Regγ ablation impedes dedifferentiation of anaplastic thyroid carcinoma and accentuates radio-therapeutic response by regulating the smad7-tgf-β pathway
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Anaplastic thyroid cancer (ATC) is the most aggressive human thyroid malignancy, characterized by dedifferentiation and resistance to radioiodine therapy. The underlying mechanisms
regulating ATC dedifferentiation are largely unknown. Here, we show that REGγ, a noncanonical proteasome activator highly expressed in ATC, is an important regulator of differentiation in
ATC cells. Ablation of REGγ significantly restored expression of thyroid-specific genes, enhanced iodine uptake, and improved the efficacy of 131I therapy in ATC xenograft models.
Mechanistically, REGγ directly binds to the TGF-β signaling antagonist Smad7 and promotes its degradation, leading to the activation of the TGF-β signal pathway. With gain- and
loss-of-function studies, we demonstrate that Smad7 is an important mediator for the REGγ function in ATC cell dedifferentiation, which is supported by expression profiles in human ATC
tissues. It seems that REGγ impinges on repression of thyroid-specific genes and promotion of tumor malignancy in ATC cells by activating the TGF-β signal pathway via degradation of Smad7.
Thus, REGγ may serve as a novel therapeutic target for allowing radioiodine therapy in anaplastic thyroid cancer patients with poor prognosis. SIMILAR CONTENT BEING VIEWED BY OTHERS DUAL
TARGETING OF MAPK AND PI3K PATHWAYS UNLOCKS REDIFFERENTIATION OF _BRAF_-MUTATED THYROID CANCER ORGANOIDS Article Open access 20 November 2023 REGULATION OF CANCER STEM CELL ACTIVITY BY
THYROID HORMONE RECEPTOR Β Article 07 March 2022 THYROID HORMONE RECEPTOR Α1: A NOVEL REGULATOR OF THYROID CANCER CELL DIFFERENTIATION Article 26 August 2023 INTRODUCTION Anaplastic thyroid
cancer (ATC) is one of the most deadly human diseases. Due to its invasive nature and resistance to radiotherapy, ATC has an extremely low cure rate with a median survival of less than 6
months [1], responsible for more than half of all thyroid cancer deaths [2]. However, patients with differentiated thyroid carcinoma have an excellent 10-year survival ranging between 80 and
95% [3]. Radioactive iodine therapy improves the survival rate of patients with differentiated metastastatic thyroid cancer. The sodium iodide symporter (NIS), a transmembrane glycoprotein
in the thyroid follicular cells, catalyzes the accumulation of iodide in the thyroid gland, allowing the treatment of differentiated thyroid cancers by radioactive iodine [4]. However, ATC
fails to express adequate amounts of thyroid-specific genes, primarily due to _NIS_ [5], leading to its resistance to radioiodine therapy. Therefore, innovative approaches for recovery of
NIS expression in poorly differentiated thyroid cancers might promote therapy via iodine uptake [6]. TGF-β functions as a tumor promoter through increasing tumor cell invasion and metastasis
in late-stage cancers. TGF-β1 is overexpressed in ATC and silencing TGF-β1 inhibits cell migration and invasion of ATC cells [7]. Smad3 activation inhibits expression of Pax8 and its
DNA-binding activity, mediating TGF-β-induced downregulation of NIS in thyroid follicular cells [8]. BRAF appears to induce secretion of TGF-β in human PTC and inhibit _NIS_ expression [9],
substantiating that TGF-β plays an important role in thyroid cancer progression. REGγ (also known as PA28γ, PSME3, Ki antigen) belongs to the 11 S family of proteasome activators to promote
ubiquitin and ATP-independent degradation of proteins [10, 11]. REGγ regulates cell cycle, inflammation, angiogenesis, and additional biological processes [12,13,14,15,16]. In addition, REGγ
is overexpressed in several tumors, including thyroid cancer, displaying oncogenic actions [17,18,19]. However, it is unclear if overexpressed REGγ in ATC promotes its malignancy. In this
study, we demonstrate that REGγ enhances dedifferentiation of ATC cells. Depletion of REGγ restored the expression of thyroid-specific genes in ATC cells and improved radioiodine uptake in
vitro and in vivo, therefore, improving 131I therapy in ATC xenograft tumors. REGγ mediates upregulation of the TGF-β pathway by degrading Smad7, since inactivation of Smad7 prevents the
recovery of thyroid-specific genes in REGγ-deficient ATC cells. Thus, inhibition of the REGγ proteasome might be a promising approach for ATC patients. METHODS CELLS K18 ATC and HEK293T
cells were purchased from American Type Culture Collection (ATCC, USA). SW1736 ATC was from James A. Fagin’s laboratory. The REGγ knockdown stable cell lines were generated by integration of
retroviral ShRNA vector specific for REGγ to produce ShR (ShRNA against REGγ) or a negative control from OriGene (Rockville, MD) to produce ShN (ShRNA as a negative control) cells. ATC cell
lines and HEK293T cell line were cultured in the 1640 and DMEM medium supplied with 10% fetal bovine serum (Gibco), respectively. The 293-REGγ inducible cell lines were previously
