Association of post-diagnostic use of cholera vaccine with survival outcome in breast cancer patients
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Download PDF Article Open access Published: 07 October 2020 Epidemiology
Association of post-diagnostic use of cholera vaccine with survival outcome in breast cancer patients Guoqiao Zheng1, Jan Sundquist1,2,3, Kristina Sundquist1,2,3 & …Jianguang Ji1 Show
authors British Journal of Cancer volume 124, pages 506–512 (2021)Cite this article
1986 Accesses
2 Altmetric
Metrics details
Subjects Breast cancerCancer epidemiology AbstractBackgroundExpensive cancer treatment calls for alternative ways such as drug repurposing to develop effective drugs. The aim of this study was to analyse the effect of post-diagnostic use of cholera
vaccine on survival outcome in breast cancer patients.
MethodsCancer diagnosis and cholera vaccination were obtained by linkage of several Swedish national registries. One vaccinated patient was matched with maximum two unvaccinated individuals based
on demographic, clinical and socioeconomic factors. We performed proportional Cox regression model to analyse the differences in overall and disease-specific survivals between the matched
patients.
ResultsIn total, 617 patients received cholera vaccine after breast cancer diagnosis. The median (interquartile range) time from diagnosis to vaccination was 30 (15–51) months and from vaccination
to the end of follow-up it was 62 (47–85) months. Among them, 603 patients were matched with 1194 unvaccinated patients. Vaccinated patients showed favourable overall survival (hazard ratio
(HR): 0.54, 95% confidence interval (CI): 0.37–0.79) and disease-specific survival (HR: 0.53, 95% CI: 0.33–0.84), compared to their unvaccinated counterpart. The results were still
significant in multiple sensitivity analyses.
ConclusionsPost-diagnostic use of cholera vaccine is associated with a favourable survival rate in breast cancer patients; this provides evidence for repurposing it against breast cancer.
Similarcontent being viewed by others Effectiveness of COVID-19 vaccines against severe COVID-19 among patients with cancer in Catalonia, Spain Article Open access 19 June 2024 The COVID-19
pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020 Article Open access 30 November 2020 Considerations for the treatment of pancreatic cancer
during the COVID-19 pandemic: the UK consensus position Article Open access 08 July 2020 Background
Breast cancer is the most common cancer found among women worldwide. Although the survival of breast cancer is increasing with the advancement of treatment, it is still the leading cause of
death due to cancer among women.1 The development of targeted therapy on breast cancer is both time-consuming and expensive. It is estimated that a typical drug development usually takes
15–18 years and costs approximately 2–3 billion dollars.2 In a clinical setting, cancer patients and their involved family members suffer from the pressure of meeting the costs of these
expensive cancer drugs financially as well as the emotional burden associated with the treatment. Some of these expensive cancer drugs are not covered by the public healthcare system in many
developing countries thus leading to a higher mortality rate among insolvent patients with breast cancer.3 In this scenario, drug repurposing is an alternative and efficient way for drug
development, which identifies the new indication of the drug outside the scope of the original medical condition. For example, raloxifene, which was originally used to treat osteoporosis,
was approved by the U.S Food and Drug Administration for invasive breast cancer treatment in 2007.4
Cholera vaccine is widely used among people travelling to regions with a high prevalence of cholera infection. Cholera toxin is composed of two subunits: the A subunit (CTA) and the B
subunit (CTB). The functional component of the vaccine is CTA. Many studies have shown that cholera toxin can suppress the proliferation of several cancer cell lines, including breast
cancer, by inhibiting growth factor signal transduction pathway or by triggering apoptosis.5 Cholera toxin has been reported to have immunomodulatory properties.6,7,8,9 In vitro experiments
have shown that recombinant CTB can activate dendritic cells and enhance antitumour immunity.6 Cholera toxin suppressed carcinogenesis in a mouse model of inflammation-driven sporadic colon
cancer.10 Recently, post-diagnostic use of cholera vaccine has been shown to be of benefit in disease-specific survival of colorectal and prostate cancers.11,12 The aim of this study was to
evaluate whether the antitumour effect of cholera vaccine could be valid in breast cancer patients by analysing data derived from several Swedish national registries. To the best of our
knowledge, this is the first national population-based cohort study on the association of post-diagnostic use of cholera vaccine and breast cancer survival, which may provide new evidence
for breast cancer treatment.
