Ginsenoside rg1 ameliorates stress-exacerbated parkinson’s disease in mice by eliminating rtp801 and α-synuclein autophagic degradation obstacle
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

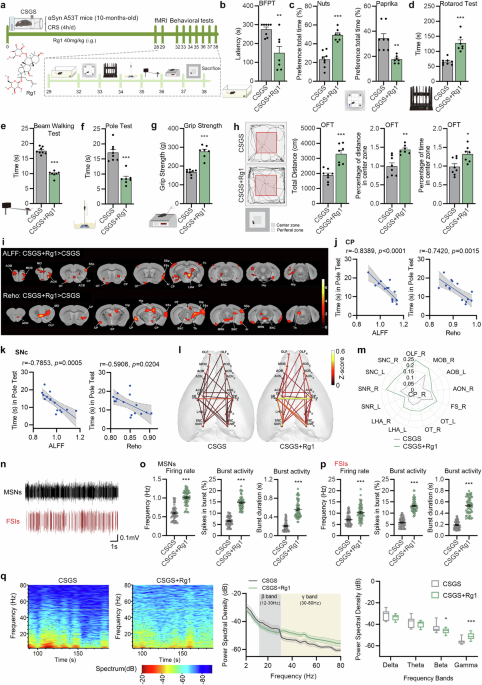
ABSTRACT Emerging evidence shows that psychological stress promotes the progression of Parkinson’s disease (PD) and the onset of dyskinesia in non-PD individuals, highlighting a potential
avenue for therapeutic intervention. We previously reported that chronic restraint-induced psychological stress precipitated the onset of parkinsonism in 10-month-old transgenic mice
expressing mutant human α-synuclein (αSyn) (hαSyn A53T). We refer to these as chronic stress-genetic susceptibility (CSGS) PD model mice. In this study we investigated whether ginsenoside
Rg1, a principal compound in ginseng notable for soothing the mind, could alleviate PD deterioration induced by psychological stress. Ten-month-old transgenic hαSyn A53T mice were subjected
to 4 weeks’ restraint stress to simulate chronic stress conditions that worsen PD, meanwhile the mice were treated with Rg1 (40 mg· kg−1 ·d−1, i.g.), and followed by functional magnetic
resonance imaging (fMRI) and a variety of neurobehavioral tests. We showed that treatment with Rg1 significantly alleviated both motor and non-motor symptoms associated with PD. Functional
MRI revealed that Rg1 treatment enhanced connectivity between brain regions implicated in PD, and in vivo multi-channel electrophysiological assay showed improvements in dyskinesia-related
electrical activity. In addition, Rg1 treatment significantly attenuated the degeneration of dopaminergic neurons and reduced the pathological aggregation of αSyn in the striatum and SNc. We
revealed that Rg1 treatment selectively reduced the level of the stress-sensitive protein RTP801 in SNc under chronic stress conditions, without impacting the acute stress response.
HPLC-MS/MS analysis coupled with site-directed mutation showed that Rg1 promoted the ubiquitination and subsequent degradation of RTP801 at residues K188 and K218, a process mediated by the
Parkin RING2 domain. Utilizing αSyn A53T+; RTP801−/− mice, we confirmed the critical role of RTP801 in stress-aggravated PD and its necessity for Rg1’s protective effects. Moreover, Rg1
alleviated obstacles in αSyn autophagic degradation by ameliorating the RTP801-TXNIP-mediated deficiency of ATP13A2. Collectively, our results suggest that ginsenoside Rg1 holds promise as a
therapeutic choice for treating PD-sensitive individuals who especially experience high levels of stress and self-imposed expectations. Access through your institution Buy or subscribe This
is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00
per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CHRONIC STRESS
INDUCES DEPRESSION-LIKE BEHAVIORS AND PARKINSONISM VIA UPREGULATING Α-SYNUCLEIN Article Open access 28 May 2025 BNIP3L/NIX-MEDIATED MITOPHAGY ALLEVIATES PASSIVE STRESS-COPING BEHAVIORS
INDUCED BY TUMOR NECROSIS FACTOR-Α Article 13 March 2023 GINSENOSIDE RG1 ALLEVIATES CHRONIC STRESS-INDUCED DEPRESSION IN RATS BY TARGETING CX43-YAP AXIS Article 07 March 2025 REFERENCES *
Przedborski S. The two-century journey of Parkinson disease research. Nat Rev Neurosci. 2017;18:251–9. CAS PubMed Google Scholar * Emamzadeh FN, Surguchov A. Parkinson’s disease:
biomarkers, treatment, and risk factors. Front Neurosci. 2018;12:612. PubMed PubMed Central Google Scholar * Lang AE, Siderowf AD, Macklin EA, Poewe W, Brooks DJ, Fernandez HH, et al.
Trial of cinpanemab in early Parkinson’s disease. N Engl J Med. 2022;387:408–20. CAS PubMed Google Scholar * Pagano G, Taylor KI, Anzures-Cabrera J, Marchesi M, Simuni T, Marek K, et al.
Trial of prasinezumab in early-stage Parkinson’s disease. N Engl J Med. 2022;387:421–32. CAS PubMed Google Scholar * Zou K, Guo W, Tang G, Zheng B, Zheng Z. A case of early onset
Parkinson’s disease after major stress. Neuropsychiatr Dis Treat. 2013;9:1067–9. PubMed PubMed Central Google Scholar * Blauwendraat C, Nalls MA, Singleton AB. The genetic architecture of
Parkinson’s disease. Lancet Neurol. 2020;19:170–8. CAS PubMed Google Scholar * Chen YP, Yu SH, Zhang GH, Hou YB, Gu XJ, Ou RW, et al. The mutation spectrum of Parkinson-disease-related
genes in early-onset Parkinson’s disease in ethnic Chinese. Eur J Neurol. 2022;29:3218–28. PubMed Google Scholar * van der Heide A, Meinders MJ, Bloem BR, Helmich RC. The impact of the
COVID-19 pandemic on psychological distress, physical activity, and symptom severity in Parkinson’s disease. J Parkinsons Dis. 2020;10:1355–64. PubMed PubMed Central Google Scholar *
Helmich RC, Bloem BR. The impact of the COVID-19 pandemic on Parkinson’s disease: hidden sorrows and emerging opportunities. J Parkinsons Dis. 2020;10:351–4. CAS PubMed PubMed Central
Google Scholar * Edwards E, Carroll C. In reply to: Helmich and Bloem (2020) “The impact of the COVID-19 pandemic on Parkinson’s disease: hidden sorrows and emerging opportunities”. J
Parkinsons Dis. 2020;10:1267–8. CAS PubMed PubMed Central Google Scholar * Zhang Z, Chu SF, Wang SS, Jiang YN, Gao Y, Yang PF, et al. RTP801 is a critical factor in the neurodegeneration
process of A53T alpha-synuclein in a mouse model of Parkinson’s disease under chronic restraint stress. Br J Pharmacol. 2018;175:590–605. CAS PubMed PubMed Central Google Scholar * Lin
XM, Pan MH, Sun J, Wang M, Huang ZH, Wang G, et al. Membrane phospholipid peroxidation promotes loss of dopaminergic neurons in psychological stress-induced Parkinson’s disease
susceptibility. Aging Cell. 2023;22:e13970. CAS PubMed PubMed Central Google Scholar * Baida G, Bhalla P, Kirsanov K, Lesovaya E, Yakubovskaya M, Yuen K, et al. REDD1 functions at the
crossroads between the therapeutic and adverse effects of topical glucocorticoids. EMBO Mol Med. 2015;7:42–58. CAS PubMed Google Scholar * Malagelada C, Jin ZH, Greene LA. RTP801 is
induced in Parkinson’s disease and mediates neuron death by inhibiting Akt phosphorylation/activation. J Neurosci. 2008;28:14363–71. CAS PubMed PubMed Central Google Scholar *
Emmanouilidou E, Elenis D, Papasilekas T, Stranjalis G, Gerozissis K, Ioannou PC, et al. Assessment of alpha-synuclein secretion in mouse and human brain parenchyma. PLoS One. 2011;6:e22225.
CAS PubMed PubMed Central Google Scholar * Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under
stress conditions. J Neurochem. 2010;113:1263–74. CAS PubMed Google Scholar * Yamada K, Iwatsubo T. Extracellular alpha-synuclein levels are regulated by neuronal activity. Mol
Neurodegener. 2018;13:9. PubMed PubMed Central Google Scholar * Molitoris JK, McColl KS, Swerdlow S, Matsuyama M, Lam M, Finkel TH, et al. Glucocorticoid elevation of
dexamethasone-induced gene 2 (Dig2/RTP801/REDD1) protein mediates autophagy in lymphocytes. J Biol Chem. 2011;286:30181–9. CAS PubMed PubMed Central Google Scholar * Yang SJ, Wang JJ,
Cheng P, Chen LX, Hu JM, Zhu GQ. Ginsenoside Rg1 in neurological diseases: from bench to bedside. Acta Pharmacol Sin. 2023;44:913–30. CAS PubMed Google Scholar * Zhang Z, Song Z, Shen F,
Xie P, Wang J, Zhu AS, et al. Ginsenoside Rg1 prevents PTSD-like behaviors in mice through promoting synaptic proteins, reducing Kir4.1 and TNF-alpha in the hippocampus. Mol Neurobiol.
2021;58:1550–63. CAS PubMed Google Scholar * Li J, Gao W, Zhao Z, Li Y, Yang L, Wei W, et al. Ginsenoside Rg1 reduced microglial activation and mitochondrial dysfunction to alleviate
depression-like behaviour via the GAS5/EZH2/SOCS3/NRF2 axis. Mol Neurobiol. 2022;59:2855–73. CAS PubMed PubMed Central Google Scholar * Zhang QS, Heng Y, Chen Y, Luo P, Wen L, Zhang Z,
et al. A novel bibenzyl compound (20C) protects mice from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid toxicity by regulating the alpha-synuclein-related inflammatory response. J
Pharmacol Exp Ther. 2017;363:284–92. CAS Google Scholar * Lyu D, Wang F, Zhang M, Yang W, Huang H, Huang Q, et al. Ketamine induces rapid antidepressant effects via the autophagy-NLRP3
inflammasome pathway. Psychopharmacology. 2022;239:3201–12. CAS PubMed Google Scholar * Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (resting-state) brain
imaging. Neuroinformatics. 2016;14:339–51. PubMed Google Scholar * Barriere DA, Ella A, Szeremeta F, Adriaensen H, Meme W, Chaillou E, et al. Brain orchestration of pregnancy and maternal
behavior in mice: a longitudinal morphometric study. Neuroimage. 2021;230:117776. PubMed Google Scholar * Deng S, Franklin CG, O’Boyle M, Zhang W, Heyl BL, Jerabek PA, et al. Hemodynamic
and metabolic correspondence of resting-state voxel-based physiological metrics in healthy adults. Neuroimage. 2022;250:118923. CAS PubMed Google Scholar * Wang J, Wang X, Xia M, Liao X,
Evans A, He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci. 2015;9:386. CAS PubMed PubMed Central Google Scholar * Yang X, Williams
JK, Yan R, Mouradian MM, Baum J. Increased dynamics of alpha-synuclein fibrils by beta-synuclein leads to reduced seeding and cytotoxicity. Sci Rep. 2019;9:17579. PubMed PubMed Central
Google Scholar * Chen C, Chu SF, Ai QD, Zhang Z, Chen NH. CKLF1/CCR5 axis is involved in neutrophils migration of rats with transient cerebral ischemia. Int Immunopharmacol. 2020;85:106577.
CAS PubMed Google Scholar * Zhou X, Zhang YN, Li FF, Zhang Z, Cui LY, He HY, et al. Neuronal chemokine-like-factor 1 (CKLF1) up-regulation promotes M1 polarization of microglia in rat
brain after stroke. Acta Pharmacol Sin. 2022;43:1217–30. CAS PubMed Google Scholar * Xu J, Ao YL, Huang C, Song X, Zhang G, Cui W, et al. Harmol promotes alpha-synuclein degradation and
improves motor impairment in Parkinson’s models via regulating autophagy-lysosome pathway. NPJ Parkinsons Dis. 2022;8:100. PubMed PubMed Central Google Scholar * Green C, Sydow A, Vogel
S, Anglada-Huguet M, Wiedermann D, Mandelkow E, et al. Functional networks are impaired by elevated tau-protein but reversible in a regulatable Alzheimer’s disease mouse model. Mol
Neurodegener. 2019;14:13. PubMed PubMed Central Google Scholar * Tuite P. Magnetic resonance imaging as a potential biomarker for Parkinson’s disease. Transl Res. 2016;175:4–16. PubMed
Google Scholar * Lv H, Wang Z, Tong E, Williams LM, Zaharchuk G, Zeineh M, et al. Resting-state functional MRI: everything that nonexperts have always wanted to know. Am J Neuroradiol.
2018;39:1390–9. CAS PubMed PubMed Central Google Scholar * Oh JY, Lee YS, Hwang TY, Cho SJ, Jang JH, Ryu Y, et al. Acupuncture regulates symptoms of Parkinson’s disease via brain neural
activity and functional connectivity in mice. Front Aging Neurosci. 2022;14:885396. CAS PubMed PubMed Central Google Scholar * Cavdar S, Ozgur M, Cakmak YO, Kuvvet Y, Kunt SK, Saglam G.
Afferent projections of the subthalamic nucleus in the rat: emphasis on bilateral and interhemispheric connections. Acta Neurobiol Exp. 2018;78:251–63. Google Scholar * Simonsen U,
Comerma-Steffensen S, Andersson KE. Modulation of dopaminergic pathways to treat erectile dysfunction. Basic Clin Pharmacol Toxicol. 2016;119:63–74. CAS PubMed Google Scholar * Pautrat A,
Rolland M, Barthelemy M, Baunez C, Sinniger V, Piallat B, et al. Revealing a novel nociceptive network that links the subthalamic nucleus to pain processing. Elife. 2018;7:e36607. PubMed
PubMed Central Google Scholar * Green AL, Paterson DJ. Using deep brain stimulation to unravel the mysteries of cardiorespiratory control. Compr Physiol. 2020;10:1085–104. PubMed Google
Scholar * Laansma MA, Bright JK, Al-Bachari S, Anderson TJ, Ard T, Assogna F, et al. International multicenter analysis of brain structure across clinical stages of Parkinson’s disease. Mov
Disord. 2021;36:2583–94. PubMed PubMed Central Google Scholar * Low A, Foo H, Yong TT, Tan LCS, Kandiah N. Hippocampal subfield atrophy of CA1 and subicular structures predict
progression to dementia in idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2019;90:681–7. PubMed Google Scholar * Hanna C, Hamilton J, Arnavut E, Blum K, Thanos PK. Brain
mapping the effects of chronic aerobic exercise in the rat brain using FDG PET. J Pers Med. 2022;12:860. PubMed PubMed Central Google Scholar * Chen H, Lei H, Xu Q. Neuronal activity
pattern defects in the striatum in awake mouse model of Parkinson’s disease. Behav Brain Res. 2018;341:135–45. PubMed Google Scholar * Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT,
Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–6. CAS PubMed PubMed Central Google Scholar *
Owen SF, Berke JD, Kreitzer AC. Fast-spiking interneurons supply feedforward control of bursting, calcium, and plasticity for efficient learning. Cell. 2018;172:683–95.e15. CAS PubMed
PubMed Central Google Scholar * Niyomrat K, Cheaha D, Nukitram J, Kumarnsit E. Locomotor activity and resting local field potential oscillatory rhythms of 6-OHDA mouse model of Parkinson’s
disease in response to acute and repeated treatments with L-dopa. Neurosci Lett. 2021;759:136007. CAS PubMed Google Scholar * Juszczak GR, Stankiewicz AM. Glucocorticoids, genes and
brain function. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:136–68. CAS PubMed Google Scholar * Fushimi S, Nohno T, Katsuyama H. Chronic stress induces type 2b skeletal muscle
atrophy via the inhibition of mTORC1 signaling in mice. Med Sci. 2023;11:19. CAS Google Scholar * Yuan T, Fu D, Xu R, Ding J, Wu J, Han Y, et al. Corticosterone mediates FKBP51 signaling
and inflammation response in the trigeminal ganglion in chronic stress-induced corneal hyperalgesia mice. J Steroid Biochem Mol Biol. 2023;231:106312. CAS PubMed Google Scholar *
Nechushtai L, Frenkel D, Pinkas-Kramarski R. Autophagy in Parkinson’s disease. Biomolecules. 2023;13:1435. CAS PubMed PubMed Central Google Scholar * Li R, Lu Y, Zhang Q, Liu W, Yang R,
Jiao J, et al. Piperine promotes autophagy flux by P2RX4 activation in SNCA/alpha-synuclein-induced Parkinson disease model. Autophagy. 2022;18:559–75. CAS PubMed Google Scholar * Zhang
F, Wu Z, Long F, Tan J, Gong N, Li X, et al. The roles of ATP13A2 gene mutations leading to abnormal aggregation of alpha-synuclein in Parkinson’s disease. Front Cell Neurosci.
2022;16:927682. CAS PubMed PubMed Central Google Scholar * Wang R, Tan J, Chen T, Han H, Tian R, Tan Y, et al. ATP13A2 facilitates HDAC6 recruitment to lysosome to promote
autophagosome-lysosome fusion. J Cell Biol. 2019;218:267–84. CAS PubMed PubMed Central Google Scholar * van der Heide A, Speckens AEM, Meinders MJ, Rosenthal LS, Bloem BR, Helmich RC.
Stress and mindfulness in Parkinson’s disease—a survey in 5000 patients. NPJ Parkinsons Dis. 2021;7:7. PubMed PubMed Central Google Scholar * Huang P, Zhang LY, Tan YY, Chen SD. Links
between COVID-19 and Parkinson’s disease/Alzheimer’s disease: reciprocal impacts, medical care strategies and underlying mechanisms. Transl Neurodegener. 2023;12:5. PubMed PubMed Central
Google Scholar * Damodaran S, Cressman JR, Jedrzejewski-Szmek Z, Blackwell KT. Desynchronization of fast-spiking interneurons reduces beta-band oscillations and imbalance in firing in the
dopamine-depleted striatum. J Neurosci. 2015;35:1149–59. PubMed PubMed Central Google Scholar * Seo HW, Suh JH, So SH, Kyung JS, Kim YS, Han CK. Subacute oral toxicity and bacterial
mutagenicity study of Korean Red Ginseng oil. J Ginseng Res. 2017;41:595–601. PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by the
National Key R&D Program of China (2022YFC3500300), the National Natural Science Foundation of China (82130109, 81973499), the CAMS Innovation Fund for Medical Sciences (CIFMS)
(2021-I2M-1-020). AUTHOR INFORMATION Author notes * These authors contributed equally: Sha-sha Wang, Ye Peng AUTHORS AND AFFILIATIONS * State Key Laboratory of Bioactive Substances and
Functions of Natural Medicines, Institute of Materia Medica & Neuroscience Center, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050, China Sha-sha
Wang, Ye Peng, Ping-long Fan, Jun-rui Ye, Wen-yu Ma, Qing-lin Wu, Hong-yun Wang, Xu Yan, Zhao Zhang, Shi-feng Chu & Nai-hong Chen * Science and Technology Innovation Center, Guangzhou
University of Chinese Medicine, Guangzhou, 510405, China Sha-sha Wang, Ping-long Fan, Wen-yu Ma, Qing-lin Wu & Nai-hong Chen * School of Pharmacy, Minzu University of China, Beijing,
100081, China Ye Peng * Shanxi Key Laboratory of Chinese Medicine Encephalopathy, National International Joint Research Center for Molecular Chinese Medicine, Shanxi University of Chinese
Medicine, Taiyuan, 030024, China Ya-juan Tian & Wen-bin He Authors * Sha-sha Wang View author publications You can also search for this author inPubMed Google Scholar * Ye Peng View
author publications You can also search for this author inPubMed Google Scholar * Ping-long Fan View author publications You can also search for this author inPubMed Google Scholar * Jun-rui
Ye View author publications You can also search for this author inPubMed Google Scholar * Wen-yu Ma View author publications You can also search for this author inPubMed Google Scholar *
Qing-lin Wu View author publications You can also search for this author inPubMed Google Scholar * Hong-yun Wang View author publications You can also search for this author inPubMed Google
Scholar * Ya-juan Tian View author publications You can also search for this author inPubMed Google Scholar * Wen-bin He View author publications You can also search for this author inPubMed
Google Scholar * Xu Yan View author publications You can also search for this author inPubMed Google Scholar * Zhao Zhang View author publications You can also search for this author
inPubMed Google Scholar * Shi-feng Chu View author publications You can also search for this author inPubMed Google Scholar * Nai-hong Chen View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS NHC, ZZ, SFC, and SSW conceived and designed the study. SSW, YP, PLF, JRY, WYM and XY performed the experiments. JRY, QLW, HYW and YJT
participated in data analysis. SSW and ZZ wrote the manuscript. NHC, SFC, ZZ, YP and WBH revised the manuscript. All the authors have read and approved the final version of the manuscript.
CORRESPONDING AUTHORS Correspondence to Zhao Zhang, Shi-feng Chu or Nai-hong Chen. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION WB FIGURES ORIGINAL RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article
under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such
publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, Ss., Peng, Y., Fan, Pl. _et al._ Ginsenoside Rg1 ameliorates stress-exacerbated
Parkinson’s disease in mice by eliminating RTP801 and α-synuclein autophagic degradation obstacle. _Acta Pharmacol Sin_ 46, 308–325 (2025). https://doi.org/10.1038/s41401-024-01374-w
Download citation * Received: 29 April 2024 * Accepted: 31 July 2024 * Published: 03 September 2024 * Issue Date: February 2025 * DOI: https://doi.org/10.1038/s41401-024-01374-w SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Parkinson’s disease (PD) * psychological stress * Rg1 * RTP801 * α-Synuclein * autophagy