generated. PLASMIDS, CONSTRUCTS, AND EXPRESSION HA-REGγ (pcDNA3.1), Flag-Smad7 (pcDNA3.1), PSG5-HA-Smad7, plvx-GFP-Smad7, plvx-Luc-G418, and NIS promoter luciferase (NIS-Luc) reporter
plasmid (pGL2-Basic) containing the −2000/ + 375 sequence of NIS promoter were constructed in our laboratory. Smad3 siRNA (F-5′-CCAGUGACCACCAGAUGAA-3′) and Smad7 siRNA
(F-5′-CUCUCUGGAUAUCUUCUAUTT-3′ and R-5′-AUAGAAGAUAUCCAGAGAGTT-3′) were synthesized by Genepharma. Plasmids or siRNA were transfected to different cells and cultured for 36 h or 72 h. IN
VITRO 131I UPTAKE OF ATC CELLS Overall, 5 × 105 ShN and ShR ATC cancer cells (SW1736 and K18) were plated in triplicates in 12-well plates. After washing with cold HBSS three times, cells
were incubated for the indicated time at 37 °C with 1 ml of HBSS containing 1 μCi carrier-free Na131I and 10 μM NaI. In control groups, cells were treated with 300 μM NaClO4, a competitive
inhibitor of NIS, for 30 min to determine the nonspecific radioiodine uptake. Then, cells were washed with ice-cold HBSS for three times, lysed in 1 ml 0.33 M NaOH. The radioactivity was
measured with a Perkinelmer 2470 gamma-counter. LUCIFERASE ASSAYS SW1736 and K18 ATC cells were washed with cold PBS three times after transfection with NIS-Luc reporter for 36 h, harvested
in the lysis buffer provided in the Luciferase Assay Kit (Promega). After one cycle of freezing and thawing, the cell lysates were centrifuged at 12,000 rpm for 10 min at 4 °C. Then 20 μl of
supernatant was added to an equal amount of luciferase assay substrate. Luminescence was measured as relative light units using the LUMIstar OPTIMA (BMG Labtech) illuminometer. WESTERN BLOT
ANALYSIS, IMMUNOPRECIPITATION, AND IN VITRO PROTEOLYTIC ANALYSIS Cells were collected in NP40 lysis buffer and minced tissues were lysed in RIPA buffer on ice for 15 min. For NP40 lysed
samples, protein concentrations were determined by BCA assay kit (Beyotime, China). Equal amount of proteins were run on a 10–12% SDS–PAGE, transferred to a nitrocellulose membrane
(Millipore, MA, USA), and then immunoblotted with the NIS (Millipore and Proteintech 24324-1-AP), Pax8 (Millipore and Bioworld BS3459), REGγ, p-Smad3, Smad3 (Proteintech), Smad7 (Abcam
ab55493 and Proteintech), or β-actin antibodies (CST 3102 and Sigma A5441) overnight. After incubation with secondary fluorescent antibodies for 1 h, the antibody-bound proteins were
analyzed by a fluorescent western blot imaging system (Odyssey). For co-immunoprecipitation assay, HEK293 cells were transiently transfected with plasmids expressing pcDNA3.1-HA-REGγ and
pcDNA3.1-Flag-Smad7 for 48 h. Then, cells were harvested in CHAPS lysis buffer. Extracts were incubated overnight with 2.5 µg of anti-HA or anti-Flag (Santa Cruz Biotechnology, Heidelberg,
Germany) antibody in the presence of Protein G beads from a Protein G Immunoprecipitation kit (Sigma-Aldrich, Buchs, Switzerland). Complexes were washed, denatured, and eluted for western
blot analysis. For in vitro proteolytic analysis, Smad7 protein was generated by in vitro translation [10]. The protein degradation experiment was conducted by incubating Smad7, 20S
proteasome (Boston Biochem), and purified REGγ for 3–6 h in 25-μl reaction volume at 30 °C with appropriate controls. The results were analyzed by western blotting. QUANTITATIVE REAL-TIME
PCR The total RNA was extracted from cells or tumor tissues using TRIZOL reagent (TaKaRa). A pool of cDNA was synthesized from 1 μg of RNA with M-MLV reverse transcriptase (Takara Co., Otsu,
Japan) as described [17]. Real-time PCR was performed using SYBR Premix Ex Taq (TaKaRa). The following parameters were used for the PCR: 95 °C for 10 min followed by 40 cycles at 95 °C for
30 s, 55 °C for 30 s, and 72 °C for 45 s. Gene expression was normalized against 18S RNA. The primers used in this study are listed in Supplementary Table 1. IMMUNOFLUORESCENCE ANALYSIS
SW1736 and K18 ATC cancer cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.01% Triton X-100 for 15 min. After blocking with 1% bovine serum in PBS for 30 min,
cells were incubated with one of the following antibodies: NIS, Pax8, Smad7, Smad3, and Smad4 (Abcam) diluted in PBS overnight at 4 °C. Then, cells were washed with PBS three times followed
by incubation with 1:500 Alexa Fluor 550 and Alexa Fluor 488 phalloidin-conjugated secondary antibodies (Invitrogen) for 1 h. Cells were stained with DAPI for 1 min. Finally, cells were
mounted on glass slides in the AquaPoly/Mount medium (Polysciences). Photomicrographs were recorded by an Olympus microscope. The percentage of positive cells was determined after counting
at least 300 cells in a double-blinded manner. HE STAINING AND IMMUNOHISTOCHEMICAL ANALYSIS Human ATC samples were fixed in 4% paraformaldehyde, dehydrated with ethanol, and then fixed in
paraffin. Approximately 4-μm-thick sections were cut and deparaffinized, rehydrated, and stained with hematoxylin–eosin (H&E). Stained slides were then evaluated using IX81 microscopy
(Olympus, Tokyo, Japan). Immunohistochemical analysis was performed with streptavidin–biotin complex (ABC) approach following the instruction by a Neobioscience kit (Shenzhen, China).
Primary antibodies were diluted in PBS as follows: anti-REGγ, anti-NIS, anti-SMAD7, and anti-Pax8. Prior to antibody incubation, an antigen was retrieved in 10 mM sodium citrate, pH 6.0, for
15 min at 100 °C in a water bath. Color reaction was visualized after addition of diaminobenzidine (DAB)-H2O2 as a substrate for peroxidase. All sections were counterstained with
hematoxylin, dehydrated, mounted, and observed under a microscope. Staining percentage was classified as negative (0–25%), weak (25–50%), moderate (50–75%), or strong (75–100%) according to
previous publication [20]. XENOGRAFT MOUSE MODELS ATC cells (5 × 106 in 0.1 mL of PBS per mouse) were inoculated subcutaneously on the right back sides of the mice. In all, 100 μl of
luciferase substrate at the concentration of 33 mg/ml was injected into the intraperitoneal cavity, ~5 min before imaging. Mice were anesthetized with isoflurane and then ventral images were
collected for 30 s to 2 min using the IVIS (Xenogen Corp., Alameda, CA). Photons emitted from the primary tumor and lung region were quantified using Living Image software (Xenogen Corp.,
Alameda, CA). 131I UPTAKE AND THERAPY OF XENOGRAFT TUMORS IN NUDE MICE Two-month-old BALB/c nude mice were used in xenograft and radiotherapy studies following the ethical and safety
guideline approved by the Animal Center at Jiangsu Institute of Nuclear Medicine. SW1736 and K18 (ShN and ShR) ATC cells with a stably integrated luciferase reporter gene (effluc) were
subcutaneously implanted into the dorsal sides (ShN to left and ShR to the right side) of BALB/c nude mice in two groups (six mice in each group). For transplanted tumors sized 6–8 mm in
diameter, Cherenkov imaging was performed for 30 min after Na131I (0.5 mCi) i.p. injection. For 131I therapy, mice were administered 1.5 mCi Na131I by a single i.p. injection. The control
mice were administered 0.9% NaI. Tumors were measured before administration of radioiodine and weekly thereafter. Tumor optical images were acquired by the IVIS imaging system, and tumor
size was quantified with Living Image software. BIOINFORMATICS ANALYSIS The samples of ATC (_n_ = 20) and PTC (_n_ = 17) were chosen from the NCBI Gene Expression Omnibus
(http://www.ncbi.nlm.nih.gov/geo) to determine the transcript levels of _REGγ, NIS, Pax8_, and _Smad7_, the accession number is GSE76039. ATC PATIENT SAMPLES Ten unnamed human ATC samples
were collected from the Second Chengdu Municipal Hospital in China. Patient organization and case access are in agreement with the ethical guidelines and requirements by the hospital.
STATISTICAL ANALYSIS All experiments were performed independently at least three times. All data were expressed as the mean ± SD. Paired Student's _t_ test or one-way ANOVA were used to
compare the differences in the data between two groups or more. _P_-value of less than 0.05 were considered to be of significance. RESULTS REGΓ DEFICIENCY AUGMENTS 131I UPTAKE IN ATC CELLS
Previous studies [21] reported anomalous REGγ expression in thyroid neoplasms, with higher levels in poorly differentiated than in well-differentiated thyroid cancers. To investigate REGγ
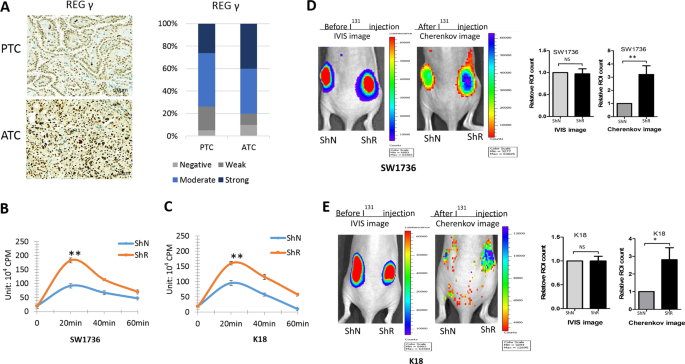
function in ATC, we analyzed histobiochemistry in ten samples by comparison with papillary thyroid cancer (PTC). REGγ displayed nuclear localization in the ATC and PTC tissues. Critically,
REGγ expression in ATC was found higher than in PTC (Fig. 1a), suggesting that REGγ may play a role in the development of poorly differentiated ATC. Therefore, we addressed whether
hyperactivation of REGγ might be involved in the dedifferentiation of ATC. First, we determined if attenuation of REGγ in ATC cells may restore radioiodine uptake. Approximately 20 min after
131I treatment, REGγ-depleted SW1736-ShR and K18-ShR cells had 94.7% and 84.2% higher radioiodine absorption, respectively, than the ShN control cells (Fig. 1b, c). Then, to substantiate
the influence of REGγ on iodine intake, we generated xenograft tumors with similar volume and monitored radioiodine absorption in vivo (Fig. 1d, e). Compared with the ShN controls, REGγ
knockdown greatly enhanced the uptake of iodine in the xenograft tumors, with an increase by 3.2- and 2.8-fold for SW1736-ShR and K18-ShR tumors, respectively (Fig. 1d, e). The results
suggest the possibility that REGγ may be involved in dedifferentiation of ATC cells. ABLATION OF REGΓ RESTORES EXPRESSION OF THYROID-SPECIFIC GENES IN ATC CELLS To further determine if REGγ
regulates dedifferentiation of ATC cells, we evaluated expression of thyroid-specific genes following RNAi of REGγ. In contrast to the control in ShN cells, expression of _TTF1_, _Pax8_,
_NIS_, _Tg, TSHR_, and _TPO_ in SW1736-ShR and K18-ShR was markedly upregulated (Fig. 2a, b). The effects of REGγ on _NIS_ transcription were also investigated using a _NIS_ luciferase (luc)
reporter construct in a Doxycycline-inducible cellular system to overexpress either a wild-type (WT) REGγ or a dominant-negative loss-of-function mutant REGγ (N151Y). Overexpression of REGγ
greatly inhibited _NIS_-luc reporter activity, while the N151Y-mutant failed to do so (Fig. 2c; Supplementary Fig. 1A). Western blot analysis validated the increase in NIS and Pax8 protein