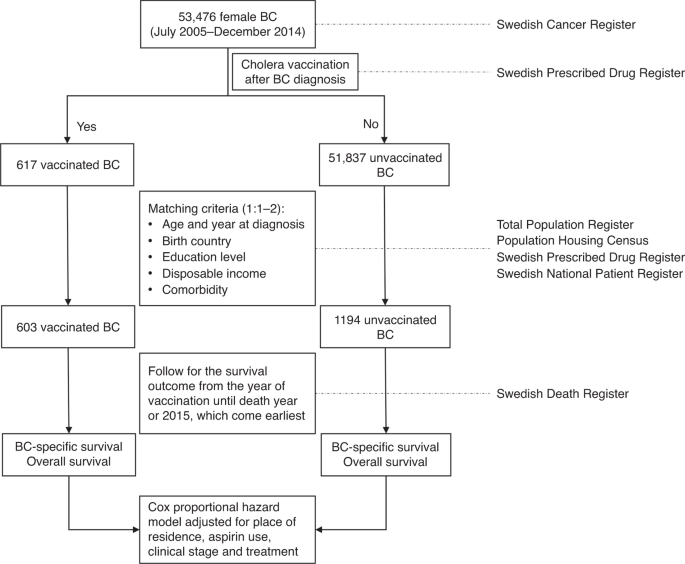
MethodsThis study was performed based on the linkage of several national Swedish registries and how the study was performed is shown in Fig. 1. Female patients, who were diagnosed with primary
invasive breast cancer, were identified from the Swedish Cancer Registry by using the Tenth Version of International Classification of Disease (ICD-10) code of C50. The clinical stage of
breast cancer at diagnosis was classified into four groups (stage I, stage II, stage II and stage IV) based on the tumour size (T), nodal status (N) and the presence of metastasis (M)
according to the seventh edition of the American Joint Committee on Cancer staging manual.13 The TNM staging system has been used in the cancer registry since 2003.
Fig. 1: Flowchart ofthe study.
BC breast cancer.
Full size imageData on post-diagnostic use of cholera vaccine were extracted from the Swedish Prescribed Drug Register. As this register was established in July 2005 and was updated until December 2014,
breast cancer patients diagnosed during only this period were included in the study. The Anatomical Therapeutic Chemical (ATC) Classification System was applied in the drug register and the
administration of cholera vaccine was identified by code “J07AE01”. The ATC code for aspirin use was B01AC06, which was also considered in our analysis, as aspirin use in breast cancer
patients was associated with decreased mortality.14 As the information on hormone receptor status was not available, we used medical treatments as a proxy for the identification of hormone
receptor status, which included treatment with anti-oestrogens (L02BA), aromatase inhibitors (L02BG) and gonadotropin-releasing hormone analogues (L02AE).
The date of death, as well as the underlying cause of death during the study period, was obtained from the Swedish Death Register. The primary outcome was death due to breast cancer (ICD-10
code: C50) and the secondary outcome was death due to all causes (ICD-10 code: A00 to Z99).
Patients’ demographic and socioeconomic factors including country of birth (Sweden, other European countries and non-European countries), educational level (1–9 years, 10–11 years and ≥12
years of education), disposable income (lowest, middle–low, middle–high, highest) and place of residence (big cites, other southern and northern cities) at diagnosis were obtained from the
Total Population Register and the Population Housing Census. Comorbidity at the diagnosis of breast cancer was extracted from the Swedish National Patient Register and the diseases for the
calculation of Charlson Comorbidity Index were considered.15
A total of 52,454 breast cancer patients were diagnosed between July 2005 and December 2014, among which 617 had post-diagnostic use of cholera vaccine. The characteristics of patients
stratified by cholera vaccination are shown in Supplementary Table 1. Considering the possibility that patients using cholera vaccine might be healthier or associated with better
socioeconomic status, we matched each vaccinated patient with at most two patients who did not receive the vaccine. The matching conditions included year of diagnosis, age at diagnosis
(5-year gap), education level, comorbidity (yes or no), disposable income and country of birth. Pearson’s Chi-square tests, or Fisher Exact tests when appropriate, were performed to compare
the difference of these characteristics between the two groups. The follow-up commenced from the date of administration of cholera vaccine for the vaccinated patients. For the unvaccinated
patients, it commenced from the date of vaccination matched in each stratum. The follow-up was terminated in the year of death or 2015, whichever came earliest. Cox proportional hazard
regression model was used to analyse the effect of post-diagnostic use of cholera vaccine on all-cause and disease-specific survival with further adjustment of clinical stage, aspirin use,
place of residence and hormone therapy. Kaplan–Meier plot was generated for disease-specific survival since the cholera vaccination.
To avoid chance findings, several sensitivity analyses were performed. The effect of competing risks as a result of death from other causes was analysed by using the sub-distribution hazards
model proposed by Fine and Gray.16 The exposure of cholera vaccine was considered with 1-year lag given that short duration of exposure is unlikely to be associated with the mortality
outcome. As they were able to travel abroad, patients who received cholera vaccine could have been healthier and associated with better socioeconomic status compared to their non-receiving
counterparts. To avoid the indication bias, effects of post-diagnostic use of antimalarial medication on the breast cancer survival were analysed by using the same matching approach. In
Sweden, malarone (atovaquone/proguanil) (ATC code: P01BB51), mefloquine (ATC code: P01BA05 and P01BC02) and doxycycline are usually recommended for the prevention of malaria. However,
doxycycline is normally used for the treatment of bacterial infection, thus it is not suitable to be included in this study.17 In addition, influence of use of cholera vaccine before breast
cancer diagnosis on the survival rate was evaluated. Finally, we performed sensitivity analyses by excluding patients with advanced breast cancer (clinical stages of III and IV) and by
including patients with hormone therapy.
All the statistical analyses were performed in SAS environment (version 9.3). The survival curve was generated in R (version 3.3.5). Statistical comparisons were two tailed and P value <
0.05 was considered statistically significant.
ResultsAmong the 617 breast cancer patients with post-diagnostic use of cholera vaccine, the median (interquartile range (IQR)) time from breast cancer diagnosis to vaccination was 30 (15–51)
months, and the median (IQR) time from vaccination to the end of follow-up was 62 (47–85) months. The median age at diagnosis of breast cancer was 64 years. In the matched setting, 603
vaccinated patients were able to match with 1194 unvaccinated individuals. The demographic, clinical and socioeconomic characteristics of the two groups are displayed in Table 1. Age at
diagnosis, year of diagnosis, birth country, education level, disposable income and comorbidity were found to be well distributed based on Pearson’s Chi-square test. As for the unmatched
factors, no significant difference was found for place of residence, use of aspirin and clinical stage. In the final regression model, these unmatched factors were adjusted. Most of the
patients were born in Sweden (92%) and diagnosed before the age of 65 years (80%). Approximately half of them had >11 years of education (54%), had the highest disposable income (41%) and
were living in big cities (53%). Nearly 15% of them had a history of aspirin use and 14% had comorbidity upon diagnosis. More than half of them were diagnosed with stage II breast
cancer.
Table 1 Characteristics of matched breast cancer patients diagnosed from 2005 to 2014.Full size tableThe Kaplan–Meier survival curve in Fig. 2 shows that the disease-specific survival in patients with cholera vaccination was better than those without. After 5 years of cholera vaccination,
the disease-specific survival (95% confidence interval (CI)) was 95.3% (93.4–97.4%) for patients with vaccination and 91.9% (90.2–93.7%) for those without. After 10 years, the survival rate
(95% CI) was 94.1% (91.8–96.5%) and 89.9% (88.0–91.9%), respectively. Table 2 displays the effects of post-diagnostic use of cholera vaccine on overall and disease-specific survival in the
matched breast cancer patients. After the respective median (IQR) follow-up time of 62 (47–85) and 62 (45–85) months, 39 vaccinated and 127 unvaccinated patients died, thus resulting in a
better overall survival for patients with vaccine (hazard ratio (HR): 0.54, 95% CI: 0.37–0.79). Considering that death was only caused by breast cancer, the difference in survival
probability was significant (HR: 0.53, 95% CI: 0.33–0.84).
Fig. 2: Kaplan–Meier plot for disease-specific survival stratified by cholera vaccination.The area within the band is the confidence interval of the survival probability.
Full size imageTable 2 Effects of post-diagnostic use of cholera vaccine on breast cancer survival.Fullsize table
Table 3 displays the results from the sensitivity analyses. While considering the effect of competing risks from other cause of death, the vaccinated patients still experienced better
survival compared to their unvaccinated counterparts (HR: 0.55, 95% CI: 0.37–0.81). By defining the exposure period as 1 year after the cholera vaccine administration, similar sets of
analyses were performed for overall (HR: 0.57, 95% CI: 0.38–0.88) and disease-specific survival (HR: 0.56, 95% CI: 0.33–0.95). A total of 1013 patients were vaccinated before their breast
cancer diagnosis. After applying the same approach, cholera vaccination before breast cancer diagnosis did not show a significant effect on the disease-specific survival (HR: 1.04, 95% CI:
0.66–1.64). When the analysis included only patients with clinical stages of I and II breast cancer, the result was still significant (HR: 0.59, 95% CI: 0.37–0.94). Among individuals with
hormone therapy, the protective nature of the vaccination showed borderline significance (HR: 0.60, 95% CI: 0.34–1.04).
Table 3 Sensitivity analyses.Full size tableNext, the effect (if any) of antimalarial medication was assessed to account for chance findings due to indication bias. Notably, 598 patients had post-diagnostic antimalarial medication,
and 130 of them had previously used cholera vaccine. To remove the protective effects of cholera vaccine, 468 unvaccinated patients were retained. After matching 444 patients with 873
individuals without antimalarial medication, we found that antimalarial medication was not significantly associated with disease-specific survival (HR: 1.14, 95% CI:
0.57–2.29).
DiscussionWith better understanding of cancer biology and more advanced technology, various antitumour drugs have been developed to fight against cancer. However, the process from drug discovery to
the ultimate approval for clinical application is usually lengthy and costly with an accompanying low success rate. Drug repurposing for oncology that studies the antitumour effects for
drugs available for other diseases is relatively cheaper and faster than the classical drug discovery process as the safety and toxicity of the drugs are already known.18 The aim of the
current study was therefore to serve the drug repurposing approach for breast cancer. To our best knowledge, it is the first nationwide population-based study evaluating the association
between post-diagnostic use of cholera vaccine and disease-specific survival in breast cancer. Consistent with the results reported for colorectal and prostate cancer,11,12 vaccinated breast
cancer patients were observed with 47% decreased hazard from breast cancer compared to the unvaccinated individuals. The results remained significant in various sensitivity analyses.
When estimating the effects of medication use on health outcomes, many issues should be considered, such as immortal time bias, indication bias, confounding, etc. In order to control
immortal time bias, we started the follow-up from the administration of cholera vaccination. Compared to breast cancer patients without cholera vaccination (Supplementary Table 1), those who
had been vaccinated tended to be younger, diagnosed more recently, born in Sweden, with longer education years, higher personal disposable income and less comorbidity, thus suggesting that
these patients might survive long enough to receive the vaccination. To control this bias, the matching strategy was used to reduce the confounding effect from those factors. Consistently,
we also observed the slightly larger proportion of early stage (I and II) breast cancer in vaccinated patients, so a sensitivity analysis only including patients with early stage breast
cancer was performed. Another important prognostic factor is the treatment for breast cancer. Despite lacking detailed treatment information, we obtained the medication of hormonal therapy
from the Swedish Prescribed Drug Register. No difference in the distribution of the therapy in the cohort stratified by cholera vaccination was found thus demonstrating the unlikely
discrepancy of breast cancer treatment in Sweden where universal healthcare is accessible for all citizens at a minimal cost. As for the indication bias, the reasons to have cholera vaccine
after breast cancer diagnosis were unknown, so we could not largely rule it out. However, we tried to investigate it by checking the survival in breast cancer patients with post-diagnostic
antimalarial vaccination as those individuals represented a group similar to those with cholera vaccination who were able to travel abroad.
The mechanism behind the association is not clear yet, but some in vitro and in vivo studies have shown some evidence of antitumour effect of cholera toxin. Suppression of cell proliferation
either by inhibiting growth factor signals or by triggering apoptosis was observed in several cancer cell lines treated with cholera toxin, including bladder,19 ovarian,20 breast,5 lung5
and pancreatic cancers,21 hepatocellular carcinoma and glioma.22 Cho-Chung et al. reported growth arrest of 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in rats treated with a
daily injection of cholera toxin, and the tumours shrank 85% in 4–5 weeks.23 Similar results were found in human breast cancer cells (MCF-7).23 Growth inhibitions both in vivo and in vitro
were dose dependent and correlated with increases of cyclic adenosine 3’:5’-monophosphate (cAMP) content and type II cAMP-dependent protein kinase activity as well as a decrease of
oestrogen-binding activity.23 In addition, acetylation of P53 protein was observed in cultured MCF-7 cells treated with CTB subunit by upregulating the expression of P300, an enzyme that
acetylates histones, and consequently it induced apoptosis.5 Antitumour effects of cholera toxin may partly be attributed to its immunomodulatory properties. It is considered to be a
promising drug in treatment of autoimmune and allergic diseases.24 Recombinant CTB subunit could promote dendritic cell maturation presenting with upregulated expression of major
histocompatibility complex class II and B7-2 on dendritic cell and enhanced secretion of interleukin (IL)-12 from dendritic cell, which is important for T cell stimulation and further
antitumour immunity.6 Suppression of carcinogenesis in a mouse model of inflammation-driven colon cancer was observed by the oral administration of cholera toxin. This finding was
accompanied with the downregulated neutrophils and upregulated regulatory T cells, IL-10 and tumour necrosis factor α in the colonic mucosa.10 This study indicated that gut microbiota
antigenic stimuli may affect the immune system and further cancer development. As for breast cancer, the correlation between gut microbiota and mammary tumorigenesis can explain the role of
immunity in our finding to some extent.25 Interestingly, immunomodulatory property was not only found in cholera vaccine but also seasonal influenza vaccines. Intratumoural injection of the
seasonal flu shot could reduce tumour growth by increasing antitumour CD8+ T cells and decreasing regulatory B cells within the tumour. In addition, lung cancer patients with influenza
infections had lower cancer-specific mortality.26 This further supported the possibility of protective effect of cholera vaccination in our study. However, we acknowledged that some
undetected variables such as smoking, physical activity, body mass index and diet can also confound the current association although consideration of other socioeconomic factors like
disposable income, educational level
and place of residence could adjust them somewhat as they are correlated to each other.27,28,29 Other observational studies and clinical trials are needed to validate the association.
The strengths and limitations of the study need to be addressed. Use of Swedish nationwide registry data provided adequate sample size and, consequently, enough statistical power to detect
the difference in survival between vaccinated and unvaccinated patients. It also enabled us to avoid information bias by providing an accurate record on the cancer identification and drug
administration. By linking several Swedish registers, a facet of demographic, clinical and socioeconomic factors, which may affect breast cancer survival, could be considered for adjustment.
Some other health-related indicators such as smoking, physical activity, body mass index and diet were not available in our study, which may affect our findings. However, consideration of
other socioeconomic factors like disposable income, educational level and place of residence can adjust them on some level. Multiple sensitivity analyses were done, which strengthened the
robustness of the results. Notably, analysis of the association between antimalarial medication and breast cancer survival was performed to avoid the indication bias, given the fact that
vaccinated patients might be healthier and associated with better socioeconomic status. Application of matching design improved the comparability between groups and, in addition, helped
avoid confounding. However, the protective effect of cholera vaccine was only observed in the matched patients who presented with specific characteristics, for example, largely with early
clinical stage and hormonal therapy (Table 1). Studies among patients with late-stage breast cancer are needed. In addition, information on hormonal receptor status is required to
investigate whether the effect is subtype specific. We were unable to analyse the dose–response effect as the variation of the patients with vaccination was very small. Further studies are
required to generalise the results to the other population and to explore the dose–response relationship between cholera vaccination and breast cancer survival.
ConclusionsBased on this nationwide study, we found that post-diagnostic use of cholera vaccine in breast cancer patients was associated with better overall and disease-specific survival. This
association was still significant after considering competing risks and 1-year lag of exposure. This study suggests that cholera vaccine may be a good candidate for drug repurposing for
breast cancer. However, our results should be interpreted carefully as some other undetected factors such as physical activity and dietary habits may have masked the current association
despite our stringent analyses. Further studies are required to validate our finding in other populations and to explore the mechanisms behind the observed associations.
References Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A. & Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
PubMed Google Scholar
Aggarwal, S., Verma, S. S., Aggarwal, S. & Gupta, S. C. Drug repurposing for breast cancer therapy: old weapon for new battle. Semin. Cancer Biol.
https://doi.org/10.1016/j.semcancer.2019.09.012 (2019).
Ramsey, S. D., Bansal, A., Fedorenko, C. R., Blough, D. K., Overstreet, K. A., Shankaran, V. et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J.
Clin. Oncol. 34, 980 (2016).
Article CAS PubMed PubMed Central Google Scholar
Pushpakom, S., Iorio, F., Eyers, P. A., Escott, K. J., Hopper, S., Wells, A. et al. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 18, 41 (2019).
Article CAS PubMed Google Scholar
Dastjerdi, M. N., Salahshoor, M. R., Mardani, M., Hashemibeni, B. & Roshankhah, S. The effect of CTB on P53 protein acetylation and consequence apoptosis on MCF-7 and MRC-5 cell lines. Adv.
Biomed. Res. 2, 24 (2013).
Isomura, I., Yasuda, Y., Tsujimura, K., Takahashi, T., Tochikubo, K. & Morita, A. Recombinant cholera toxin B subunit activates dendritic cells and enhances antitumor immunity. Microbiol.
Immunol. 49, 79–87 (2005).
Article CAS PubMed Google Scholar
Kawamura, Y. I., Kawashima, R., Shirai, Y., Kato, R., Hamabata, T., Yamamoto, M. et al. Cholera toxin activates dendritic cells through dependence on GM1‐ganglioside which is mediated by
NF‐κB translocation. Eur. J. Immunol. 33, 3205–3212 (2003).
Article CAS PubMed Google Scholar
Lavelle, E. C., Jarnicki, A., McNeela, E., Armstrong, M. E., Higgins, S. C., Leavy, O. et al. Effects of cholera toxin on innate and adaptive immunity and its application as an
immunomodulatory agent. J. Leukoc. Biol. 75, 756–763 (2004).
Article CAS PubMed Google Scholar
Sun, J. B., Czerkinsky, C. & Holmgren, J. Mucosally induced immunological tolerance, regulatory T cells and the adjuvant effect by cholera toxin B subunit. Scand. J. Immunol. 71, 1–11
(2010).
Article CAS PubMed Google Scholar
Doulberis, M., Angelopoulou, K., Kaldrymidou, E., Tsingotjidou, A., Abas, Z., Erdman, S. E. et al. Cholera-toxin suppresses carcinogenesis in a mouse model of inflammation-driven sporadic
colon cancer. Carcinogenesis 36, 280–290 (2014).
Article PubMed PubMed Central Google Scholar
Ji, J., Sundquist, J. & Sundquist, K. Association between post-diagnostic use of cholera vaccine and risk of death in prostate cancer patients. Nat. Commun. 9, 2367 (2018).
Article PubMed PubMed Central Google Scholar
Ji, J., Sundquist, J. & Sundquist, K. Cholera vaccine use is associated with a reduced risk of death in patients with colorectal cancer: a population-based study. Gastroenterology 154,
86.e1–92.e1 (2018).
Article Google Scholar
Edge, S. B., Byrd, D. R., Carducci, M. A., Compton, C. C., Fritz, A. & Greene, F. AJCC Cancer Staging Manual (Springer, New York, 2010).
Holmes, M. D., Chen, W. Y., Li, L., Hertzmark, E., Spiegelman, D. & Hankinson, S. E. Aspirin intake and survival after breast cancer. J. Clin. Oncol. 28, 1467 (2010).
Article CAS PubMed PubMed Central Google Scholar
Quan, H., Sundararajan, V., Halfon, P., Fong, A., Burnand, B., Luthi, J.-C. et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 43,
1130–1139 (2005).
Fine, J. P. & Gray, R. J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94, 496–509 (1999).
Article Google Scholar
Lindqvist, L. & Lindkvist, P. Råd och profylax vid resa. https://lakemedelsboken.se/kapitel/antibiotika_och_reseprofylax/rad_och_profylax_vid_resa.html#l2_61 (2018).
Bertolini, F., Sukhatme, V. P. & Bouche, G. Drug repurposing in oncology—patient and health systems opportunities. Nat. Rev. Clin. Oncol. 12, 732 (2015).
Article PubMed Google Scholar
Zheng, X., Ou, Y., Shu, M., Wang, Y., Zhou, Y., Su, X. et al. Cholera toxin, a typical protein kinase A activator, induces G1 phase growth arrest in human bladder transitional cell carcinoma
cells via inhibiting the c‑Raf/MEK/ERK signaling pathway. Mol. Med. Rep. 9, 1773–1779 (2014).
Article CAS PubMed Google Scholar
Han, X., Papadopoulos, A. J., Jones, T., Devaja, O. & Raju, K. S. Cholera toxin‐induced alteration of the phenotype and behaviour of an ovarian carcinoma cell line, SR8. Immunol. Cell Biol.
77, 377–384 (1999).
Article CAS PubMed Google Scholar
Ohmura, E., Wakai, K., Isozaki, O., Murakami, H., Onoda, N., Emoto, N. et al. Inhibition of human pancreatic cancer cell (MIA PaCa-2) growth by cholera toxin and 8-chloro-cAMP in vitro. Br.
J. Cancer 67, 279 (1993).
Article CAS PubMed PubMed Central Google Scholar
Li, Y., Yin, W., Wang, X., Zhu, W., Huang, Y. & Yan, G. Cholera toxin induces malignant glioma cell differentiation via the PKA/CREB pathway. Proc. Natl Acad. Sci. USA 104, 13438–13443
(2007).
Article CAS PubMed PubMed Central Google Scholar
Cho-Chung, Y. S., Clair, T., Shepheard, C. & Berghoffer, B. Arrest of hormone-dependent mammary cancer growth in vivo and in vitro by cholera toxin. Cancer Res. 43, 1473–1476 (1983).
CAS PubMed Google Scholar
Sánchez, J. & Holmgren, J. Cholera toxin—a foe & a friend. Indian J. Med. Res. 133, 153 (2011).
PubMed PubMed Central Google Scholar
Lakritz, J. R., Poutahidis, T., Mirabal, S., Varian, B. J., Levkovich, T., Ibrahim, Y. M. et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget 6, 9387 (2015).
Article PubMed PubMed Central Google Scholar
Newman, J. H., Chesson, C. B., Herzog, N. L., Bommareddy, P. K., Aspromonte, S. M., Pepe, R. et al. Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to
hot and serves as an immunotherapy for cancer. Proc. Natl Acad. Sci. USA 117, 1119–1128 (2020).
Article CAS PubMed Google Scholar
Oftedal, S., Vandelanotte, C. & Duncan, M. J. Patterns of diet, physical activity, sitting and sleep are associated with socio-demographic, behavioural, and health-risk indicators in adults.
Int. J. Environ. Res. Public Health 16, 2375 (2019).
Article PubMed Central Google Scholar
Fredj, S. B., Ghammem, R., Maatoug, J., Zammit, N., Hasni, Y., Chelly, S. et al. Association between physical inactivity and socioeconomic factors and lifestyle among Tunisian adolescents.
Endocr. Abstr. 63, P977 (2019).
Novak, D., Lovro, Š., Antala, B., Emeljanovas, A., Mieziene, B., Milanović, I. et al. The associations between socioeconomic status and lifestyle factors in European adolescents: a
population-based study. Acta Facultatis Educationis Phys. Universitatis Comen. 57, 111–124 (2017).
Article Google Scholar
Download references
AcknowledgementsWe thank Patrick Reilly for language editing.
Author informationAuthors and Affiliations Center for Primary Health Care Research, Lund University/Region Skåne, Malmö, Sweden
Guoqiao Zheng, Jan Sundquist, Kristina Sundquist & Jianguang Ji
Department of Family Medicine and Community Health, Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Jan Sundquist & Kristina Sundquist
Center for Community-based Healthcare Research and Education (CoHRE), Department of Functional Pathology, School of Medicine, Shimane University, Shimane, Japan
Jan Sundquist & Kristina Sundquist
AuthorsGuoqiao ZhengView author publications You can also search for this author inPubMed Google Scholar
Jan SundquistView author publications You can also search for this author inPubMed Google Scholar
Kristina SundquistView author publications You can also search for this author inPubMed Google Scholar
Jianguang JiView author publications You can also search for this author inPubMed Google Scholar
ContributionsDesign: J.J., G.Z.; acquisition of data: J.S., K.S.; statistical analysis and interpretation: all authors; manuscript writing: G.Z. and all other authors; approval of the final text: all
authors.
Corresponding author Correspondence to Guoqiao Zheng.
Ethics declarations Ethics approval and consent to participateAs this study was based on anonymous information from the Swedish national registries and study participants were never contacted, it was approved by the Ethics Committee of Lund University
without requirement for informed consent. Through advertisements in the major newspapers, people could chose to opt out before the research database were constructed. The study was performed
in accordance with the Declaration of Helsinki.
Consent for publicationNot applicable.
Data availabilityThe use of these data is governed by an agreement with the Swedish National Board of Health and Welfare with J.S., which does not allow redistribution of original data. Anyone who is
interested in the data set should contact the Swedish National Board of Health and Welfare and apply for the access to the data set (https://www.socialstyrelsen.se/statistics). If anyone
gets the approval, they can get access to the database in the same manner as the authors. The project database is located at Center for Primary Health Care in Malmö, Sweden.
Competinginterests
The authors declare no competing interests.
Funding informationNot applicable.
Additional informationNote This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons
Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary informationSupplementary table1Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third
party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
About this articleCite this article Zheng, G., Sundquist, J., Sundquist, K. et al. Association of post-diagnostic use of cholera vaccine with survival outcome in breast cancer patients. Br J
Cancer 124, 506–512 (2021). https://doi.org/10.1038/s41416-020-01108-9
Download citation
Received: 06 April 2020
Revised: 30 August 2020
Accepted: 16 September 2020
Published: 07 October 2020
Issue Date: 19 January 2021
DOI: https://doi.org/10.1038/s41416-020-01108-9
Share this article Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article.
Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative