, followed by staining with 0.1% oil red O staining solution (Sigma-Aldrich) for 15 min. The cells were then washed
with water, and images were captured under a light microscope (Olympus IX51; Olympus Corporation, Central Valley, PA, USA). For lipid quantification, isopropanol was added to each well to
dissolve the red dye. After 10 min, the absorbance values were measured at 510 nm (Molecular Devices). HISTONE EXTRACTION HepG2 cells were seeded at a concentration of 5 × 106 in 100-mm
dishes. After the cells reached 70% confluency (~2 × 108 cells), the cells were treated for 18 h with the indicated concentrations of OPA, with or without metabolites of rutin. The histone
proteins from HepG2 cells were extracted using a Histone Extraction Kit (Abcam, Cambridge, MA, USA) according to the manufacturer’s protocol. IN VITRO HAT AND HISTONE DEACETYLASE (HDAC)
ASSAYS HAT and HDAC activities in the HeLa nuclear extracts (BioVision, Milpitas, CA, USA) were assessed using a commercially available kit according to the manufacturer’s protocol
(BioVision). To examine the inhibitory effect of DHT on p300, 100 ng of p300 recombinant protein (Enzo Life Science) was used as the enzyme source instead of the nuclear extracts. For
autoradiography-based in vitro HAT activity assays, either the HeLa cell nuclear extract or the p300 recombinant protein was incubated with HAT assay buffer (50 mM HEPES, pH 8.0; 10%
glycerol; 1 mM dithiothreitol [DTT]; 1 mM phenylmethylsulfonyl fluoride; and 10 mM sodium butyrate), 1 µL of [3H] acetyl-CoA, and 5 µg of biotinylated-H4 peptide (Millipore, Billerica, MA,
USA) along with metabolites of rutin at the indicated concentrations at 30 °C for 1 h. The reactions were stopped by adding 5× sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS–PAGE) sample buffer. Proteins from each sample were then separated on 15% SDS–PAGE gels and visualized via autoradiographic analysis. QUANTITATIVE REVERSE-TRANSCRIPTION POLYMERASE CHAIN
REACTION (QRT-PCR) Cells were seeded in 24-well plates at 5 × 104 cells/well. At ~70% confluency, the cells were treated with the indicated concentrations of DHT in the presence or absence
of OPA. After an 18-h incubation, total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). qRT-PCR was performed using an I Cycler iQ system (Bio-Rad Laboratories,
Hercules, CA, USA) with SYBR Green PCR master mix (Thermo Fisher Scientific, Waltham, MA, USA). PCR amplification was carried out in triplicate using the primers listed in Supplementary
Table 1. mRNA levels were normalized to those of glyceraldehyde-3-phosphate dehydrogenase (_GAPDH_) mRNA, and relative expression levels were calculated using the comparative ΔΔCT method.
WESTERN BLOT ANALYSIS Cells were grown and treated in a similar fashion as those used for qRT-PCR. Cell extracts were then prepared using lysis buffer (Cell Signaling, Danvers, MA, USA)
containing protease and phosphatase inhibitors (Roche, Basel, Switzerland) and were incubated on ice for 30 min. The lysates were centrifuged at 20,000 × _g_ for 20 min at 4 °C. The cell
lysates were separated on SDS–PAGE gels and transferred to cellulose membranes (Whatman, Dassel, Germany). The membranes were blocked with 5% (_w_/_v_) skimmed milk (BD Biosciences, Sparks,
MD, USA) solution in 1× phosphate buffered saline (PBS) containing Tween-20 (PBST) for 1 h. The blocked membranes were incubated overnight with the indicated primary antibodies at 4 °C
(Supplementary Table S2). The membranes were then washed with 1× PBST, incubated with the appropriate secondary anti-rabbit or anti-mouse horseradish peroxidase-conjugated antibody (Thermo
Scientific, Rockford, IL, USA) for 1 h, and visualized using Fusion Solo 6 S (Vilber Lourmat, Collegien, France) with an enhanced chemiluminescence detection reagent (Thermo Scientific).
HISTONE TAIL BINDING ASSAY In the biotinylated histone peptide-binding assay, 1 µg of biotin-labeled Lys acetylated histone H3 (AcH3) tail peptide (Anaspec, Fermont, CA, USA) was bound to
NeutrAvidin (Pierce); pull-down assays were performed with flag-tagged recombinant p300 (1041–1161 amino acid) containing the bromodomain (Active Motif, Carlsbad, CA, USA). The binding assay
was performed at 4 °C for 2 h in binding buffer [20 mM Tris-HCl (pH 7.1), 120 mM KCl, 1 mM DTT, 1 mM ethylenediaminetetraacetic acid, 0.1% Nonidet P-40 (NP-40), 10% glycerol, and 1 mM
PMSF]. The histone tail and protein complexes were separated via SDS–PAGE and detected using a flag antibody (Sigma). The biotinylated AcH3 tail was stained with Ponceau S solution (Sigma)
to demonstrate the loading control. DRUG AFFINITY RESPONSIVE TARGET STABILITY (DARTS) ASSAY The DARTS assay was performed according to a previously described protocol [23]. HepG2 cells were
incubated in 10-cm dishes until 80% confluency, followed by lysis in M-PER buffer (Pierce, Rockford, IL, USA), which contains 30 mM NaCl, protease, and phosphatase inhibitors. After
centrifugation (14,000 × _g_, 10 min, 4 °C), 10× TNC buffer [500 mM Tris-HCl (pH 8.0), 500 mM NaCl, 100 mM CaCl2] was added to the lysates prior to the determination of protein concentration
using a DC protein assay kit (Bio-Rad Laboratories), according to the manufacturer’s instructions. The proteins in the supernatant (2 μg/μL) were digested with pronase (1:1,000,000 protein
to pronase ratio) for 30 min. Digestion was stopped by adding 5× Laemmli sample buffer, followed by denaturation at 95 °C for 5 min. CELL AND LIVER TISSUE FRACTIONS Cells were seeded at a
concentration of 5 × 106 in 100-mm dishes. After reaching ~70% confluency (~2 × 108 cells), the cells were treated with the indicated concentrations of OPA, with or without the metabolites
of rutin, for 18 h. Harvested cells were lysed in lysis buffer [10 mM Tris (pH 7.4), 10 mM NaCl, 3 mM MgCl2, 0.5% NP-40, and protease inhibitor cocktail (Roche)] at 3000 r/min and 4 °C for 3
min, and the resulting supernatants were used as cytosol fractions. Nuclear fractions from HepG2 cells were obtained by following the manufacturer’s protocol (Abcam). Liver tissues obtained
at the end of the animal experiments were washed with ice-cold PBS and lysed according to the manufacturer’s protocol (Abcam) to obtain the cytosolic and nuclear fractions. Briefly, tissues
were weighed and cut into small pieces. To obtain the cytosolic fractions, tissue pieces were homogenized in pre-extraction buffer containing DTT using a glass tissue homogenizer (Thomas
Scientific, NJ, USA). Extraction buffer was added to the pellets, and the resulting suspensions were incubated on ice for 15 min and were then further homogenized using a Teflon pestle
(Thomas Scientific). The suspensions were then centrifuged at 14,000 r/min at 4 °C for 10 min, and the supernatants were used as nuclear fractions. Each nuclear fraction was used for the HAT
assays. ANIMAL EXPERIMENTS Seven-week-old male _ob/ob_ mice were purchased from Orient Bio (Seongnam, Gyeonggi, Korea) and housed at the College of Medicine of Ulsan University. All animal
experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Ulsan University Guide for the
Animal Care and Use Committee (2017-02-148). A total of 20 male _ob/ob_ mice were divided into three groups (2 or 3 mice/cage) and housed in a temperature- and humidity-controlled room with
a 12-h light/dark cycle, with free access to food and water. After a 1-week acclimation period, saline (control group), 10 mg, or 20 mg/kg DHT was injected into the lateral tail vein of each
mouse once every alternate day. Body weights were measured at the beginning of the experiment and subsequently every week for 6 weeks. At the end of the experiment, mice were sacrificed by
cervical dislocation under anesthesia (100 mg/kg ketamine + 5 mg/kg xylazine), and blood samples were collected for serum isolation via abdominal heart puncture. After laparotomy, the liver,
retroperitoneal fat, and epididymal fat were weighed, and a portion of the liver from each mouse was fixed in 4% formalin solution for hematoxylin and eosin (H&E) staining. The
remaining livers were used for the experiments as indicated. H&E STAINING Liver specimens were fixed in 4% buffered formalin, embedded in paraffin, and cut into 4–5-μm-thick sections,
which were stained with H&E. Lipid accumulation in each liver specimen was assessed through microscopic observation using an Eclipse 80i microscope (Nikon Instruments, Inc., Melville,
NY, USA). MEASUREMENT OF TRIGLYCERIDES (TGS), TOTAL CHOLESTEROL, AND LOW-DENSITY LIPOPROTEIN CHOLESTEROL (LDL-C) The serum levels of TGs, total cholesterol, and LDL-C were measured
enzymatically using a commercial kit (Asan Pharm, Seoul, Korea). PROTEIN–LIGAND DOCKING SIMULATIONS FOR THE E1A BINDING PROTEIN P300 (EP300) To identify the active site in EP300, the
three-dimensional X-ray crystallographic structure of EP300 was downloaded from the Protein Data Bank (http://www.rcsb.org/pdb/home/home.do) (PDB ID: 4BHW) [24]. The three-dimensional
structure of DHT used as a ligand for docking was downloaded from the PubChem site (CID: 9958) (https://pubchem.ncbi.nlm.nih.gov) [25]. The specific simulation methods were described in
detail in a previous study by Chung et al. [11]. STATISTICAL ANALYSIS Data were analyzed using Student’s _t_-test for comparing two groups or one-way analysis of variance (ANOVA) with
Tukey’s multiple comparison test for comparing multiple groups, and values are expressed as the means ± standard deviations (SD) or means ± SEM. Statistical analyses were conducted using
SPSS ver. 20 (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant at _P_ < 0.05, _P_ < 0.01, and _P_ < 0.001. RESULTS DHT HAS THE MOST SIGNIFICANT
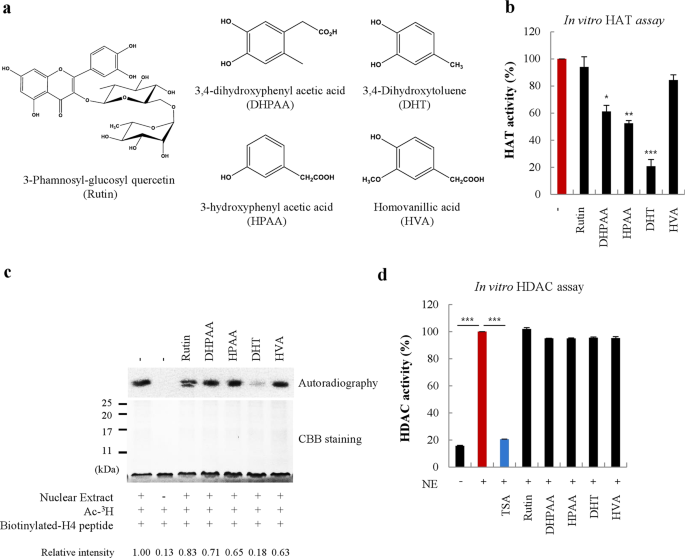
INHIBITORY EFFECT ON HAT ACTIVITY Initially, to test whether rutin and its metabolites (Fig. 1a) can modulate HAT activity, we measured HAT activity using a cell-free system. Rutin (100 µM)
or one of its metabolites, including DHPAA, HPAA, DHT, and HVA, was incubated with HeLa nuclear extract. The results indicated that DHT had the most significant inhibitory effect on HAT
activity compared to that of the other metabolites (Fig. 1b). To confirm the anti-HAT activity of DHT, an autoradiography assay was performed using radiolabeled H4 tail peptides. Consistent
with the results of the in vitro HAT activity assay, DHT exhibited the most powerful HATi effect (Fig. 1c), although the effects of DHPAA and HPAA in the cell-free system and the
autoradiography assay were slightly different from the in vitro HAT activity. To ensure that the activities of rutin and its metabolites, including DHT, were specifically directed against
HATs, we assessed their effects on HDAC activities. Trichostatin A (TSA), used as a positive control, efficiently inhibited nuclear HDAC activities, whereas rutin and its metabolites did not
affect HDAC activity (Fig. 1d). Together, these data showed that DHT, among the rutin metabolites, most efficiently abrogated HAT activity without affecting HDAC activity. DHT SELECTIVELY
INHIBITS P300 ACETYLTRANSFERASE ACTIVITY To further confirm the effect of DHT as a HATi, the changes in both HAT and HDAC activities were measured with DHT at various concentrations in a
cell-free system. DHT diminished HAT activity in a dose-dependent manner (Fig. 2a), whereas HDAC activity was not affected by DHT (Fig. 2b). Next, we examined the enzyme specificity of DHT.
The HAT activities of p300, CREB-binding protein (CBP), and p300/CBP-associated factor (pCAF) were measured either in the absence or presence of increasing concentrations of DHT. DHT was
shown to be a highly efficient inhibitor of p300 acetyltransferase activity (Fig. 2c). The p300 inhibitory effect of DHT was supported by the results of an autoradiography assay using a
radiolabeled H4 tail peptide (Fig. 2d). Collectively, these results showed that DHT specifically inhibited p300 acetyltransferase activity. THE ANTILIPOGENIC EFFECT OF DHT IS ASSOCIATED WITH
ITS HAT INHIBITORY CAPACITY We previously demonstrated that HAT activity increases in NAFLD in vitro and in vivo [11]. To determine whether the rutin metabolites attenuated the increased
HAT activity found in HepG2 cells undergoing lipid accumulation, we measured the change in HAT activity after exposing an OPA-induced NAFLD model to rutin metabolites. HepG2 cells with or
without various rutin metabolites were treated with OPA for 24 h. Nuclear extracts were then used to evaluate HAT enzyme activity. OPA-induced HAT activity was significantly inhibited by
DHPAA, HPAA, DHT, and HVA (Fig. 3a). Interestingly, similar to the previous result (Fig. 1b), DHT showed the strongest HATi effect. To observe the correlation between HATi activity and
intracellular lipid accumulation in HepG2 cells, oil red O staining was performed under the same conditions as above. The inhibition of lipid accumulation by rutin metabolites was also
similar to that of the observed HATi activity (Fig. 3b). The results showed that the metabolites were not cytotoxic at the concentration used (Fig. 3c). To check the effect of different
concentrations of DHT on HAT activity and lipid accumulation, we treated cells with 25–50 μM DHT and remeasured their corresponding HATi effects and degrees of inhibition of lipid
accumulation. DHT showed both HATi and antilipogenic effects in a dose-dependent manner (Fig. 3d, e). Next, RT-qPCR was conducted to determine whether DHT was implicated in the expression of
acetyl-CoA carboxylase (_ACC_), ATP-citrate lyase (_ACLY_), fatty acid synthase (_FASN_), and sterol regulatory element-binding protein 1c (_SREBP1c_). As expected, there was a significant
increase in the mRNA expression of these genes in the OPA-treated group; mRNA expression significantly decreased in OPA-treated groups that were exposed to DHT (Fig. 3f). These data suggest
that DHT decreased lipid accumulation in hepatic cells by abrogating the transcription of lipogenesis-related genes via its HATi capacity. DHT BINDS TO THE BROMODOMAIN OF P300 AND INHIBITS
P300–HISTONE COMPLEX FORMATION Previously, we demonstrated the selective p300 inhibitory effect of DHT. We then examined the binding affinity of DHT and p300 through docking simulation. The
most likely binding site on p300 was the catalytic pocket for the bromodomain. We identified the top five docking structures with free energy values between −7.181 kcal/mol and −6.547
kcal/mol (Fig. 4a). In particular, the most likely binding residues were PRO1074, TYR1089, MET1124, ASN1127, ALA1128, and TYR1131 (Fig. 4b). To confirm DHT-p300 binding at the molecular
level, we conducted a DARTS assay using HepG2 cells. The binding of chemicals to specific proteins increases protein stability and resistance to proteases. The cells were treated with DHT,
and the lysates were then digested with pronase, a mixture of proteases, as indicated. DARTS analysis revealed that DHT directly bound to p300, and the resistance of p300 to pronase
increased proportionately to the DHT concentration (Fig. 4c). It is well established that the bromodomain of p300 is responsible for its histone binding [26]. Based on this, we hypothesized
that docking of DHT in the p300 bromodomain would inhibit histone binding with p300 and would consequently suppress histone acetylation. To support our hypothesis, we performed a histone
tail binding assay. Biotinylated AcH3 and flag-tagged p300 recombinant protein containing the bromodomain were incubated with or without DHT (Fig. 4d). Then, biotinylated AcH3 was
immunoprecipitated with avidin beads, and the bound p300 protein was detected using an anti-flag antibody. Remarkably, our results demonstrated that the binding of p300 and histone H3
decreased as the concentration of DHT increased (Fig. 4d). To observe whether a decrease in the binding of p300 and histone protein would actually lead to a reduction in histone acetylation,
we detected the histone acetylation status following DHT treatment. DHT effectively obstructed the OPA-induced hyperacetylation of the histone subunits H3K8, H3K36, H4K8, and H4K16 (Fig.
4e). Together, these data indicate that DHT interrupted p300-histone protein binding by docking at the histone binding site of p300 and consequentially inhibiting hyperacetylation of histone
proteins caused by OPA-induced lipid accumulation. DHT ADMINISTRATION IMPROVES THE NAFLD PATHOGENIC FEATURES IN VIVO To strengthen the previously gathered data, we examined the effect of
DHT on significant NAFLD features. A dose of 10 or 20 mg/kg DHT was injected into the lateral tail vein once every alternate day for 6 weeks in _ob/ob_ mice (Fig. 5a). The DHT-injected mouse
groups demonstrated a lower average body weight than the control mouse group (Fig. 5b). The average weight gain was also significantly lower in the DHT-injected groups than in the control
group (Fig. 5c), and there were no significant differences in daily food intake between the DHT-injected mice and the control mice (_P_ < 0.05) (Fig. 5d). Enlarged tissue sizes of the
liver and retroperitoneal and epididymal fat and increased tissue mass were observed in the control group but not in the DHT-injected group (Fig. 5e, f). In H&E-stained liver tissue,
less lipid accumulation was detected in DHT-injected mice than in control mice (Fig. 5g). In the blood test, TG, LDL-C, alkaline phosphatase (ALP), and alanine aminotransferase (ALT) levels
effectively decreased following DHT treatment (Fig. 5h). Moreover, the mRNA expression of lipogenic genes, such as _FASN_, _ACLY_, and _PPARγ_, decreased in the DHT-injected groups (Fig.
5i). These results established that DHT administration ameliorated NAFLD peculiarities in _ob/ob_ mice. DISCUSSION NAFLD is one of the major health concerns worldwide. Its development and
progression are affected by various factors, such as genetics, epigenetics, and environmental factors. A large number of studies have provided evidence to support that the critical
components implicated in the progression of NAFLD pathogenesis are epigenetically regulated [27,28,29]. In addition, recent studies have shown that epigenetic modifications are reprogrammed
for NAFLD [30, 31]. For this reason, there is increasing interest in developing new preventive or therapeutic applications that target NAFLD based on epigenetics owing to its reversible
properties. In particular, epigenetic regulation using natural compounds with favorable safety profiles is expected to be a novel preventive or therapeutic strategy for metabolic syndromes,
including NAFLD [7, 32, 33]. In a preliminary study, we observed that sprouts containing a large amount of rutin, which has known beneficial effects against metabolic diseases, show anti-HAT
capacity. Based on our preliminary observations, in this study, we elucidated the in-depth epigenetic mechanism underlying NAFLD control by rutin and its metabolites at the molecular level.
Initially, we investigated the anti-HAT efficacy of rutin and its metabolites, namely, DHPAA, HPAA, DHT, and HVA. Our data showed that DHPAA, HPAA, and DHT showed potential in effectively
inhibiting HAT activity. In particular, DHT, a phenol derivative of rutin, demonstrated the most potent HAT inhibitory effect among all the rutin metabolites. Several small molecules
targeting HAT that have been derived from natural products, such as anacardic acid, curcumin, and garcinol [34, 35], contain phenolic structures that are prone to oxidation; thus, it is
occasionally difficult to distinguish whether their efficacy in disease models is due to the antioxidant effect or the anti-HAT effect. Chen et al. reported that DHT had the most significant
inhibitory effect on LPS-induced NO production and inflammatory cytokine production among rutin metabolites [20]. Recently, a study indicated that DHT is a plausible rutin colonic
metabolite that suppresses oxidative stress by enhancing peroxiredoxin-6 protein expression, a protein responsible for the cellular antioxidant defense system [21]. These studies allow us to
indirectly or directly infer that the beneficial effects of DHT are mediated by its antioxidant effect. However, to the best of our knowledge, no studies have shown the efficacy of DHT as a
HAT inhibitor. Our data clearly demonstrate that HAT activity is regulated in a DHT dose-dependent manner. Based on the above facts, we believe that it is justifiable to identify the
bioactive effects of DHT based on its HAT inhibitory capacity. The acetylation modification of proteins in the liver is associated with a broad range of cellular activities. This is
supported by a study showing that ~1000 proteins in human liver tissue are subjected to lysine acetylation modification [36]. The development and progression of NAFLD is no exception. A
pregnant woman’s high-fat diet was shown to trigger the development of NAFLD in the fetal liver; statistically significant hyperacetylations and gross alterations of acetylation at the
H3K14, H3K9, and H3K18 subunits have also been observed [37]. Global histone H3 acetylation also increases in a serotonin reuptake inhibitor-induced NAFLD model [38]. We recently reported
that increased HAT activity following treatment with a mixture of oleic and palmitic acids (OPA) induces histone hyperacetylation and promotes NAFLD phenotypes such as steatosis and
lipogenic gene expression in HepG2 cells [39]. Our data showed that histones H3 and H4 were hypoacetylated in an OPA-induced NAFLD model in HepG2 cells. This result was attributed to the
increased HAT activity in the model. Interestingly, DHT effectively blocked the phenomena through its anti-HAT activity, especially by selectively inhibiting p300. According to our data, the
effect of HATs appeared to be tissue-specific; in the differentiation of preadipocytes (3T3-L1) to adipocytes, pCAF activity was more significant than p300 activity (data not shown). P300
dynamically upregulates many transcription factors, including _SREBP1c_ and _PPARγ_, which are responsible for lipogenesis [40, 41], and consequently enhances the expression of lipogenic
genes in NAFLD [42]. Thus, regardless of its molecular mechanism, these results show that inhibition of p300 activity can serve as a key to prevent the development and progression of NAFLD.
In the present study, we provided a plausible mechanism underlying p300 inhibition caused by the derivative of a natural compound. To explain this, we proved the mode of action of DHT as a
p300 inhibitor through the DARTS assay and docking simulations. The results demonstrated that DHT bound to p300, and the docking site was predicted to be a bromodomain located in the p300
catalytic pocket. The domain not only increases p300 activity by providing a docking surface for acetyl-CoA, the intracellular acetyl donor [43], but also plays an important role in
activating the target level of gene transcription through binding with histone H3 [44]. The bromodomain has emerged as a novel therapeutic target for cancer, cardiovascular disease, and type
2 diabetes, and its inhibitors, such as apabetalone and JQ-1, disrupt the interaction of the domain with acetylated histones [45]. We elucidated that DHT directly interrupted the access of
the p300 bromodomain to histone H3 using the histone tail binding assay. Therefore, the results shown in our study suggested that DHT inhibited p300 activity by obstructing the binding of
both acetyl-CoA and the histone tail to the p300 bromodomain. Physically, acetylation of the histone tail changes the positive charge of lysine residues to negative, thereby weakening its
electrostatic affinity to DNA, resulting in conformational changes in euchromatin and enhancing gene transcription [46, 47]. Thus, from an epigenetic point of view, our hypothesis that DHT
prevents the onset and progression of NAFLD through its p300 inhibitory capacity is firmly supported by the compelling results obtained in this study. Our data showed that DHT induced
inhibition of the hyperacetylation of histone proteins, including H3K9, H3K36, H4K8, and H4K16, and decreased expression of lipogenic genes, such as _ACC1_, _ACLY_, _FASN_, and _SREBP1c_.
Consistent with our results, we recently reported that tannic acid, a plant-derived polyphenol, suppresses HAT, notably p300 activity, hyperacetylation of histone marks, and lipogenic gene
expression and ameliorates NAFLD [11]. Based on the present findings and the previous literature, it can be postulated that the beneficial effects of DHT on the development and progression
of NAFLD are closely associated with DHT-dependent epigenetic regulation of the lipid metabolic pathway. In conclusion, we have not only provided powerful evidence that DHT is a new p300
inhibitor but also elucidated a plausible scenario describing the underlying molecular mechanism controlling NAFLD through DHT (Fig. 6). However, we did not observe whether DHT affected the
occupancy of p300 on the promoter region of the lipogenic genes. Considering that this study provides strong evidence to support DHT-mediated epigenetic regulation in controlling NAFLD,
further relevant in-depth studies are needed. REFERENCES * Shankar E, Kanwal R, Candamo M, Gupta S. Dietary phytochemicals as epigenetic modifiers in cancer: promise and challenges. Semin
Cancer Biol. 2016;40–41:82–99. Article PubMed PubMed Central CAS Google Scholar * Fotbolcu H, Zorlu E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J
Gastroenterol. 2016;22:4079–90. Article CAS PubMed PubMed Central Google Scholar * Sun C, Fan JG, Qiao L. Potential epigenetic mechanism in non-alcoholic fatty liver disease. Int J Mol
Sci. 2015;16:5161–79. Article CAS PubMed PubMed Central Google Scholar * Tariq A, Mussarat S, Adnan M. Review on ethnomedicinal, phytochemical and pharmacological evidence of Himalayan
anticancer plants. J Ethnopharmacol. 2015;164:96–119. Article CAS PubMed Google Scholar * Choi WJ, Kim SK, Park HK, Sohn UD, Kim W. Anti-inflammatory and anti-superbacterial properties
of sulforaphane from shepherd’s purse. Korean J Physiol Pharmacol. 2014;18:33–9. Article CAS PubMed PubMed Central Google Scholar * Lee Y-H, Kwak J, Choi H-K, Choi K-C, Kim S, Lee J, et
al. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int J Mol Med. 2012;30:69–74. CAS PubMed Google
Scholar * Shankar S, Kumar D, Srivastava RK. Epigenetic modifications by dietary phytochemicals: implications for personalized nutrition. Pharmacol Ther. 2013;138:1–17. Article CAS PubMed
Google Scholar * Wang G-L, Salisbury E, Shi X, Timchenko L, Medrano EE, Timchenko NA. HDAC1 cooperates with C/EBPα in the inhibition of liver proliferation in old mice. J Biol Chem.
2008;283:26169–78. Article CAS PubMed PubMed Central Google Scholar * Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea
polyphenol,(-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat–fed mice. J Nutr. 2008;138:1677–83. Article CAS PubMed Google Scholar
* Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP, et al. Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/western-style diet-induced obesity
and metabolic syndrome in mice. J Agric Food Chem. 2011;59:11862–71. Article CAS PubMed PubMed Central Google Scholar * Chung MY, Song JH, Lee J, Shin EJ, Park JH, Lee SH, et al. Tannic
acid, a novel histone acetyltransferase inhibitor, prevents non-alcoholic fatty liver disease both in vivo and in vitro model. Mol Metab. 2019;19:34–48. Article CAS PubMed Google Scholar
* Asrih M, Jornayvaz FR. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J Endocrinol. 2013;218:R25–36. Article CAS PubMed Google
Scholar * Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients. 2017;9:387. Article PubMed Central
CAS Google Scholar * Li Z, Li Y, Zhang HX, Guo JR, Lam CWK, Wang CY, et al. Mitochondria‐mediated pathogenesis and therapeutics for non‐alcoholic fatty liver disease. Mol Nutr Food Res.
2019;63:1900043. Article CAS Google Scholar * Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty
liver disease. Oxid Med Cell Longev. 2018;2018:7864316. * Simões IC, Fontes A, Pinton P, Zischka H, Wieckowski MR. Mitochondria in non-alcoholic fatty liver disease. Int J Biochem Cell Biol.
2018;95:93–9. Article PubMed CAS Google Scholar * Al-Dhabi NA, Arasu MV, Park CH, Park SU. An up-to-date review of rutin and its biological and pharmacological activities. EXCLI J.
2015;14:59–63. PubMed PubMed Central Google Scholar * Sharma S, Ali A, Ali J, Sahni JK, Baboota S. Rutin: therapeutic potential and recent advances in drug delivery. Expert Opin Investig
Drugs. 2013;22:1063–79. Article CAS PubMed Google Scholar * Jaganath IB, Jaganath IB, Mullen W, Edwards CA, Crozier A. The relative contribution of the small and large intestine to the
absorption and metabolism of rutin in man. Free Radic Res. 2006;40:1035–46. Article CAS PubMed Google Scholar * Su KY, Yu CY, Chen YP, Hua KF, Chen YLS. 3, 4-Dihydroxytoluene, a
metabolite of rutin, inhibits inflammatory responses in lipopolysaccharide-activated macrophages by reducing the activation of NF-κB signaling. BMC Complement Alter Med. 2014;14:21. Article
CAS Google Scholar * Morales AM, Mukai R, Murota K, Terao J. Inhibitory effect of catecholic colonic metabolites of rutin on fatty acid hydroperoxide and hemoglobin dependent lipid
peroxidation in Caco-2 cells. J Clin Biochem Nutr. 2018;63:175–80. * Giménez‐Bastida JA, Zielinski H, Piskula M, Zielinska D, Szawara‐Nowak D. Buckwheat bioactive compounds, their derived
phenolic metabolites and their health benefits. Mol Nutr Food Res. 2017;61:1600475. Article CAS Google Scholar * Lomenick B, Hao R, Jonai N, Chin RM, Aghajan M, Warburton S, et al. Target
identification using drug affinity responsive target stability (DARTS). Proc Natl Acad Sci U S A. 2009;106:21984–9. Article CAS PubMed PubMed Central Google Scholar * Sharma R, Zhou
MM. Partners in crime: The role of tandem modules in gene transcription. Protein Sci. 2015;24:1347–59. Article CAS PubMed PubMed Central Google Scholar * Wang Y, Xiao J, Suzek TO, Zhang
J, Wang J, Bryant SH. PubChem: a public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009;37:W623–33. Article CAS PubMed PubMed Central Google
Scholar * Manning ET, Ikehara T, Ito T, Kadonaga JT, Kraus WL. p300 forms a stable, template-committed complex with chromatin: role for the bromodomain. Mol Cell Biol. 2001;21:3876–87.
Article CAS PubMed PubMed Central Google Scholar * da Silva RP, Kelly KB, Al Rajabi A, Jacobs RL. Novel insights on interactions between folate and lipid metabolism. Biofactors.
2014;40:277–83. Article PubMed CAS Google Scholar * Lu SC, Alvarez L, Huang Z-Z, Chen L, An W, Corrales FJ, et al. Methionine adenosyltransferase 1A knockout mice are predisposed to
liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci U S A. 2001;98:5560–5. Article CAS PubMed PubMed Central Google Scholar * Sinton MC,
Hay DC, Drake AJ. Metabolic control of gene transcription in non-alcoholic fatty liver disease: the role of the epigenome. Clin Epigenet. 2019;11:104. Article CAS Google Scholar * Feng
D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–9. Article CAS
PubMed PubMed Central Google Scholar * Ferreira DM, Simão AL, Rodrigues CM, Castro RE. Revisiting the metabolic syndrome and paving the way for micro RNA s in non‐alcoholic fatty liver
disease. FEBS J. 2014;281:2503–24. Article CAS PubMed Google Scholar * Legeay S, Rodier M, Fillon L, Faure S, Clere N. Epigallocatechin gallate: a review of its beneficial properties to
prevent metabolic syndrome. Nutrients. 2015;7:5443–68. Article CAS PubMed PubMed Central Google Scholar * Shanak S, Saad B, Zaid H. Metabolic and epigenetic action mechanisms of
antidiabetic medicinal plants. Evid Based Complement Alternat Med. 2019;2019:3583067. * Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK. Small molecule modulators of histone
acetyltransferase p300. J Biol Chem. 2003;278:19134–40. Article CAS PubMed Google Scholar * Marcu MG, Jung YJ, Lee S, Chung EJ, Lee MJ, Trepel J, et al. Curcumin is an inhibitor of p300
histone acetylatransferase. Med Chem. 2006;2:169–74. Article CAS PubMed Google Scholar * Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. Regulation of cellular metabolism by protein
lysine acetylation. Science. 2010;327:1000–4. Article CAS PubMed PubMed Central Google Scholar * Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R, et al. Developmental
origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol. 2008;41:91–102. Article CAS PubMed PubMed Central Google
Scholar * De Long NE, Hardy DB, Ma N, Holloway AC. Increased incidence of non‐alcoholic fatty liver disease in male rat offspring exposed to fluoxetine during fetal and neonatal life
involves the NLRP3 inflammasome and augmented de novo hepatic lipogenesis. J Appl Toxicol. 2017;37:1507–16. Article PubMed CAS Google Scholar * Chung S, Hwang JT, Park JH, Choi HK. Free
fatty acid-induced histone acetyltransferase activity accelerates lipid accumulation in HepG2 cells. Nutr Res Pr. 2019;13:196–204. Article CAS Google Scholar * Gelman L, Zhou G, Fajas L,
Raspé E, Fruchart JC, Auwerx J. p300 interacts with the N-and C-terminal part of PPARγ2 in a ligand-independent and-dependent manner, respectively. J Biol Chem. 1999;274:7681–8. Article CAS
PubMed Google Scholar * Giandomenico V, Simonsson M, Grönroos E, Ericsson J. Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors. Mol Cell
Biol. 2003;23:2587–99. Article CAS PubMed PubMed Central Google Scholar * Tian Y, Wong VWS, Chan HLY, Cheng ASL. Epigenetic regulation of hepatocellular carcinoma in non-alcoholic fatty
liver disease. Semin Cancer Biol 2013;23:471–82. Article CAS PubMed Google Scholar * Maksimoska J, Segura-Peña D, Cole PA, Marmorstein R. Structure of the p300 histone acetyltransferase
bound to acetyl-coenzyme A and its analogues. Biochemistry. 2014;53:3415–22. Article CAS PubMed Google Scholar * Delvecchio M, Gaucher J, Aguilar-Gurrieri C, Ortega E, Panne D.
Structure of the p300 catalytic core and implications for chromatin targeting and HAT regulation. Nat Struct Mol Biol. 2013;20:1040–6. Article CAS PubMed Google Scholar * Cochran AG,
Conery AR, Sims RJ. Bromodomains: a new target class for drug development. Nat Rev Drug Discov. 2019;18:609–28. Article CAS PubMed Google Scholar * Chang L, Takada S. Histone acetylation
dependent energy landscapes in tri-nucleosome revealed by residue-resolved molecular simulations. Sci Rep. 2016;6:34441. Article CAS PubMed PubMed Central Google Scholar * Zhang F,
Huang Q, Yan J, Chen Z. Histone acetylation induced transformation of B-DNA to Z-DNA in cells probed through FT-IR spectroscopy. Anal Chem. 2016;88:4179–82. Article CAS PubMed Google
Scholar Download references ACKNOWLEDGEMENTS This study was supported by the Main Research Program (E-0150301) of the Korea Food Research Institute (KFRI), funded by the Ministry of
Science, ICT & Future Planning. AUTHOR INFORMATION Author notes * These authors contributed equally: Jangho Lee, Ji-Hye Song. AUTHORS AND AFFILIATIONS * Korea Food Research Institute,
Jeollabuk-do, 55365, Republic of Korea Jangho Lee, Min-Yu Chung, Jae Ho Park, Jin-Taek Hwang & Hyo-Kyoung Choi * Department of Biomedical Sciences, Asan Medical Center, University of
Ulsan College of Medicine, Seoul, 05505, Republic of Korea Ji-Hye Song * Korea Research Institute of Bioscience and Biotechnology, Daejeon, 34141, Republic of Korea Jin-Hyuk Lee * Department
of Bioinformatics, Korea University of Science and Technology, Daejeon, 34113, Republic of Korea Jin-Hyuk Lee * Major of Food Science and Biotechnology, Division of Bio-convergence, Kyonggi
University, Suwon, 16227, Republic of Korea Tae-Gyu Nam * Department of Food Biotechnology, Korea University of Science and Technology, Daejeon, 34113, Republic of Korea Jin-Taek Hwang
Authors * Jangho Lee View author publications You can also search for this author inPubMed Google Scholar * Ji-Hye Song View author publications You can also search for this author inPubMed
Google Scholar * Min-Yu Chung View author publications You can also search for this author inPubMed Google Scholar * Jin-Hyuk Lee View author publications You can also search for this author
inPubMed Google Scholar * Tae-Gyu Nam View author publications You can also search for this author inPubMed Google Scholar * Jae Ho Park View author publications You can also search for
this author inPubMed Google Scholar * Jin-Taek Hwang View author publications You can also search for this author inPubMed Google Scholar * Hyo-Kyoung Choi View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS HKC and JTH designed the research. JL, JHS, MYC, and HKC performed the research. JL, JHL, TGN, and JHP contributed to the
data analysis. JL, HKC, and JTH wrote the paper. CORRESPONDING AUTHORS Correspondence to Jin-Taek Hwang or Hyo-Kyoung Choi. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lee, J., Song, JH., Chung, MY. _et
al._ 3,4-dihydroxytoluene, a metabolite of rutin, suppresses the progression of nonalcoholic fatty liver disease in mice by inhibiting p300 histone acetyltransferase activity. _Acta
Pharmacol Sin_ 42, 1449–1460 (2021). https://doi.org/10.1038/s41401-020-00571-7 Download citation * Received: 24 August 2020 * Accepted: 02 November 2020 * Published: 10 December 2020 *
Issue Date: September 2021 * DOI: https://doi.org/10.1038/s41401-020-00571-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * non-alcoholic fatty
liver disease * rutin metabolites * 3,4-dihydroxytoluene * p300 histone acetyltransferase * epigenetic regulation * OPA-induced NAFLD model * _ob/ob_ mice