expression in SW1736 and K18 ATC cells, with stable knockdown of REGγ (Fig. 2d). Furthermore, immunofluorescent analyses displayed more intensive staining of NIS and Pax8 in ATC-ShR cells
than in ATC-ShN cells (Fig. 2e, f). Together, our results indicate that blockade of REGγ action has the potential to induce re-differentiation of ATC cells, as demonstrated by restoration of
NIS transcription. REGΓ SUPPRESSES THYROID-SPECIFIC GENES BY PROMOTING THE TGF-Β/SMAD SIGNAL PATHWAY To address the molecular mechanism by which REGγ negatively regulates thyroid-specific
genes, we performed a high-throughput proteomic screen of REGγ targets or effectors by antibody array analysis (FullMoon BioSystem) [15]. Among the top ten proteins differentially expressed
in REGγ WT and REGγ KO MEFs, we found approximately threefold higher levels of active Smad3 (phosphor-Ser 204) [22] in WT than in KO cells (Fig. 3a). We performed western blot analysis to
confirm the correlation between REGγ and p-Smad3 in ATC cells. Consistently, the p-Smad3 levels were markedly higher in ShN SW1736 and K18 ATC cells than in ShR cells (Fig. 3b). Moreover, we
validated that nuclear Smad3 is much more abundant in REGγ containing ShN than in REGγ-silenced ShR ATC cells following TGF-β treatments (Fig. 3c, d; Supplementary Fig. 1B–C). In contrast,
SB-431542, an inhibitor of the TGF-β type I receptor, abolished nuclear accumulation of Smad3 in all the cells (Fig. 3c, d; Supplementary Fig. 1B–C), reminiscent of REGγ ablation. Silencing
Smad3 markedly increased the expression of _NIS_, _Pax_8, _TTF1_, and _TSHR_ in K18 and SW1736 ATC cells (Fig. 3e; Supplementary Fig. 1D), consistent with a previous report that TGF-β/Smad3
signaling is a potent repressor of _Pax8_/_NIS_ in normal epithelial thyroid cells [8]. To substantiate the role of Smads on NIS expression, _NIS_ luciferase reporter assays were performed
in the presence of Smad2 or Smad3. Either greatly inhibited the activitiy of the _NIS_ reporter in SW1736 or K18 ATC cells (Supplementary Fig. 1E). Indeed, TGF-β signal transduction is
crippled in ShR cells since we found de-repression of thyroid-specific genes (_NIS_, _Pax_8, _TTF1_, and _TSHR_) in both K18 and SW1736 ShR cells compared with ShN ATCs (Fig. 3f;
Supplementary Fig. 2A), suggesting that REGγ might be a critical regulator of the TGF-β signal pathway. Consistently, expression of classical TGF-β target genes, including _PAI-1_ and
_CTGF_, was markedly higher in SW1736 and K18 ShN than in ShR cells after TGF-β treatments (Supplementary Fig. 2B, C). In contrast, _NIS_ and _Pax_8 expression, negatively regulated by REGγ,
displayed a marked decrease in a time-dependent manner following the TGF-β activation in ShN cells, while maintained higher levels in ShR cells (Supplementary Fig. 2D, E). The percentage of
nuclear Smad3/Smad4 positive cells was significantly decreased with REGγ knockdown (Supplementary Fig. 3A, B). These data indicate that REGγ positively regulates the activity of the
TGF-β/Smad signal pathway in ATC cells. Thus, restoration of thyroid-specific gene expression in REGγ-silenced ATC cells may depend on the inhibition of the TGF-β/Smad signal pathway. REGΓ
NEGATIVELY REGULATES SMAD7 BY DIRECTLY PROMOTING ITS DEGRADATION Although Smad3 is a key effector regulated by REGγ, it is not a direct target of the REGγ proteasome. By searching for
negative regulators of the TGF-β pathway, we observed striking accumulation of Smad7 protein (Fig. 4a), but no increase in mRNA expression (Fig. 4b) in ATC cells with stable RNAi depletion
of REGγ. Increased expression of Smad7 in REGγ-deficient K18 and SW1736 cells was validated by immunofluorescence (Fig. 4c; Supplementary Fig. 4A). The percentage of Smad7-positive cells was
about threefold higher in both REGγ-ShR K18 and SW1736 cells than in the corresponding ShN cells (Fig. 4c; Supplementary Fig. 4A). These results support the concept that REGγ negatively
regulates the protein levels of Smad7 in ATC cells. To determine if REGγ mediates Smad7 degradation, we analyzed the dynamics of Smad7 stability in the presence of cycloheximide, a protein
synthesis inhibitor. In ATC cells lacking REGγ, Smad7 protein is much more stable with a significantly slower decay rate than in the REGγ WT control cells (Fig. 4d, e), suggesting that REGγ
is required for degradation of Smad7 in these cells. We carried out gain-of-function experiments using the doxycycline-inducible 293 cells system [10] to overexpress either WT REGγ or the
REGγ-N151Y mutant. Induction of REGγ triggered degradation of Smad7 (Fig. 4f), whereas induced expression of the REGγ-N151Y mutant failed to do so (Fig. 4f). We validated physical
interactions between REGγ and Smad7 in cells by reciprocal coimmunoprecipitation experiments (Supplementary Fig. 4B, C). To determine if the Smad7 is a direct target of the REGγ proteasome,
we performed cell-free proteolysis with purified proteins. Incubation of in vitro-translated Smad7 with 20S proteasome alone or REGγ alone exhibited no significant degradation of Smad7, but
a combination of REGγ and 20S proteasome promoted rapid turnover of Smad7 (Fig. 4g). Taken together, our results demonstrate that REGγ can directly interact with Smad7 and promote its
degradation in vitro and in SW1736 or K18 ATC cells. CLINICAL RELEVANCE OF REGΓ/SMAD7-DEPENDENT REGULATION OF THYROID-SPECIFIC GENES IN ATC To substantiate that REGγ-mediated regulation of
thyroid-specific genes is Smad7 dependent, we performed loss- and gain-of-function experiments in ATC cells. Depletion of Smad7 by RNAi markedly downregulated the expression of _NIS_,
_Pax8_, _TTF1_, _Tg_, and _TSHR_, which is more dramatic in REGγ-defective ShR than in ShN K18 and SW1736 cells (Fig. 5a; Supplementary Fig. 4D). Depletion of Smad7 eliminated the
differences in thyroid-specific gene expression between ShR and ShN ATC cells, suggesting that REGγ action relies on Smad7. In contrast, stable overexpression of Smad7 upregulated the
expression of _NIS_, _Pax8_, _TTF1_, _Tg_, and _TSHR_, with a greater increase in REGγ ShN cells than in REGγ ShR K18 or SW1736 ATC cells (Fig. 5b; Supplementary Fig. 4E). Interestingly,
overexpression of Smad7 recovered thyroid-specific gene transcription in ShN cells to levels equivalent to those in control ShR cells (Fig. 5b; Supplementary Fig. 4E). These results
demonstrate that Smad7 mediates REGγ-dependent regulation of thyroid-specific gene expression in ATC cells. To understand the clinical significance of the REGγ pathway in ATC, we performed
bioinformatics analyses using the NCBI Gene Expression Omnibus database to compare transcription levels of _REGγ, NIS, Pax8_, and _Smad7_ in human ATC and PTC samples. Indeed, transcription
levels of _REGγ_ are higher (_P_ < 0.001) in ATC than that in PTC, while NIS and Pax8 were lower in ATC than PTC (Fig. 5c), representing an inverse correlation between REGγ and NIS/Pax8
in human ATC samples. However, _Smad7_ mRNA levels showed no significant difference between ATC and PTC (Supplementary Fig. 5A), suggesting a posttranscriptional regulation. Next, we
evaluated the protein levels of REGγ, NIS, Pax8, and Smad7 in the human ATC samples by immunohistochemistry comparison with normal thyroid tissues. There was very weak staining of REGγ
accompanied with dramatically higher NIS, Pax8, and Smad7 expression in normal human thyroid tissues (Fig. 5d). On the contrary, the REGγ in ATC was significantly elevated, associated with
drastically reduced expression of NIS, Pax8, and Smad7 (Fig. 5d). Quantified analysis of the IHC data supports that REGγ expression is negatively correlated with NIS, Pax8, and Smad7 protein
levels in human ATC (Fig. 5d), implicating REGγ-Smad7 regulation in ATC tissues. REGΓ ABROGATION ACCENTUATES 131I THERAPEUTIC SENSITIVITY TO XENOGRAFT ATC TUMORS Given REGγ-dependent
regulation of _NIS_ and radioiodine uptake, we evaluated if the therapeutic effect of 131I can be modified in an ATC cell xenograft model with altered REGγ expression. Since REGγ-KO tumors
grow slower [18, 20], a cohort of ShN and ShR ATC cells were inoculated individually at different time point so that both tumor types would reach 6–8 mm in diameter simultaneously. Following
transplantation of paired ShN and ShR ATC tumors to animals, Cherenkov imaging analysis with Na131I ensured absorption of radioiodine (Fig. 1d, e). Dynamic evaluation of tumor regression by
luciferase activities showed greater decrease in SW1736 ShR than in ShN tumors at days 14 and 21 post therapy (Fig. 6a). Similar results were observed for K18 tumors with a marked reduction
of bioluminescence signals in the ShR tumors compared with ShN tumors at days 14 and 21 (Fig. 6b). At the final day of 131I treatments, we harvested and measured actual sizes of the paired
tumors. Noticeably, immunostaining showed that Pax8, NIS, and Smad7 were still significantly higher in REGγ-deleted tumors than ShN tumors (Supplementary Figs 5B, C). The volume of tumors
derived from SW1736 and K18 ShR cells had greater reduction than the tumors from ShN control cells with an approximately threefold difference in tumor volumes on average (Fig. 6c–f;
Supplementary Fig. 6A, B). Since the tumor promoting effect of REGγ-positive ShN cells yielded 1.5-fold higher growth rate benefit over ShR cells, we subtracted the growth differences
between 131I-treated vs. untreated groups to evaluate the net effect of radiotherapy. The results disclosed a greater growth inhibition in treated ShR tumors, indicating more efficient
radioiodine therapy in sensitized ShR groups (Fig. 6g, h). Taken together, REGγ inhibition could be a promising direction for treating ATC in the future. DISCUSSION In this study, we have
demonstrated that REGγ is an important factor in the regulation of thyroid-specific genes in ATC by directly targeting Smad7 for proteasome-mediated degradation and enhancing the TGF-β
signal pathway, which negatively regulates thyroid-specific genes. The significance of REGγ-depletion restoration of ATC differentiation is highlighted by increased uptake of radioiodine and
improved effects of 131I treatment in the ATC xenograft tumor model. Thus, REGγ appears to be important for driving transformation of ATC from pre-existing differentiated thyroid cancer.
This study provides molecular basis that targeting a noncanonical proteasome degradation pathway or directly targeting p-Smad3 may be new directions for ATC therapy. ATC has the most
aggressive progression among thyroid malignancies due to its dedifferentiation; however, the molecular mechanisms of ATC are far from understood. Mutations of various pathways, including
p53, Braf, Ras, PTEN/PI3K, and Wnt-β-catenin have been described as potential drivers for ATC [23]. Current therapeutic approaches commonly aim at inhibiting cell proliferation pathways or
restoring the function of tumor suppressors. However, single-modality therapy such as Braf inhibitor, PI3K inhibitor, or multimodal treatments has limited effects on ATC, and the mean
survival time from diagnosis to death continues to remain at about 6 months [23]. Therefore, there is an urgent need for novel therapies against ATC. Here, we demonstrate for the first time
that REGγ is a regulator for _Pax8_ and _NIS_ transcription. We have provided proof-of-principle evidence that inhibiting REGγ activity greatly improves iodine uptake in ATC cells in vitro
and in vivo, which benefits 131I therapy in ATC tumor model. Given that REGγ-mediated Smad7 degradation induces NIS repression, combinational therapy to target REGγ and other proteins such
as BRAF V600E might be promising in the future. It is well established that the TGF-β/Smad pathway plays a critical role in ATC progression and metastasis. Indeed, blocking TGF-β1 inhibits
growth and invasion of ATC cells [7, 24]. ATC malignancy caused by TGF-β is probably based on epithelial-to-mesenchymal transition [25, 26]. However, TGF-β also acts as an inhibitor of cell
proliferation by suppressing function of c-Myc and enhancing expression of CDK inhibitors such as p15INK4B, p21CIP1, and p27KIP1 [27], particularly exhibiting tumor suppressive effects in
healthy noncancerous cells and in early-stage cancerous cells [28]. Since ATC frequently derives from PTC and can co-exist with PTC, in which the TGF-β pathway may function differently than
it does in ATC alone. In addition, numerous reports suggest that Smad4 deficiency drives cancer metastasis and tumor malignancy [29,30,31]. Thus, the function of the TGF-β signal pathway is
further complicated by Smad-dependent and Smad-independent pathways [32]. Since TGF-β exerts both tumor suppressive and a metastasis-promoting functions, use of TGF-β receptor inhibitors for
ATC patients seems not to be an optimal choice in anticancer therapy. Smad7 is a key negative regulator of TGF-β signaling by binding the MH2 domain and blocking R-Smad activation [33]. The
key function of Smad7 is to control activated Smad2/3 and prevent nuclear translocation. In this study, we observed that the upregulation of _NIS_ and _Pax8_ after siSmad3 was almost
similar to the extent induced by REGγ knockdown in ATC cells, implying that selective targeting Smad3 may be applicable for restoration of the sensitivity to radioiodine therapy. Considering
REGγ knockout mice can survive and breed normally, we assume that targeting REGγ may be an alternative option for ATC therapy in the future with less side effects than inhibition of the
conventional ubiquitin–proteasome degradation system. In addition, Smad7 has TGF-β-independent function in promoting pluripotency by amplifying STAT3 activation [34]. Thus, our study
suggests: (1) targeting Smad7 downstream of TGF-β but not directly affecting receptor levels, may be an economical approach. (2) Proteasome inhibition has proven anticancer effects in ATC
[35, 36], the narrow-scoped REGγ proteasome may be specific. It is also noticeable that REGγ was reported to interact with E3-ubiquitin ligase Smurf1 and mediates its degradation [37].
Interestingly, Smad7 also function by binding Smurf2 to TβRI, controlling its turnover and mitigating TGF-β signaling [38]. Given the net effects of Smad7 and Smurf1 on TGF-β/Smad signal
pathways are consistent, it is likely that Smad7 and Smurfs may contribute together to the activation of TGF-β signaling affected by REGγ in SW1736 and K18 ATC cells. Loss of
thyroid-specific gene expression, especially _NIS_, contributes to lack of response to radioiodine ablation therapy in ATC, a reason for the high lethality of ATC patients. WTp53 was shown
to upregulate NIS expression [39], while BrafV600E and HDAC inhibited its transcription [6, 9]. Coincident with our previous findings [16, 40], abrogation of REGγ enhances p53 levels in
multiple cell types. Whether REGγ may also regulate NIS expression in ATC via the p53 pathway is not clear. Taken together, our results demonstrate a critical role of REGγ in regulating ATC
cell differentiation. Antagonizing REGγ greatly restores expression of thyroid-specific genes, intensifies iodide uptake and accentuates cellular sensitivity to radioiodine therapy in ATC
(Fig. 7). Molecularly, REGγ acts by activating TGF-β/Smad signaling via proteasome-dependent degradation of Smad7 (Fig. 7), adding an additional layer of the regulatory mechanism in a
ubiquitin-independent manner. Therefore, this study presents evidences that blocking REGγ activity may be an alternative therapeutic strategy for anaplastic thyroid carcinoma. REFERENCES *
Onoda N, Sugino K, Higashiyama T, Kammori M, Toda K, Ito K-I, et al. The safety and efficacy of weekly paclitaxel administration for anaplastic thyroid cancer patients: a nationwide
prospective study. Thyroid. 2016;26:1293–9. Article CAS Google Scholar * Hsu K-T, Yu X-M, Audhya AW, Jaume JC, Lloyd RV, Miyamoto S, et al. Novel approaches in anaplastic thyroid cancer
therapy. Oncologist. 2014;19:1148–55. Article CAS Google Scholar * Links T, Van Tol K, Jager P, Plukker JTM, Piers D, Boezen H, et al. Life expectancy in differentiated thyroid cancer: a
novel approach to survival analysis. Endocr Relat Cancer. 2005;12:273–80. Article CAS Google Scholar * Boland A, Ricard M, Opolon P, Bidart J-M, Yeh P, Filetti S, et al.
Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res. 2000;60:3484–92. CAS PubMed Google Scholar * Hébrant A, Dom
G, Dewaele M, Andry G, Trésallet C, Leteurtre E, et al. mRNA expression in papillary and anaplastic thyroid carcinoma: molecular anatomy of a killing switch. PLoS ONE. 2012;7:e37807. Article
Google Scholar * Ke C-C, Liu R-S, Chi C-W, Lee C-H. HDAC inhibitor induces re-expression of thyroid specific genes as well as differentiating in anaplastic thyroid cancer. J Nucl Med.
2013;54(supplement 2):1341–1341. Google Scholar * Zhang K, Liu X, Hao F, Dong A, Chen D. Targeting TGF-β1 inhibits invasion of anaplastic thyroid carcinoma cell through SMAD2-dependent
S100A4-MMP-2/9 signalling. Am J Transl Res. 2016;8:2196. CAS PubMed PubMed Central Google Scholar * Costamagna E, García B, Santisteban P. The functional interaction between the paired
domain transcription factor Pax8 and Smad3 is involved in transforming growth factor-β repression of the sodium/iodide symporter gene. J Biol Chem. 2004;279:3439–46. Article CAS Google
Scholar * Riesco-Eizaguirre G, Rodríguez I, De la Vieja A, Costamagna E, Carrasco N, Nistal M, et al. The BRAFV600E oncogene induces transforming growth factor β secretion leading to sodium
iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res. 2009;69:8317–25. Article CAS Google Scholar * Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O’Malley
BW. Ubiquitin-and ATP-independent proteolytic turnover of p21 by the REGγ-proteasome pathway. Mol Cell. 2007;26:831–42. Article Google Scholar * Li X, Lonard DM, Jung SY, Malovannaya A,
Feng Q, Qin J, et al. The SRC-3/AIB1 coactivator is degraded in a ubiquitin-and ATP-independent manner by the REGγ proteasome. Cell. 2006;124:381–92. Article CAS Google Scholar * Zhang Z,
Zhang R. Proteasome activator PA28γ regulates p53 by enhancing its MDM2‐mediated degradation. EMBO J. 2008;27:852–64. Article CAS Google Scholar * Xu J, Zhou L, Ji L, Chen F, Fortmann K,
Zhang K, et al. The REGγ-proteasome forms a regulatory circuit with IκBɛ and NFκB in experimental colitis. Nat Commun. 2016;7:10761. Article CAS Google Scholar * Liu S, Lai L, Zuo Q, Dai
F, Wu L, Wang Y, et al. PKA turnover by the REGγ-proteasome modulates FoxO1 cellular activity and VEGF-induced angiogenesis. J Mol Cell Cardiol. 2014;72:28–38. Article CAS Google Scholar
* Dong S, Jia C, Zhang S, Fan G, Li Y, Shan P, et al. The REGγ proteasome regulates hepatic lipid metabolism through inhibition of autophagy. Cell Metab. 2013;18:380–91. Article CAS
Google Scholar * Li L, Zhao D, Wei H, Yao L, Dang Y, Amjad A, et al. REGγ deficiency promotes premature aging via the casein kinase 1 pathway. Proc Natl Acad Sci. 2013;110:11005–10. Article
CAS Google Scholar * He J, Cui L, Zeng Y, Wang G, Zhou P, Yang Y, et al. REGγ is associated with multiple oncogenic pathways in human cancers. BMC Cancer. 2012;12:75. Article CAS
Google Scholar * Li L, Dang Y, Zhang J, Yan W, Zhai W, Chen H, et al. REGγ is critical for skin carcinogenesis by modulating the Wnt/β-catenin pathway. Nat Commun. 2015;6:6875. Article CAS
Google Scholar * Wang Q, Gao X, Yu T, Yuan L, Dai J, Wang W, et al. REGγ controls Hippo signaling and reciprocal NF-κB–YAP regulation to promote colon cancer. Clin Cancer Res.
2018;24:2015–25. Article CAS Google Scholar * Wang Q, Gao X, Yu T, Yuan L, Dai J, Wang W, et al. REGγ controls Hippo signaling and reciprocal NF-κB-YAP regulation to promote colon cancer.
Clin Cancer Res. 2018;2986:2017. clincanres Google Scholar * Okamura T, Taniguchi S-i, Ohkura T, Yoshida A, Shimizu H, Sakai M, et al. Abnormally high expression of proteasome activator-γ
in thyroid neoplasm. J Clin Endocrinol Metab. 2003;88:1374–83. Article CAS Google Scholar * Millet C, Yamashita M, Heller M, Yu L-R, Veenstra TD, Zhang YE. A negative feedback control of
transforming growth factor-β signaling by glycogen synthase kinase 3-mediated Smad3 linker phosphorylation at Ser-204. J Biol Chem. 2009;284:19808–16. Article CAS Google Scholar * Reddi
HV, Kumar A, Kulstad R. Anaplastic thyroid cancer–an overview of genetic variations and treatment modalities. Adv Genom Genet. 2015;5:43–52. Article CAS Google Scholar * Li X, Zhuang J,
Zhang K, Liu Y, Dong A. Galunisertib, a TGF-β receptor (TβR)-I inhibitor, suppresses growth and invasion of an anaplastic thyroid cancer 8505C cell in vitro and in vivo. Int J Clin Exp
Pathol. 2016;9:7050–9. CAS Google Scholar * Papageorgis P. TGFβ signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol. 2015;2015:587193. Article
Google Scholar * Ma R, Bonnefond S, Morshed SA, Latif R, Davies TF. Stemness is derived from thyroid cancer cells. Front Endocrinol. 2014;5:114. Article Google Scholar * Kubiczkova L,
Sedlarikova L, Hajek R, Sevcikova S. TGF-β–an excellent servant but a bad master. J Transl Med. 2012;10:183. Article CAS Google Scholar * Syed V. TGF‐β Signaling in Cancer. J Cell
Biochem. 2016;117:1279–87. Article CAS Google Scholar * Papageorgis P, Cheng K, Ozturk S, Gong Y, Lambert AW, Abdolmaleky HM, et al. Smad4 inactivation promotes malignancy and drug
resistance of colon cancer. Cancer Res_._ 2011;71:998–1008. Article CAS Google Scholar * Voorneveld PW, Kodach LL, Jacobs RJ, Liv N, Zonnevylle AC, Hoogenboom JP, et al. Loss of SMAD4
alters BMP signaling to promote colorectal cancer cell metastasis via activation of Rho and ROCK. Gastroenterology. 2014;147:196–208. e113. Article CAS Google Scholar * Liu J, Cho S-N,
Akkanti B, Jin N, Mao J, Long W, et al. ErbB2 pathway activation upon Smad4 loss promotes lung tumor growth and metastasis. Cell Rep. 2015;10:1599–613. Article CAS Google Scholar *
Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577. Article CAS Google Scholar * Yan X, Liao H, Cheng M, Shi X, Lin X, Feng
X-H, et al. Smad7 protein interacts with receptor-regulated Smads (R-Smads) to inhibit transforming growth factor-β (TGF-β)/Smad signaling. J Biol Chem. 2016;291:382–92. Article CAS
Google Scholar * Yu Y, Gu S, Li W, Sun C, Chen F, Xiao M, et al. Smad7 enables STAT3 activation and promotes pluripotency independent of TGF-β signaling. Proc Natl Acad Sci.
2017;114:10113–8. Article CAS Google Scholar * Altmann A, Markert A, Askoxylakis V, Schning T, Jesenofsky R, Eisenhut M, et al. Antitumor effects of proteasome inhibition in anaplastic
thyroid carcinoma. J Nucl Med. 2012;53:1764. Article CAS Google Scholar * Mehta A, Zhang L, Boufraqech M, Zhang Y, Patel D, Shen M, et al. Carfilzomib is an effective anticancer agent in
anaplastic thyroid cancer. Endocr Relat Cancer. 2015;22:319–29. Article CAS Google Scholar * Nie J, Wu M, Wang J, Xing G, He F, Zhang L. REGγ proteasome mediates degradation of the
ubiquitin ligase Smurf1. FEBS Lett. 2010;584:3021–7. Article CAS Google Scholar * Zhang Z, Fan Y, Xie F, Zhou H, Jin K, Shao L, et al. Breast cancer metastasis suppressor OTUD1
deubiquitinates SMAD7. Nat Commun. 2017;8:2116. Article Google Scholar * Liu L, Li D, Chen Z, Yang J, Ma Y, Cai H, et al. Wild-type P53 induces sodium/iodide symporter expression allowing
radioiodide therapy in anaplastic thyroid cancer. Cell Physiol Biochem. 2017;43:905–14. Article CAS Google Scholar * Liu J, Yu G, Zhao Y, Zhao D, Wang Y, Wang L, et al. REGγ modulates p53
activity by regulating its cellular localization. J Cell Sci. 2010;123:4076–84. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr. James A. Fagin (Memorial
Sloan Kettering Cancer Center) for kindly providing SW1736 cell line. This work was supported by the National Basic Research Program of China (2015CB910402, 2016YFC0902102), the National
Natural Science Foundation of China (31200878, 31730017, 91629103, 81471066, 31401012, 81672883, 81401837, and 31670882), the Science and Technology Commission of Shanghai Municipality
(17ZR1407900, 11ZR1410000, 14430712100, 14ZR1411400, and 16ZR1410000), Shanghai Rising-Star Program (16QA1401500), the applied Basic Research Program of Science and Technology Department of
Sichuan Province (2015JY0038). We also thank ECNU Multifunctional Platform for Innovation (011) for keeping and raising mice. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Shanghai Key
Laboratory of Regulatory Biology, Institute of Biomedical Sciences, School of Life Sciences, East China Normal University, 500 Dongchuan Road, Shanghai, 200241, China Chan Jiao, Ke Li, Riqun
Fang, Lei Yuan, Kaixuan Shi, Linan Pan, Qiannan Guo, Xiao Gao, Geng Chen, Qingwei Wang, Di Zuo, Wei Wu, Xiaoshuang Wang, Lei Li, Yongyan Dang & Xiaotao Li * Department of Orthopedic
Oncology, Changzheng Hospital, The Second Military Medical University, 415 Fengyang Road, Shanghai, 200003, China Lin Li & Jianru Xiao * Department of Pathology, the Second Chengdu
Municipal Hospital, Chengdu, 610017, China Pei Zhang * Jiangsu Institute of Nuclear Medicine, Key laboratory of Nuclear Medicine, Ministry of Health, 20 Qian Rong Road, Wuxi, Jiangsu,
214063, China Li Zhang & Shichen Xu * Institute of Life & Health Sciences, Chubu University, 1200 Matsumoto-cho, Kasugai, Aichi, 487-8501, Japan Shanlou Qiao * Department of
Molecular and Cellular Biology, Dan L. Duncan Cancer Center, Baylor College of Medicine, One Baylor Plaza, Houston, TX, 77030, USA Robb Moses & Xiaotao Li Authors * Chan Jiao View author
publications You can also search for this author inPubMed Google Scholar * Lin Li View author publications You can also search for this author inPubMed Google Scholar * Pei Zhang View
author publications You can also search for this author inPubMed Google Scholar * Li Zhang View author publications You can also search for this author inPubMed Google Scholar * Ke Li View
author publications You can also search for this author inPubMed Google Scholar * Riqun Fang View author publications You can also search for this author inPubMed Google Scholar * Lei Yuan
View author publications You can also search for this author inPubMed Google Scholar * Kaixuan Shi View author publications You can also search for this author inPubMed Google Scholar *
Linan Pan View author publications You can also search for this author inPubMed Google Scholar * Qiannan Guo View author publications You can also search for this author inPubMed Google
Scholar * Xiao Gao View author publications You can also search for this author inPubMed Google Scholar * Geng Chen View author publications You can also search for this author inPubMed
Google Scholar * Shichen Xu View author publications You can also search for this author inPubMed Google Scholar * Qingwei Wang View author publications You can also search for this author
inPubMed Google Scholar * Di Zuo View author publications You can also search for this author inPubMed Google Scholar * Wei Wu View author publications You can also search for this author
inPubMed Google Scholar * Shanlou Qiao View author publications You can also search for this author inPubMed Google Scholar * Xiaoshuang Wang View author publications You can also search for
this author inPubMed Google Scholar * Robb Moses View author publications You can also search for this author inPubMed Google Scholar * Jianru Xiao View author publications You can also
search for this author inPubMed Google Scholar * Lei Li View author publications You can also search for this author inPubMed Google Scholar * Yongyan Dang View author publications You can
also search for this author inPubMed Google Scholar * Xiaotao Li View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to
Lei Li, Yongyan Dang or Xiaotao Li. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Co-first authors: Chan Jiao, Lin Li Edited by J.P. Medema SUPPLEMENTARY
INFORMATION SUPPLEMENTAL FIGURE LEGEND UNTRACKED SUPPLEMENTAL FIGURE 1–6 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jiao, C., Li, L., Zhang, P. _et al._ REGγ ablation impedes dedifferentiation of
anaplastic thyroid carcinoma and accentuates radio-therapeutic response by regulating the Smad7-TGF-β pathway. _Cell Death Differ_ 27, 497–508 (2020).
https://doi.org/10.1038/s41418-019-0367-9 Download citation * Received: 27 January 2019 * Revised: 17 May 2019 * Accepted: 30 May 2019 * Published: 26 June 2019 * Issue Date: February 2020 *
DOI: https://doi.org/10.1038/s41418-019-0367-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative