BMSCs pre-treatment ameliorates inflammation-related tissue destruction in LPS-induced rat DIC model
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

This study aimed to investigate the effect of bone marrow-derived mesenchymal stem cells (BMSCs) on disseminated intravascular coagulation (DIC) model rats and to further explore the
underlying mechanism. A rat model of lipopolysaccharide (LPS)-induced DIC was successfully established, as indicated by impaired plasma hemostatic parameters and damaged organ functions in
rats. Importantly, pre-treatment with rat allogeneic BMSCs before LPS injection significantly alleviated systemic intravascular coagulation, reduced plasma levels of organ dysfunction
indicators and pro-inflammatory cytokines, suppressed fibrin microthrombi formation, ameliorated liver, heart, and renal injuries, and increased 24-hour survival rates in LPS-induced DIC
rats. The protection of BMSCs against DIC was in a moderately dose-dependent manner. Further investigation revealed that BMSCs co-cultured with peripheral blood mononuclear cells (PBMCs)
significantly inhibited the LPS-stimulated PBMCs proliferation and the release of pro-inflammatory cytokines from PBMCs. Of note, upregulation of immunosuppressive factors including
indoleamine 2,3-dioxygenase and interleukin-10, which was induced by interferon-γ, contributed to BMSCs-mediated inhibition of LPS-stimulated PBMCs proliferation. These effects do not depend
on the direct cell–cell contact. In conclusion, BMSCs pre-treatment ameliorates inflammation-related tissue destruction in LPS-induced DIC model rats. The protection of BMSCs may be
attributed to their anti-inflammatory and immunomodulatory properties, which render BMSCs a promising source for stem cell-based therapeutic approaches in inflammation-related DIC.
Disseminated intravascular coagulation (DIC) is a devastating clinical condition caused by unbalanced activation between coagulation and fibrinolysis1. It typically occurs as an acute
complication in patients with underlying life-threatening illnesses, such as severe infection, severe sepsis, solid or hematologic malignancies, severe trauma, placental abruption, and
obstetric calamities2. DIC is characterized by systemic activation of coagulation, potentially leading to thrombotic obstruction of small and midsize vessels, thereby contributing to
multiple organ failure (MOF)3. MOF involves disturbed microcirculation owing to the formation of numerous microthrombi in several organs and is one of the most common complications of DIC4.
The pathophysiology of DIC is multifactorial involving intertwined feedback loops between the coagulant, immune, and inflammatory pathways5. Although DIC is a serious disease, there is no
gold standard for its diagnosis, no single biomarker by which DIC can be clearly diagnosed, and no anticoagulants have been recommended for the treatment of DIC in worldwide6.
Mesenchymal stem cells (MSCs) are multipotent non-hematopoietic progenitor cells that can differentiate into bone marrow stromal cells, adipocytes, osteoblasts, chondrocytes, tenocytes,
neurons, skeletal myocytes, and cells of visceral mesoderm7,8. MSCs have become promising candidates for the development of novel allogeneic cell-based therapeutic strategies in harnessing
inflammation in the repair or regeneration of various damaged tissues9,10. Until now, most cell-based therapies were conducted using the well-characterized bone marrow-derived MSCs (BMSCs).
Growing evidence has revealed that BMSCs display profound immunomodulatory and anti-inflammatory capacities11,12,13. Furthermore, BMSCs can exert immunosuppressive and anti-inflammatory
effects both in vitro and in vivo by inhibiting the proliferation and function of innate and adaptive immune cells, such as natural killer (NK) cells, dendritic cells, and T and B
lymphocytes14,15,16,17. To date, various studies have demonstrated that soluble factors including transforming growth factor β1 (TGF-β1), interleukin-10 (IL-10), cyclooxygenase-2 (COX-2),
inducible nitric oxide synthase (iNOS), indoleamine 2,3-dioxygenase (IDO), and hepatocyte growth factor (HGF), either produced constitutively by MSCs or as a result of cross-talk with target
immune cells, have been attributed to the immunomodulatory properties of MSCs18. Interestingly, the pro-inflammatory cytokine interferon-γ (IFN-γ), secreted by activated T cells, is capable
of regulating the immunomodulatory functions of BMSCs via regulation of a variety of immunosuppressive factors, including COX-2, iNOS, IDO, and IL-107,19,20. In addition, the unique
immunomodulatory and anti-inflammatory effects of MSCs have been demonstrated in several animal disease models related to inflammation, such as bowel disease18, periodontitis21, and
sepsis22. Taken together, these observations have provided convincing evidence that BMSCs-based therapy may be potential for the treatment of DIC.
The aim of present study was to investigate the in vivo effect of BMSCs pre-treatment on inflammation-related DIC rat model induced by lipopolysaccharide (LPS) and to explore whether their
anti-inflammatory protection of BMSCs against DIC was associated with their immunomodulatory effect on peripheral blood mononuclear cells (PBMCs) proliferation.
Adult pathogen-free male Wistar rats (6–7 weeks old, weighing 160–170 g) were obtained from the Laboratory Animal Center of Shandong University (Jinan, Shandong, China). The experimental
procedures were approved by the Animal Care and Use Committee of Shandong University. All rats were kept per cage with free access to food and water, and a 12/12 h light/dark cycle, with an
ambient temperature of 20–25 °C.
Isolation of BMSCs from Wistar rats was performed according to our previous studies23. The morphological characteristics of BMSCs were examined using an inverted microscope.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed to draw the growth curve of the BMSCs cultured for 1–8 days as previously described24. Flow cytometry and
differentiation assays were carried out according to our previous studies23 to verify BMSCs based on established criteria25. The fourth passage BMSCs were used in subsequent experiments.
The establishment DIC rat model was performed according to the methods described by our previous studies23,26. Rats were anesthetized with an intraperitoneal injection of pentobarbital
sodium salt (30 mg/kg, Sigma-Aldrich). Experimental DIC models were treated with sustained intravenous infusion of 3 mg/kg LPS diluted in 1 ml saline for 1 h via the tail vein. The control
group was injected with the same volume of saline. The laboratory diagnosis of DIC in rats was performed according to a previous study27.
Sixty male rats were randomly divided into five treatment groups and one control group (n = 10/group). (1) Control: rats were pre-treated with 1 ml culture medium (vehicle of allogeneic
BMSCs) and then administered a sustained intravenous infusion of 1 ml normal saline (vehicle of LPS) for 1 h via tail vein; (2) LPS: rats were pre-treated with 1 ml culture medium and then
treated with a sustained intravenous infusion of 3 mg/kg LPS diluted in 1 ml saline for 1 h via tail vein; (3) LPS + BMSCs: rats were pre-treated with 1 × 103, 1 × 104, 1 × 105, and 1 × 106
allogeneic BMSCs in 1 ml cultured medium via tail vein for 3 consecutive days (three times at intervals of 24 h) before LPS injection. LPS was injected via tail vein at the end of 24 h after
last BMSCs injection.
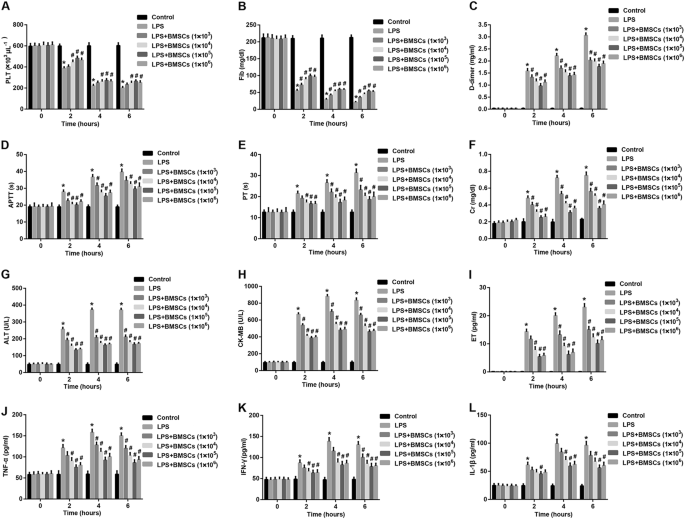
The blood sample was withdrawn from the abdominal aorta at 0 h (before), 2 h, 4 h, and 6 h after LPS injection. Platelet (PLT) counts were performed with an automated device for animals
(Celltac, MEK-5128, Nihon Kohden Co., Tokyo, Japan). d-dimer levels were determined by the quantitative latex agglutination test (Diatron, Tokyo, Japan). Fibrinogen (Fib) levels, activated
partial thromboplastin time (APTT), and prothrombin time (PT) were measured using an ACL-9000 Coagulation Analyzer. Plasma levels of creatinine (Cr), alanine aminotransferase (ALT), and
creatinine kinase-MB (CK-MB) were measured with commercially available kits (Sigma) with a fully automated clinical chemistry analyzer (Hitachi 912, Boehringer Mannheim, Germany) according
to manufacturer’s instructions. Plasma levels of endothelin (ET) were determined with a commercial enzyme-linked immunosorbent assay (ELISA) kit (Wako, Osaka, Japan).
After experiments, the rats were killed and their organs (including the heart, liver, and kidney) were harvested and prepared for histological studies. A part of the sections were stained
with hematoxylin and eosin (HE), and the others were stained with phosphotungstic acid hematoxylin (PTAH) according to the routine staining procedure. Fibrin was red-stained in HE staining
and violet blue-stained in PTAH staining. They were examined under a light microscope (Olympus, Japan).
Quantitative evaluation of the heart injury was performed according to a pathologic score system (neutrophil infiltration, hemorrhage, and necrosis) ranging from 0 (normal) to 6 (severe) as
previously described28. Liver injury of each section was assessed according to Suzuki’s criteria29, which accounts for the following three separate criteria: vascular congestion, hepatocyte
vacuolization, and necrosis. The scores from 0 (none) to 4 (severe) for each of the three criteria were then combined, leaving a combined liver injury score ranging from 0 (normal) to 12
(severe). Quantitative evaluation of the renal injury was performed based on histological parameters: tubular necrosis, interstitial edema, loss of brush border, and casts formation. The
scoring system used was 0, absent; 1, present; and 2, marked, as described previously30. A minimum of 10 fields for each section were examined and assigned for the severity of changes.
Blinded analysis of the histological samples was performed by two experts (Department of Cardiovascular Surgery, Qilu Hospital of Shandong University).
The levels of IL-1β, IL-10, tumor necrosis factor-α (TNF-α), and IFN-γ in the cell supernatant after 72 h of incubation and plasma levels of TNF-α, IFN-γ, and IL-1β were determined by ELISA
kits purchased from R&D Systems (Minneapolis, MN, USA). All conditions were performed in triplicate.
Fresh blood (4 ml) was drawn from the rat inferior vena cava at the end of 24 h after last BMSCs injection. Next, blood was heparinized for the isolation of PBMCs by a Ficoll-Hypaque density
gradient centrifugation. In brief, the mixture of lymphocyte isolation liquid (Ficoll, GE Healthcare Bioscience, Piscataway, New Jersey, USA), serum-free RPMI 1640 (Gibco, USA), and fresh
blood (1: 1: 1, v/v/v) was centrifuged at 2000 × g for 20 min at 25 °C. After that, the mononuclear cell layer (white layer) was carefully aspirated by a syringe and re-suspended in 3 ml of
RPMI 1640 medium containing 10% fetal calf serum (FCS, Hyclone, USA). The active PBMCs accounted for 95% after trypan blue dyeing. PBMCs were finally adjusted the concentration of 2 ×
106/ml.
PBMCs isolated from Wistar rats were randomly divided into six treatment groups and one control group for co-culture with BMSCs under LPS stimulation.
(1) PBMCs (control): PBMCs (100 μl, 2 × 106/ml) + RPMI 1640 medium/10% FCS (100 μl);
(2) PBMCs + LPS: PBMCs (100 μl, 2 × 106/ml) + RPMI 1640 medium/10% FCS (100 μl) + LPS (final concentration, 0.5 μg/ml, Difco Laboratory, USA);
(3–6) PBMCs + LPS + BMSCs (1 × l02/1 × l03/1 × l04/1 × l05): PBMCs (100 μl, 2 × 106/ml) + BMSCs (corresponding 1 × l03/1 × l04/1 × l05/1 × l06/ml, 100 μl) + LPS (final concentration, 0.5
μg/ml).
3H-TdR incorporation assay was performed to evaluate the proliferation capacity of PBMCs isolated from Wistar rats in co-culture with or without LPS or BMSCs. In brief, PBMCs were plated
into 96-well plates (2 × 106/ml, 200 μl/well) and treated with or without BMSCs isolated from rats, IL-10-neutralizing antibody (cat. no MAB417, 10 μg/ml, R&D Systems), TGF-β1-neutralizing
antibody (cat. no. 1D11, 10 μg/ml, R&D Systems), indomethacin (a COX-2 inhibitor, cat. no. 9758, 20 μM, Sigma-Aldrich), l-NAME (N-nitro-l-arginine methyl ester, an iNOS inhibitor, cat. no.
M18168, 1 mM, Meryer, China), 1-MT (1-methyl-l-tryptophan, an IDO inhibitor, cat. no. 860646, 500 μM, Sigma-Aldrich), and antibody IFN-γ (cat. no. ab133566, Abcam, USA) followed by the
stimulation of LPS (0.5 μg/ml, Difco Laboratory, USA) or not. After 72 h of incubation, 20 μl 3H-TdR (10 μci/ml) was added into each well for 8 h of incubation. PBMCs were harvested onto
glass fiber filter paper, and the counts per minute were determined using a Wallac TriLux 1450 MicroBeta microplate scintillation counter (PerkinElmer, Waltham, MA, USA). All conditions were
performed in triplicate.
Protein was isolated from BMSCs and subjected to western blot analysis as previously described18. Results were analyzed using Alpha View Analysis Tools (AlphaViewSA software version 3.2.2,
ProteinSimple, Santa Clara, CA). β-actin served as the loading control.
IDO activity was analyzed by measuring the concentration of kynurenine in BMSCs culture in response to IFN-γ (PeproTech) or co-culture with PBMCs in the absence or presence of 0.5 μg/ml LPS
using a high-pressure liquid chromatography technique according to a previous study31.
Data were expressed as mean ± standard deviation from three independent experiments. Statistical analyses were performed using SPSS statistical software package standard version 16.0 (SPSS,
Inc., Chicago, IL, USA). Experiments were performed in triplicate. Statistical differences between two independent groups were determined using Student’s t test. For multiple groups, the
statistical analyses were performed by one-way ANOVA followed by Tukey’s test. Survival analysis was performed by using the Kaplan–Meier method. P