The essential roles of mps1 in spermatogenesis and fertility in mice
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Monopolar spindle 1 (MPS1), which plays a critical role in somatic mitosis, has also been revealed to be essential for meiosis I in oocytes. Spermatogenesis is an important process
involving successive mitosis and meiosis, but the function of MPS1 in spermatogenesis remains unclear. Here, we generated _Mps1_ conditional knockout mice and found that _Ddx4_-cre-driven
loss of _Mps1_ in male mice resulted in depletion of undifferentiated spermatogonial cells and subsequently of differentiated spermatogonia and spermatocytes. In addition, _Stra8_-cre-driven
ablation of _Mps1_ in male mice led to germ cell loss and fertility reduction. Spermatocytes lacking _M__ps__1_ have blocked at the zygotene-to-pachytene transition in the prophase of
meiosis I, which may be due to decreased H2B ubiquitination level mediated by MDM2. And the expression of many meiotic genes was decreased, while that of apoptotic genes was increased.
Moreover, we also detected increased apoptosis in spermatocytes with _Mps1_ knockout, which may have been the reason why germ cells were lost. Taken together, our findings indicate that MPS1
is required for mitosis of gonocytes and spermatogonia, differentiation of undifferentiated spermatogonia, and progression of meiosis I in spermatocytes. SIMILAR CONTENT BEING VIEWED BY
OTHERS RAD51 IS ESSENTIAL FOR SPERMATOGENESIS AND MALE FERTILITY IN MICE Article Open access 15 March 2022 DISTINCT ROLES OF HASPIN IN STEM CELL DIVISION AND MALE GAMETOGENESIS Article Open
access 06 October 2021 TCFL5 DEFICIENCY IMPAIRS THE PACHYTENE TO DIPLOTENE TRANSITION DURING SPERMATOGENESIS IN THE MOUSE Article Open access 29 June 2022 INTRODUCTION Spermatogenesis occurs
within the testis seminiferous epithelium and consists of the following stages in mice: mitosis of spermatogonia, meiosis of spermatocytes, and maturation of haploid spermatozoa1.
Initially, spermatogonial stem cells undergo a series of mitosis events and then differentiate into B-type spermatogonia. They then enter meiosis I, becoming primary spermatocytes, as
indicated by STRA8 expression2,3,4. Meiosis I involves the stages leptotene, zygotene, pachytene, diplotene, and diakinesis, which can be distinguished by different expression patterns of
SYCP35,6. Moreover, at the leptotene stage, double-strand breaks occur and activate γH2AX and SPO117,8, while at zygonema, RAD51 is highly enriched at the break sites in the chromosome
strands9. During the pachytene stage, meiotic homologous chromosomes combine and form the synaptonemal complex. SYCP3 localizes along the lateral chromosome axis, and SYCP1 loads onto the
center to connect the two homologous chromosomes5,6,9. Subsequently, diplonema occurs, which features sex bodies marked by γH2AX10, and is followed by diakinesis. Monopolar spindle 1 (MPS1)
is a kinase that is conserved from yeast to mammals that plays a critical role in preventing erroneous chromosome segregation through the spindle assembly checkpoint (SAC) complex11,12,13.
During mitosis, MPS1 directly binds to unattached kinetochores through CDC80, after which it phosphorylates and recruits other SAC proteins, such as BUB3, BUBR1, and MAD2, to the
kinetochore14,15,16,17. This process inhibits anaphase-promoting complex/cyclosome (APC/C) activation until all microtubules stably attach to the kinetochores at anaphase onset18. MPS1 has
important functions not only in mitosis but also in meiosis19. In previous research, a mutant mouse strain was generated that expresses a form of ΔNMPS1 with a 107-amino acid deletion at the
N-terminus. The N-terminus is involved in binding to the kinetochore20,21. Without MPS1 localization, precocious APC/C activation leads to the missegregation or nondisjunction events
observed in mouse oocytes due to failure of SAC control and chromosome alignment in meiosis I20. Furthermore, MPS1 kinase activation is essential for cohesin protection in a manner dependent
on the localization of SGO2 to the centromere in mouse oocytes22. However, whether _M__ps__1_ plays an essential role in mouse spermatogenesis, especially in mitosis and male meiosis, is
still unknown. To investigate the function of MPS1 in the regulation of spermatogenesis, we generated two conditional _Mps1_ knockout strains and found that _M__ps__1_ is required for
mitosis of gonocytes and spermatogonia, differentiation of undifferentiated spermatogonia, and progression of meiosis I in spermatocytes. RESULTS _MPS1_ WAS REQUIRED FOR POSTNATAL MITOTIC
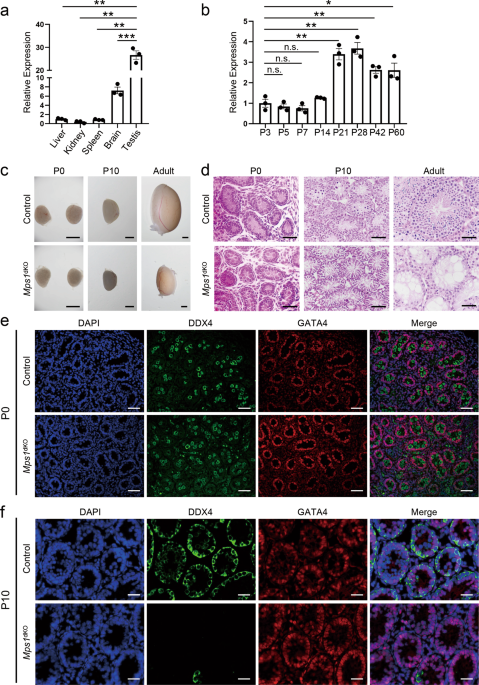
DIVISIONS OF MALE GERM CELLS To explore _Mps1_’s function in spermatogenesis, we first characterized the spatiotemporal expression pattern of _Mps1_ in mice. Quantitative polymerase chain
reaction (qPCR) analysis of different adult tissues showed higher _Mps1_ expression levels in testes than in other tissues (Fig. 1a). In testes, _Mps1_ expression levels in the mouse testes
aged from P3 (postnatal day 3) to P60 showed a dramatic increase from P21, when the male mice started to sexually mature (Fig. 1b). Using the published testes RNA-seq dataset from Soumillon
et al.23, we further found that _Mps1_ is especially enriched in spermatocytes (Fig. S1a). All these data suggested that _Mps1_ may have important roles in spermatogenesis. For studying the
role of _Mps1_, we generated a conditional _Mps1-_knockout mouse strain. Specifically, we first generated _Mps1_fl/fl mice by inserting two _LoxP_ sites in intron 5 and intron 8 of the
_Mps1_ gene, which caused deletion of exons 6–8 and a frameshift mutation driven by Cre recombinase (Fig. S1b). Then, by mating _Mps1_ floxed mice with _Ddx4_-Cre mice, which specifically
express Cre recombinase in germ cells as early as embryonic day 15.5 (E15.5)24, we obtained germ cell-specific _Mps1_-knockout (_Mps1_dKO) mice. Successful knockout was verified by PCR (Fig.
S1c, d). The _Mps1_dKO mice grew normally like the control mice, and we focused on the spermatogenesis of male mice. First, we examined the morphology of testes from newborn (P0) to adult
mice (Fig. 1c). There was no difference between _Mps1_dKO and control mice in terms of testis size at P0. At P10, the testes of _Mps1_dKO and control mice started to become different in
size, with the testes of _Mps1_dKO mice being slightly smaller than those of control mice. The difference became much more significant in adult mice. Then, to determine the reason, we
assessed the composition of cell types in seminiferous tubules, which are the sites of spermatogenesis (where germ cells develop into spermatozoa). Using hematoxylin–eosin (H&E)
staining, we were able to distinguish two different types of cells on the basis of morphology, i.e., triangle-like Sertoli cells and round germ cells. At P0, both types of cells were visible
in _Mps1_dKO and control seminiferous tubules (Fig. 1d). However, there were few germ cells (round-shaped) left in the seminiferous tubules at P10, and no germ cells were present in adult
_Mps1_dKO mice; only triangle-like Sertoli cells remained (Fig. 1d). Moreover, many empty seminiferous tubules existed in adult _Mps1_dKO mice. Consistently, no mature sperm were found in
the cauda epididymides of _Mps1_dKO mice (Fig. S1e). To further identify the cell types that disappeared, we employed immunostaining on seminiferous tubule sections of P0 and P10 mice. We
used two kinds of marker genes to distinguish cell types: GATA4 for Sertoli cells and DDX4 for germ cells. Consistent with the H&E staining results, DDX4-positive germ cells and
GATA4-positive Sertoli cells were present in both control and _Mps1_dKO P0 mice (Fig. 1e). However, almost no DDX4-positive germ cells were found in the _Mps1_dKO P10 testes (Fig. 1f).
Nonetheless, Sertoli cells (GATA4-positive) remained unaffected in the _Mps1_dKO testes, as expected. Taken together, the results indicate that _Mps1_ knockout in early-stage male germ cells
results in severe germ cell loss. Previous studies have revealed the important roles of MPS1 in mitosis in somatic cells. Given that germ cells are generated via mitosis of gonocytes and
spermatogonia, the findings suggest that the male germ cell depletion in _Mps1_dKO mice might be due to disruption of postnatal mitosis. POSTNATAL DISRUPTION OF _MPS1_ LED TO SEVERE DEFECTS
IN SPERMATOGENESIS AND SIGNIFICANTLY REDUCED MALE FERTILITY Since knocking out _Mps1_ in _Mps1_dKO mouse testes triggered such dramatic germ cell loss that almost no spermatocytes could be
observed, the role of MPS1 in the meiosis of spermatocytes remained unclear. To investigate the function of MPS1 in meiosis, we knocked out the _Mps1_ gene in germ cells by mating
_Stra8_-cre mice, which specifically express Cre recombinase at P3 (early spermatogonia) and exhibit increased expression until P7 (preleptotene spermatocytes) in testes25. This strain
(_Mps1_sKO) was verified by PCR (Fig. S2a). The knockout efficiency of _Mps1_ was confirmed by using Western blotting, which revealed that the protein levels of MPS1 were significantly
reduced in the testes of the _Mps1_sKO mice (Fig. S2b). As we observed, the _Mps1_sKO mice were mostly normal, but their testes were different from those of the control mice. The testes of
_Mps1_sKO and control mice were similar in size and mass at P10, but the testes of _Mps1_sKO mice became smaller and lighter than those of control mice at 2 months and 6 months (Fig. 2a, b).
Specifically, the testis weight/body weight ratio was significantly reduced from 0.37% in control mice to 0.12% in _Mps1_sKO mice at 2 month (32.4%) and from 0.30% to 0.08% at 6 month
(28.3%) (Fig. 2b). In addition, the epididymides were detectably smaller in _Mps1_sKO mice than in control mice (Fig. S2c). Histologically, the seminiferous tubules of _Mps1_sKO mice
displayed decreased diameters, reduced cell numbers, and increased empty seminiferous tubule percentages at 2 and 6 months (Fig. 2c, d). The average diameter of _Mps1_sKO seminiferous
tubules was reduced to 76% and 61% of that of the control mice at 2 and 6 months, respectively (Fig. 2d). At 2 months and 6 months, 35% and 68% of _Mps1_sKO seminiferous tubules were
degenerated with aberrant spermatogenesis, respectively, while degenerated tubules were hardly detected in the control mice in the time window we examined (Fig. 2d). Next, we examined
whether _Stra8_-Cre-mediated _Mps1_ ablation affected sperm production and male fertility. H&E staining of the cauda epididymis, which is the primary storage site for mature sperm,
showed that the density of mature sperm was largely reduced in adult (2 months and 6 months) _Mps1_sKO mice compared to control mice (Fig. 2e). To validate this finding, we released and
counted all mature sperm from the cauda epididymides of 6-month-old mice. Strikingly, the average sperm number of _Mps1_sKO mice was decreased to approximately one-tenth of that of control
mice (Fig. 2f). We then assessed the fertility of male _Mps1_sKO and control mice. Three-month-old male control and _Mps1_sKO mice were bred with two adult wild-type females for 3 continuous
months (_n_ = 5). Whereas the control males produced litters of normal size over this period, the _Mps1_sKO males showed significantly reduced fertility. The total number of litters
produced by the control mice was 20 (average number of litters: 4 ± 1.26), and the total number of pups was 141 (average litter size: 7.05 ± 2.5). However, 3 of 5 _Mps1_sKO mice were
infertile during this time period, and another two _Mps1_sKO mice each produced only one litter (average number of litters: 0.4 ± 0.49); the total pup number dropped to 9 (average litter
size: 4.5 ± 2.27) (Fig. 2g). Thus, postnatal deletion of _Mps1_ led to lower productivity of mature sperm and significantly reduced male fertility, suggesting that the reductions in average
litter number and size might have been caused by reductions in sperm numbers in the testes of _Mps1_sKO males. DELETION OF _MPS1_ WITHIN DIFFERENTIATING SPERMATOGONIA VIA _STRA8_-CRE
REVEALED A ROLE FOR _MPS1_ IN SPERMATOGONIAL DIFFERENTIATION To determine the mechanism of severe cell loss and fertility reduction in _Mps1_sKO mice, it was necessary to investigate which
cell types were lost and when they began to disappear during spermatogenesis. We examined the expression of germ cell markers in testes of mice at P6, P10, and 2 months by immunostaining
analysis. The DDX4 staining data showed that the number of _Mps1_sKO mouse germ cells per seminiferous tubule had decreased to 66% and 57% of that in control mice by P6 and P10, respectively
(Fig. 3a, b), although the testis weight did not differ, as previously mentioned (Fig. 2b). The impact was greater in 2-month-old _Mps1_sKO mice, which retained only 24% of the germ cells
in control mice. On the other hand, the number of PLZF-positive cells per tubule, representing undifferentiated spermatogonia, was the same between _Mps1_sKO mice and controls at different
ages, as expected (Fig. 3c, d; Fig. S3a, b). We further investigated the fates of _Mps1_sKO spermatogonia via immunostaining for c-KIT, a marker of differentiated spermatogonia. The results
indicated that the c-KIT-positive cell number in _Mps1_sKO testes was reduced to 70% of that in control testes at P6, which suggested that spermatogonial differentiation may have been
affected in _Mps1_sKO mice (Fig. 3e, f). In summary, postnatal deletion of _Mps1_ via _Stra8_-Cre does not affect the number of undifferentiated spermatogonia but plays a role in
spermatogonial differentiation during spermatogenesis. LOSS OF _MPS1_ COMPROMISED THE PROGRESSION OF MEIOSIS I AND DELAYED THE TRANSITION FROM ZYGOTENE TO PACHYTENE Meiosis is a key feature
of spermatogenesis that begins at approximately P10. STRA8 and SYCP3 are a marker of meiosis initiation and a synaptonemal complex component, respectively. Immunostaining analysis showed
that the numbers of STRA8-expressing and SYCP3-expressing cells per seminiferous tubule in _Mps1_sKO mice were significantly reduced to approximately 56% and 45% of those in control mice at
P10. Similarly, the staining results at 2 month revealed that _Mps1_sKO mice had only 30% of the STRA8-positive and 38% of the SYCP3-positive cells of control mice (Fig. 4a, b). According to
the spermatocyte composition in the seminiferous tubules, spermatid development in mice has been divided into 12 stages26,27. To determine the exact stage of meiosis that was affected by
_Mps1_ knockout, we performed H&E staining on testes of 6-month-old mice to trace the seminiferous epithelium stages of the testicular tubules. We found that spermatogenesis seemed
delayed at stage IV, during which pachytene spermatocytes should appear and localize inside of the seminiferous lumen. There were fewer pachytene spermatocytes (marked as P) in
_Mps1_-deletion testes than in control testes. Apoptotic pachytenes (marked as AP) were also observed (Fig. 5a). Although some spermatocytes survived in stage IV, fewer round and elongated
spermatids formed in _Mps1_sKO testes than in control testes. In addition, meiotic spermatocytes (marked as M) were rarely observed in stage XII _Mps1_sKO testes. To further identify the
delayed stages in the prophase of meiosis, meiotic stage progression was assessed from chromosomal spreads of spermatocytes that were prepared from testes of 1-month male mice and
immunostained with anti-SYCP3 and anti-γH2AX antibodies (Fig. 5b, c). In both the _Mps1_sKO and control groups, all five stages from leptotene to diakinesis were observed based on the
immunostaining patterns of SYCP3 and γH2AX in spermatocytes. The proportion of pachytene spermatocytes was reduced from 68.25% in control mice to 37.85% in _Mps1_sKO mice, while the
proportions of spermatocytes in diplotene and diakinesis were reduced from 8.08% in control mice to 3.70% in _Mps1_sKO mice and from 1.56% in control mice to 0.28% in _Mps1_sKO mice,
respectively. On the other hand, spermatocytes of _Mps1_sKO mice accumulated in leptotene (control mice: 7.24%; _Mps1_sKO mice: 22.55%) and in zygotene (control mice: 14.97%; _Mps1_sKO mice:
35.61%). These data indicated that the transition of zygotene spermatocytes to pachytene spermatocytes was impaired. This finding was investigated further via examination of H3 core histone
phosphorylation at serine 10 (H3pSer10). This type of phosphorylation is coincident with metaphase progression, and the signal then becomes more intense throughout chromatin during the
diakinesis phase and the first meiotic metaphase (MI) in spermatocytes28. We found that the numbers of H3pSer10-positive germ cells were dramatically reduced in _Mps1_sKO testes (Fig. S4a,
b) and that the percentages of H3pSer10-positive metaphase and anaphase spermatocytes were significantly lower in tubule sections from _Mps1_sKO mice than in those from control mice (Fig.
S4b, right). This finding suggested that loss of _Mps1_ compromised the progression of meiosis I. Collectively, the evidence indicated that deletion of _Mps1_ during meiosis resulted in a
dramatic decrease in the number of germ cells, which may have been caused by defects in the meiosis process. In particular, the transition from the zygotene to the pachytene stage might have
been delayed or partially disrupted in the spermatocytes of _Mps1_sKO mice. Previously, Xu et al.6 found H2B ubiquitination regulates the progression of meiosis I by promoting chromatin
relaxation, and its E3 ligase _Rnf20_ knockout mice had similar phenotypes, such as the smaller size of testes, loss of germ cells and spermatocytes arrested at the prophase of meiosis I. We
examined the levels of H2BK120 mono-ubiquitination in the testes of control and _Mps1_sKO mice and noticed that it decreased in _Mps1_sKO testes at 2 months of age compared with that in
control testes (Figs. 5d and S4c). MPS1 has been reported to regulate the level of H2BK120ubi mediated by MDM2, another E3 ubiquitin ligase promoting the ubiquitination of H2B28,29, and
knockdown of _Mps1_ by RNAi diminished MDM2 expression in U2OS cells30. As expected, the expression level of MDM2 was downregulated in _Mps1_sKO testes, compared to that in control testes
(Fig. 5e). In a word, loss of _Mps1_ compromised the prophase of meiosis I progress which may be due to decreased H2B ubiquitination level mediated by MDM2. THE EXPRESSION OF MEIOSIS-RELATED
GENES AND APOPTOSIS-RELATED GENES WAS ALTERED IN THE TESTES OF _MPS1_ SKO MICE To investigated the changes in gene expression after _Mps1_ deletion, we performed RNA sequencing of P10
testes from control and _Mps1_sKO mice. We found that there were more downregulated genes than upregulated genes in _Mps1_sKO mice (Fig. 6a and Table S2). Using cut-offs of a fold change
greater than 1.5 and a _P_ value less than 0.01, 92 genes were identified as downregulated, and 24 genes were identified as upregulated (Fig. 6b). We then performed Gene Ontology (GO)
analysis to identify the enriched biological processes for each group of differentially expressed genes (DEGs). Notably, for the downregulated genes, the top ten enriched terms were all
related to cell division. More importantly, seven of them were meiosis-related, including the meiotic cell cycle, synapsis, and chromosome segregation terms, among others. The most abundant
term, the meiotic cell cycle, was enriched for 21 (22.8%) of the 92 downregulated genes (Fig. 6c and Table S3). To fully discover the biological pathways affected, we relaxed the cut-off for
upregulated genes to 1.2-fold, and another 475 genes were identified as upregulated. Of these, 8 out of 189 genes were associated with the apoptosis term. Unsurprisingly, apoptosis stood
out as one of the enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) terms for the upregulated genes (Fig. 6d and Table S4). To validate the expression changes in these genes, we
examined 13 of them (_Mps1_, _Stra8_, _Sycp3_, _Meioc_, _Sycp1_, _Syce1_, _Stag3_, _Prdm9_, _Tnf_, _Capn1_, _Ptpn13_, _Sptal_, and _Tnfrsfla_) by qPCR and detected consistent changes in
_Mps1_sKO testes (Fig. 6e). Therefore, the transcriptomic data indicated that widespread impacts on meiosis and apoptosis resulted from _Mps1_ deletion. _MPS1_ DELETION ELEVATED APOPTOSIS IN
TESTES In order to determine the fates of germ cells with _Mps1_ loss, we conducted a TUNEL assay to investigate whether there were more cells undergoing apoptosis in _Mps1_sKO testes than
in control testes. On the one hand, there were more seminiferous tubules with TUNEL-positive cells in _Mps1_sKO testes than in control testes (Fig. 6f–i). The percentage of TUNEL-positive
tubules increased from 22.8% in control mice to 28.1% in _Mps1_sKO mice at P10 and from 18.1% to 32.1% at 2 months. On the other hand, the percentage of TUNEL-positive cells per seminiferous
tubule was also increased in the _Mps1_sKO testes (Fig. 6f–i). At P10, on average, 1.06% of the cells in control seminiferous tubules were TUNEL positive, while 1.39% of the cells in
_Mps1_sKO tubules were TUNEL positive. At 2-month of age, the average percentage of TUNEL-positive cells was 0.19% in control tubules, while 1.60% in _Mps1_sKO tubules. Overall, the TUNEL
assay results showed that many more germ cells in seminiferous tubules underwent apoptosis in _Mps1_sKO mice than in control mice, which indicated that the significant reductions in male
germ cell numbers in _Mps1_sKO mice were partly due to increased apoptosis. DISCUSSION MPS1, also known as TTK protein kinase, has been well studied in the context of mitosis in somatic
cells14,31 and has also been reported to function in meiosis I during oogenesis20. Nevertheless, it remains unexplored whether _Mps__1_ plays roles in male spermatogenesis. Here, we explored
MPS1 function in mouse spermatogenesis for the first time by utilizing conditional _Mps1_ knockout in DDX4-positive and STRA8-positive germ cells and demonstrated that MPS1 is critical for
spermatogonial differentiation and for the progression of meiosis I spermatocytes. We also revealed that _Mps__1_ may play its role partially by promoting male germ cell apoptosis. STRA8
expression begins at P3 in the early stage of spermatogonia and lasts through the preleptotene stage25. _Stra8_-Cre mice have been used to investigate gene functions during male meiosis. In
our study, we deleted _Mps1_ in STRA8-positive male germ cells and revealed its function in the meiotic cell cycle. However, we also detected germ cell loss at P6 in _Mps1_sKO seminiferous
tubules. Germ cells at this stage included predominantly undifferentiated and differentiated spermatogonial cells that had not entered the meiotic process. Therefore, _Stra8_-Cre _Mps1_
knockout affected not only meiosis but also the differentiation of spermatogonial cells. These results suggest that a spermatocyte-specific Cre knock-in mouse line may be more suitable than
the knockout mouse line in the current study for research focusing exclusively on meiosis. Apoptosis is a major form of programmed cell death critical for development and the damage
response32. Activation of the TNF pathway can induce distinct cellular outcomes, including cell death, inflammation, and cell survival, in a context-dependent manner33. In our study, the TNF
signaling pathway was upregulated in _Mps1_sKO testes, which might have contributed to the increased apoptosis in _Mps1_sKO testes. Given that loss of _Mps1_ compromised the progression of
meiosis I and increased apoptosis in seminiferous tubules, it seems likely that some germ cells that fail to complete meiosis I may undergo cell death in the absence of MPS1 expression.
Previous studies on _Mps1_ function in cell division have mainly illustrated its function in the SAC. Hached and his colleagues revealed that _Mps1_ functions in SAC control and chromosome
segregation in oocyte meiosis I20. Our data demonstrate _Mps__1_’s role in the prophase of the meiotic cell cycle, showing that _Mps1_ ablation in this stage results in blockade of the
zygotene-to-pachytene transition, which may be due to decreased H2B ubiquitination level mediated by MDM2. We also found that the percentages of H3pSer10-positive metaphase and anaphase
spermatocytes were significantly decreased in tubule sections from _Mps1_sKO mice, which suggests that _Mps__1_ may play a role in the progression from prophase to metaphase and anaphase.
Further studies investigating the underlying mechanisms by which MPS1 plays roles in SAC control and chromosome segregation during male meiosis I are needed to complete our knowledge of MPS1
function in male germ cells. MATERIALS AND METHODS ANIMALS _Mps1_ floxed conditional knockout mice were generated by targeted homologous recombination (Shanghai Model Organisms). Homology
arms, _LoxP_ sites, and selection markers were introduced into the targeting vector via a recombineering-based method. BAC DNA was purchased from the Sanger Institute. Then, the linearized
vector was electroporated into ESCs and screened with G418 and Ganc. The correct clones were then injected into C57BL/6J blastocysts. After mating and identification by PCR with the primers
listed below, we obtained _Mps1_-floxed mice. All mice and samples were selected randomly and proceeded in arbitrary order. No formal randomization techniques were used. All mice used and
samples used in IF, H&E, sequencing analysis were only labeled with mouse ID numbers and no genotypes or treatments were indicated. Genotypes and treatments were labeled after the
completion of data acquisition and analysis. The mice were handled according to the university guidelines, and all animal procedures were approved by the Tongji University Institutional
Animal Care and Use Program Advisory Committee (protocol number: IACUC-017-006). GENOTYPING PRIMERS The primers used to identify the _Mps1_ floxed genotypes were _Mps1_-For (forward primer)
and _Mps1_-Re (reverse primer). The floxed band was 353 bp, and the wild-type band was 167 bp. The primers used to identify the _Mps1_ deletion alleles were _Mps1_-min-For and _Mps1_-Re,
which produced a 319 bp band. The band for _Ddx4_ was 240 bp, and that for _Stra8_-Cre was 180 bp. The primers for qPCR are listed in Table S1. _Mps1_-For: TTGCTTGGTAGTTCTGTGGACT _Mps1_-Re:
AGCAGCTGTAAGTGCAGGAG _Mps1_-min-For: CTGCGCTAACCTTGTGTTGG _Stra8_-For: GTGCAAGCTGAACAACAGGA _Stra8_-Re: AGGGACACAGCATTGGAGTC _Ddx4-_For: CACGTGCAGCCGTTTAAGCCGCGT _Ddx4_-Re:
TTCCCATTCTAAACAACACCCTGAA. HISTOLOGY AND IMMUNOFLUORESCENCE For paraffin sectioning, testes were fixed in Bouin’s trichrome at 4 °C overnight. After washing in phosphate-buffered saline
(PBS), the testes were dehydrated with gradient concentrations of ethanol and xylene, embedded in paraffin, and cut into 5 μm-thick sections. H&E staining was performed and was followed
by deparaffinization and rehydration. The slides were imaged with a Nikon microscope. For frozen sectioning, testes were fixed in 4% PFA solution at 4 °C overnight and then dehydrated with
30% sucrose solution for another 2 days. The testes were dipped in O.C.T. compound and then quickly frozen in liquid nitrogen. Frozen sections with a thickness of 7 μm were subjected to
heat-induced antigen retrieval (sodium citrate, pH = 6) in a water bath for 20 min at 98 °C. The sections were cooled down slowly in retrieval buffer until they reached room temperature.
Then, they were blocked and permeated in 5% bovine serum albumin with 0.2% Triton in PBS for 1 h before being incubated with primary antibodies overnight at 4 °C. After washing in PBS three
times, the sections were incubated with secondary antibodies at room temperature for 2 h. After staining with DAPI (Beyotime) for 5 min, the sections were mounted with a fluorescent mounting
medium (Beyotime). All steps were performed with protection from light. Images were obtained with an Olympus microscope and processed with ImageJ and Photoshop software. SPERMATOCYTE
SURFACE SPREADING Testes from 1-month-old mice were subjected to a spermatocyte surface spreading assay as described by the previous research34. Briefly, the tunica albuginea was removed,
and the tubules were placed into the hypotonic buffer (30 mM Tris, 50 mM sucrose, 17 mM trisodium citrate dihydrate, 5 mM EDTA, 0.5 mM DTT, and 0.5 mM PMSF, pH = 8.2) for 30–60 min.
Subsequently, the tubules were torn into pieces in sucrose buffer (100 mM sucrose, pH = 8.2) and pipetted repeatedly to make a suspension. The cell suspension was smeared onto glass slides
containing 1% PFA and 0.15% Triton X-100, pH = 9.2. The slides were dried overnight in a closed box with high humidity. Finally, the slides were washed twice for 2 min in 0.4% Photoflo
(Kodak) and dried at room temperature. The slides were kept at −20 °C. ANTIBODY The antibody used in immunofluorescence staining were as follows: anti-DDX4 (ab13840, Abcam), anti-GATA4
(ab124265 Abcam), anti-c-KIT (25-1171-82, eBioscience) anti-SYCP3 (ab97672, Abcam), anti-STRA8 (ab49602, Abcam), anti-γH2AX (ab2893, Abcam), anti-H3pSer10 (ab5176, Abcam), anti-MPS1
(ab11108, Abcam), anti-H2BK120ubi (#5546, CST), anti-MDM2 (sc-965, Santa Cruz), anti H2B (ab1790, Abcam), and anti-ACTIN (sc-47778, Santa Cruz). All fluorophore-conjugated secondary
antibodies were obtained from Jackson Immuno Research. RNA-SEQ AND ANALYSIS Total RNA was extracted from whole testes at P10. RNA-Seq libraries were constructed from 1 μg of RNA using a
NEBNext UltraTM RNA Library Prep Kit (NEB, USA) following the manufacturer’s instructions. Sequencing was performed on an Illumina NovaSeq platform with 150 bp paired-end reads. DEG analysis
was performed with the DESeq2 R package. GO and KEGG enrichment analysis of the DEGs was implemented with the clusterProfiler R package. Libraries constructing, sequencing, and data
analyzing were performed by Novogene Co., Ltd. (Tianjin, China). SPERM COUNTING Cauda epididymides from 6-month-old mice were removed and cut in 500 μl of DMEM. The medium was incubated at
37 °C for 7 min to release sperm. After diluting them 50 times, the sperm were analyzed with a cell counter (Countstar). ASSESSMENT OF FERTILITY To test fertility, 3-month-old conditional
knockout and control males were paired with two random adult wild-type females for at least three months. The numbers of offspring from each pregnancy were recorded. TUNEL ASSAY A TUNEL
assay was performed following the instructions of an Apoptosis Detection Kit (Millipore). Sections after deparaffinization and rehydration were incubated with proteinase K (20 μg/ml) for 15
min and washed 2 times in PBS. Equilibration buffer was immediately applied and kept on the sections for at least 15 s. The excess liquid was gently tapped off, and the working-strength TDT
enzyme was pipetted onto each section. The sections were incubated in a humidified chamber at 37 °C for 1 h and then placed in a stop buffer for 10 min. After washing 3 times in PBS, the
sections were incubated with an anti-digoxigenin conjugate for 30 min at room temperature while protected from light and were washed in PBS 3 times again. After staining with DAPI (Beyotime)
for 5 min, the sections were mounted with a fluorescent mounting medium. The slides were viewed with a Leica fluorescence microscope. STATISTICAL ANALYSIS All statistical data were analyzed
by using GraphPad Prism. Sample sizes were described in figure legends. The variances of the two groups were compared by one-way ANOVA, and the _P_ value was determined with a two-tailed
unpaired Student’s _t_ test or with Welch correction. Results were shown as mean ± s.e.m. Significance was shown as *_P_ < 0.05, **_P_ < 0.01, ***_P_ < 0.001. DATA AVAILABILITY All
sequencing data that support the findings of this study have been deposited in the NCBI Gene Expression Omnibus (GEO) under accession number GSE165143. REFERENCES * La, H. M. & Hobbs, R.
M. Mechanisms regulating mammalian spermatogenesis and fertility recovery following germ cell depletion. _Cell Mol. Life Sci._ 76, 4071–4102 (2019). Article CAS Google Scholar *
Anderson, E. L. et al. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. _Proc. Natl Acad. Sci. USA_ 105, 14976–14980 (2008).
Article CAS Google Scholar * Feng, C. W., Bowles, J. & Koopman, P. Control of mammalian germ cell entry into meiosis. _Mol. Cell Endocrinol._ 382, 488–497 (2014). Article CAS Google
Scholar * Li, X. et al. The roles of retinoic acid in the differentiation of spermatogonia and spermatogenic disorders. _Clin. Chim. Acta_ 497, 54–60 (2019). Article CAS Google Scholar
* Xu, K. et al. Mettl3-mediated m(6)A regulates spermatogonial differentiation and meiosis initiation. _Cell Res._ 27, 1100–1114 (2017). Article CAS Google Scholar * Xu, Z. et al. H2B
ubiquitination regulates meiotic recombination by promoting chromatin relaxation. _Nucleic Acids Res._ 44, 9681–9697 (2016). Article CAS Google Scholar * Keeney, S., Giroux, C. N. &
Kleckner, N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. _Cell_ 88, 375–384 (1997). Article CAS Google Scholar *
Jiang, H. et al. MOF influences meiotic expansion of H2AX phosphorylation and spermatogenesis in mice. _PLoS Genet._ 14, e1007300 (2018). Article Google Scholar * Baudat, F., Imai, Y.
& de Massy, B. Meiotic recombination in mammals: localization and regulation. _Nat. Rev. Genet._ 14, 794–806 (2013). Article CAS Google Scholar * Fernandez-Capetillo, O. et al. H2AX
is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. _Dev. Cell_ 4, 497–508 (2003). Article CAS Google Scholar * Liu, X. & Winey, M. The
MPS1 family of protein kinases. _Annu. Rev. Biochem._ 81, 561–585 (2012). Article CAS Google Scholar * Manic, G., Corradi, F., Sistigu, A., Siteni, S. & Vitale, I. Molecular
regulation of the spindle assembly checkpoint by kinases and phosphatases. _Int. Rev. Cell Mol. Biol._ 328, 105–161 (2017). Article CAS Google Scholar * Pachis S. T. & Kops G. Leader
of the SAC: molecular mechanisms of Mps1/TTK regulation in mitosis. _Open Biol_. 8, 180109 https://doi.org/10.1098/rsob.180109 (2018). * Hiruma, Y. et al. Competition between MPS1 and
microtubules at kinetochores regulates spindle checkpoint signaling. _Science_ 348, 1264–1267 (2015). Article CAS Google Scholar * Ji, Z., Gao, H. & Yu, H. CELL DIVISION CYCLE.
Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. _Science_ 348, 1260–1264 (2015). Article CAS Google Scholar * Sun, T. et al. Cellular abundance of
Mps1 and the role of its carboxyl terminal tail in substrate recruitment. _J. Biol. Chem._ 285, 38730–38739 (2010). Article CAS Google Scholar * Sudakin, V., Chan, G. K. & Yen, T. J.
Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. _J. Cell Biol._ 154, 925–936 (2001). Article CAS Google Scholar * Izawa, D.
& Pines, J. Mad2 and the APC/C compete for the same site on Cdc20 to ensure proper chromosome segregation. _J. Cell Biol._ 199, 27–37 (2012). Article CAS Google Scholar * Marston, A.
L. & Wassmann, K. Multiple duties for spindle assembly checkpoint kinases in meiosis. _Front. Cell Dev. Biol._ 5, 109 (2017). Article Google Scholar * Hached, K. et al. Mps1 at
kinetochores is essential for female mouse meiosis I. _Development_ 138, 2261–2271 (2011). Article CAS Google Scholar * Wang, W. et al. Structural and mechanistic insights into Mps1
kinase activation. _J. Cell Mol. Med._ 13, 1679–1694 (2009). Article Google Scholar * El Yakoubi, W. et al. Mps1 kinase-dependent Sgo2 centromere localisation mediates cohesin protection
in mouse oocyte meiosis I. _Nat. Commun._ 8, 694 (2017). Article Google Scholar * Soumillon, M. et al. Cellular source and mechanisms of high transcriptome complexity in the mammalian
testis. _Cell Rep._ 3, 2179–2190 (2013). Article CAS Google Scholar * Gallardo, T., Shirley, L., John, G. B. & Castrillon, D. H. Generation of a germ cell-specific mouse transgenic
Cre line, Vasa-Cre. _Genesis_ 45, 413–417 (2007). Article CAS Google Scholar * Sadate-Ngatchou, P. I., Payne, C. J., Dearth, A. T. & Braun, R. E. Cre recombinase activity specific to
postnatal, premeiotic male germ cells in transgenic mice. _Genesis_ 46, 738–742 (2008). Article CAS Google Scholar * Ahmed, E. A. & de Rooij, D. G. Staging of mouse seminiferous
tubule cross-sections. _Methods Mol. Biol._ 558, 263–277 (2009). Article Google Scholar * Meistrich, M. L. & Hess, R. A. Assessment of spermatogenesis through staging of seminiferous
tubules. _Methods Mol. Biol._ 927, 299–307 (2013). Article CAS Google Scholar * Minsky, N. & Oren, M. J. M. C. The RING domain of Mdm2 mediates histone ubiquitylation and
transcriptional repression. _Mol. Cell._ 16, 631–639 (2004). Article CAS Google Scholar * Yu, Z. C., Huang, Y. F. & Shieh, S. Y. Requirement for human Mps1/TTK in oxidative DNA damage
repair and cell survival through MDM2 phosphorylation. _Nucleic Acids Res._ 44, 1133–1150 (2016). Article CAS Google Scholar * Huang, Y. F., Chang, M. D. & Shieh, S. Y. TTK/hMps1
mediates the p53-dependent postmitotic checkpoint by phosphorylating p53 at Thr18. _Mol. Cell Biol._ 29, 2935–2944 (2009). Article CAS Google Scholar * Ji, Z., Gao, H. & Yu, H.
Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. _Science_ 348, 1260–1264 (2015). Article CAS Google Scholar * Fuchs, Y. & Steller, H. Programmed
cell death in animal development and disease. _Cell_ 147, 742–758 (2011). Article CAS Google Scholar * Li, M., Sun, S., Priest, J., Bi, X. & Fan, Y. Characterization of TNF-induced
cell death in Drosophila reveals caspase- and JNK-dependent necrosis and its role in tumor suppression. _Cell Death Dis._ 10, 613 (2019). Article Google Scholar * Peters, A. H., Plug, A.
W., van Vugt, M. J. & de Boer, P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. _Chromosome Res._ 5, 66–68 (1997). Article CAS
Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank present and past members of the laboratory for their contribution to this study. This research is funded by the
National Key R&D Program of China (2017YFA0103301), and the Fundamental Research Funds for the Central Universities (Tongji University, Grant No. 22120180521). AUTHOR INFORMATION AUTHORS
AND AFFILIATIONS * Research Center for Translational Medicine, Shanghai East Hospital, School of Medicine, School of Life Sciences and Technology, Tongji University, Shanghai, 200092, China
Qiang Fang, Xue-Lin Chen, Lei Zhang, Ya-Bin Li, Tian-Zeng Sun, Chen-Xin Yang, Jian-Feng Chang, Xiao-Mei Yang & Feng Sun Authors * Qiang Fang View author publications You can also search
for this author inPubMed Google Scholar * Xue-Lin Chen View author publications You can also search for this author inPubMed Google Scholar * Lei Zhang View author publications You can also
search for this author inPubMed Google Scholar * Ya-Bin Li View author publications You can also search for this author inPubMed Google Scholar * Tian-Zeng Sun View author publications You
can also search for this author inPubMed Google Scholar * Chen-Xin Yang View author publications You can also search for this author inPubMed Google Scholar * Jian-Feng Chang View author
publications You can also search for this author inPubMed Google Scholar * Xiao-Mei Yang View author publications You can also search for this author inPubMed Google Scholar * Feng Sun View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Feng Sun and Xiaomei Yang designed the experiments; Fang Qiang performed most of the
experiments; Xuelin Chen and Lei Zhang performed part of the experiments. Yabin Li, Chenxin Yang, and Tianzeng Sun performed part of the immunofluorescence experiments. Jianfeng Chang
contributed to the data analysis. Feng Sun, Fang Qiang, and Xiaomei Yang wrote the paper. All authors reviewed and approved the paper. CORRESPONDING AUTHORS Correspondence to Xiao-Mei Yang
or Feng Sun. ETHICS DECLARATIONS ETHICS APPROVAL AND CONSENT TO PARTICIPATE All animal procedures were approved by the Tongji University Institutional Animal Care and Use Program Advisory
Committee (Protocol number: IACUC-017-006). CONFLICT OF INTEREST The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. Edited by A. Stephanou SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL TABLE S1 TABLE S2 TABLE S3 TABLE S4
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Fang, Q., Chen, XL., Zhang, L. _et al._ The essential roles of _M__ps__1_ in spermatogenesis and fertility in mice. _Cell Death Dis_ 12, 531 (2021).
https://doi.org/10.1038/s41419-021-03815-4 Download citation * Received: 04 February 2021 * Revised: 05 May 2021 * Accepted: 10 May 2021 * Published: 24 May 2021 * DOI:
https://doi.org/10.1038/s41419-021-03815-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative