Pharmacophore hybridisation and nanoscale assembly to discover self-delivering lysosomotropic new-chemical entities for cancer therapy
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Integration of the unique advantages of the fields of drug discovery and drug delivery is invaluable for the advancement of drug development. Here we propose a self-delivering one-component
new-chemical-entity nanomedicine (ONN) strategy to improve cancer therapy through incorporation of the self-assembly principle into drug design. A lysosomotropic detergent (MSDH) and an
autophagy inhibitor (Lys05) are hybridised to develop bisaminoquinoline derivatives that can intrinsically form nanoassemblies. The selected BAQ12 and BAQ13 ONNs are highly effective in
inducing lysosomal disruption, lysosomal dysfunction and autophagy blockade and exhibit 30-fold higher antiproliferative activity than hydroxychloroquine used in clinical trials. These
single-drug nanoparticles demonstrate excellent pharmacokinetic and toxicological profiles and dramatic antitumour efficacy in vivo. In addition, they are able to encapsulate and deliver
additional drugs to tumour sites and are thus promising agents for autophagy inhibition-based combination therapy. Given their transdisciplinary advantages, these BAQ ONNs have enormous
potential to improve cancer therapy.
The growing exploration of nanomedicine has contributed greatly to cancer treatment over the past few decades1. Both carrier-assisted nanomedicines and carrier-free nanomedicines are being
developed to improve drug-intrinsic kinetics and safety profiles2,3,4,5. However, there are several limitations associated with these nanotherapeutic approaches6,7. First, complexity and
toxicity due to their multicomponent natures have severely hampered the clinical translation of many nanoformulations6. Second, most drugs used in conventional delivery studies were approved
decades ago and some are no longer first-line treatments5,8. Third, nanoformulations that are designed for recently developed therapeutic agents, especially new-chemical entities, may
encounter patent issues. Finally, not all drugs can be structurally modified. These limitations can be addressed in the context of medicinal chemistry9. Compared with nanomedicine, which
focuses on delivery profiles for drug research and development, medicinal chemistry commits to the discovery of drug entities in earlier stages10. Although drug discovery technologies have
generated numerous drug leads and candidates, problems surrounding drug kinetics, metabolism and toxicology remain challenging11,12. These challenges may also be solved relatively easily by
nanotechnologies from the field of nanomedicine. To take advantage of this transdisciplinary connection, we herein integrate the principle of nanotechnology into initial drug design and
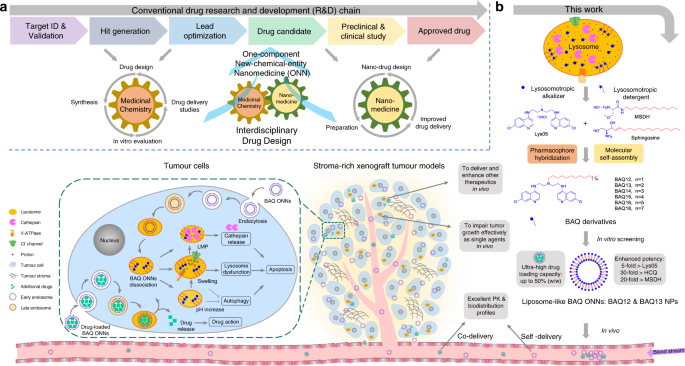
develop a one-component new-chemical-entity nanomedicine (ONN) strategy (Fig. 1a). In this strategy, the drug design follows both conventional drug design strategies and molecular
self-assembly principles so that designed drugs are endowed with advantages from the perspectives of both drug discovery and drug delivery.
a An interdisciplinary drug design strategy is proposed to integrate the conventional fields of medicinal chemistry and nanomedicine. Drugs are named as one-component new-chemical-entity
nanomedicines (ONNs), which are designed according to the strategies of conventional drug design and molecular self-assembly so that they could acquire the advantages from the perspectives
of both drug discovery and drug delivery. b The proof-of-concept experiment in this work: discovery of self-delivering lysosomotropic bisaminoquinoline (BAQ) derivatives for cancer therapy.
The BAQ derivatives, generated from the hybridisation of lysosomotropic detergents and the BAQ-based autophagy inhibitor, can self-assemble into BAQ ONNs that show enhanced functions in
vitro, excellent delivery profiles and significant in vivo therapeutic effects as single agents. Moreover, they also possess high drug-loading efficiency to deliver the additional drug into
tumour sites, thus generating a promising application of combination therapy.
To perform a proof-of-concept experiment, we chose lysosomes as therapeutic cancer targets13,14,15. Cancer cell lysosomes are hypertrophic and easily ruptured and are more fragile than
normal lysosomes16. Lysosomal membrane permeabilization (LMP) can directly trigger cell death by enabling the release of proteolytic enzymes (i.e. cathepsins) into the cytoplasm; therefore,
lysosomotropic detergents that can induce LMP have been developed for tumour treatment17. Moreover, lysosomal inhibition has considerable potential as an anticancer strategy because it
interferes with autophagy, an important pathway for the stress response and drug resistance of established tumours18,19,20,21,22. The lysosomotropic alkalizers chloroquine (CQ) and
hydroxychloroquine (HCQ) are commonly used autophagy inhibitors that have been tested in multiple clinical trials against various cancer types. However, their efficacy is considered
insufficient, particularly when they are used as single agents23. To discover more effective autophagy inhibitors, McAfee et al. synthesised a bisaminoquinoline (BAQ) derivative, Lys05, that
can impair tumour growth both in vitro and in vivo as a single agent24.
In this work, we adopt the strategies of pharmacophore hybridisation and molecular self-assembly to design a series of lipophilic cationic BAQ derivatives (Fig. 1b and Supplementary Fig.
1)25,26. BAQ12 and BAQ13 are selected to construct ONNs because of their potential to be therapeutic agents and self-assembling building blocks. These BAQ ONNs display excellent anticancer
activity in vitro, with enhanced effects on lysosomal disruption, lysosomal dysfunction and autophagy inhibition. Moreover, as nanodrugs, the BAQ ONNs exhibit the expected self-delivering
profiles. These advantages from the perspectives of both drug discovery and drug delivery ultimately contribute to the significant anticancer activity of these compounds as single agents in
gastrointestinal cancer models in vivo. In addition, the BAQ ONNs display promise for applications in combination therapy with napabucasin, as they play dual roles as both therapeutic agents
and delivery carriers. With their multidisciplinary integration and ingenious functional superposition, BAQ ONNs will emerge as good alternatives for improvement of cancer treatment.
BAQ12–BAQ18 were designed via hybridisation of the key structural elements of the lysosomotropic autophagy inhibitor Lys05 and the lysosomotropic detergent MSDH to achieve pharmacological
fusion (Fig. 1b). Based on self-assembly principles, we envisioned that the inclusion of long hydrophobic tails with the cationic BAQ heads would drive them to form nanoparticles (NPs)26,27.
Since BAQ heads have a calculated pKa of 8.4, this self-assembly should be dependent on the surroundings’ pH, wherein NPs are formed under neutral conditions and are dissociated into free
building blocks after protonation in acidic environments.
The compounds (BAQ12–BAQ18) were synthesised and structurally confirmed by 1H NMR, 13C NMR and HRMS spectra (Supplementary Fig. 1). In contrast to Lys05 and MSDH, BAQ12–BAQ18 were not
completely soluble in water as free base or hydrochloride salt forms, which is a definite defect that prevents them from being ideal drugs. However, the lipophilic cations allowed for
spontaneous self-assembly in water via nanoprecipitation, which resulted in homogeneous opalescent NP solutions. The assembled NPs of BAQ12–BAQ18 had similar nanoscale characteristics,
including their sizes (100–140 nm), polydispersity index (PDI) values