Synthetic phosphoethanolamine-modified oligosaccharides reveal the importance of glycan length and substitution in biofilm-inspired assemblies
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Bacterial biofilm matrices are nanocomposites of proteins and polysaccharides with remarkable mechanical properties. Efforts understanding and tuning the protein component have been
extensive, whereas the polysaccharide part remained mostly overlooked. The discovery of phosphoethanolamine (pEtN) modified cellulose in _E. coli_ biofilms revealed that polysaccharide
functionalization alters the biofilm properties. To date, the pattern of pEtN cellulose and its mode of interactions with proteins remains elusive. Herein, we report a model system based on
synthetic epitomes to explore the role of pEtN in biofilm-inspired assemblies. Nine pEtN-modified oligosaccharides were synthesized with full control over the length, degree and pattern of
pEtN substitution. The oligomers were co-assembled with a representative peptide, triggering the formation of fibers in a length dependent manner. We discovered that the pEtN pattern
modulates the adhesion of biofilm-inspired matrices, while the peptide component controls its stiffness. Unnatural oligosaccharides tune or disrupt the assembly morphology, revealing
interesting targets for polysaccharide engineering to develop tunable bio-inspired materials. SIMILAR CONTENT BEING VIEWED BY OTHERS PHOSPHORYLASE-CATALYZED SYNTHESIS AND SELF-ASSEMBLED
STRUCTURES OF CELLULOSE OLIGOMERS IN THE PRESENCE OF PROTEIN DENATURANTS Article 02 December 2021 BIOENGINEERING APPROACH FOR THE DESIGN OF MAGNETIC BACTERIAL CELLULOSE MEMBRANES Article
Open access 07 November 2024 _BACILLUS SUBTILIS_ EPSA-O: A NOVEL EXOPOLYSACCHARIDE STRUCTURE ACTING AS AN EFFICIENT ADHESIVE IN BIOFILMS Article Open access 02 October 2024 INTRODUCTION
Bacteria secrete various biomolecules to create extensive networks of extracellular matrix (ECM). These biofilms, often associated with pathogenic infections1, have gained popularities for
their remarkable mechanical properties, transforming bacteria into elegant biofactories of smart materials2,3,4,5. The major components of the ECM of _Escherichia coli (E. coli)_ biofilms
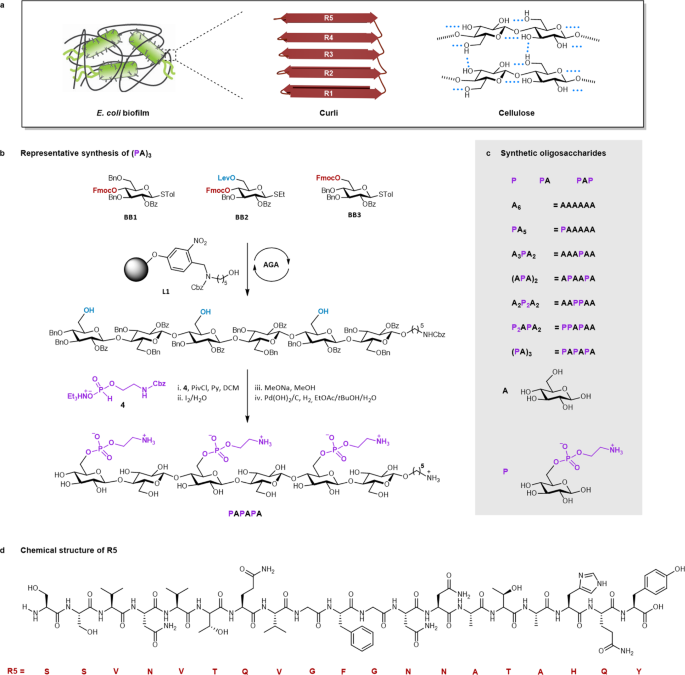
are curli fibrils—bacterial functional amyloids6,7—and cellulose8,9 (Fig. 1a). Genetic engineering approaches10 permitted the programming of bacterial amyloid production11,12 to generate
tunable bioplastics13. Similar strategies to tune the production of bacterial polysaccharides4,14,15, the other major components of bacteria biofilms, are limited by complex biosynthetic
pathways. Recently, it was discovered that some bacteria (e.g., _E. coli_ and _Salmonella enterica_) produce chemically modified cellulose bearing phosphoethanolamine (pEtN) substituents16.
The composite of curli and pEtN cellulose generates biofilms with enhanced elasticity and adhesion to bladder epithelial cells17. This exciting discovery suggests that the carbohydrate
component tunes the biofilm properties and may be the basis for tailoring cellulosic materials for applications in tissue engineering, biotechnology, and the food industry18,19. With the
genes responsible for the pEtN modification identified16, genetically engineered bacteria could be imagined for the production of specifically modified cellulose14,20,21,22. Several
fundamental aspects remain to be elucidated before pEtN cellulose can be exploited to its full potential. Approximately half of the glucose units of cellulose are substituted at the C-6
hydroxyl group with a pEtN moiety and the ratio of curli-to-pEtN cellulose varies among different _E. coli_ strains23. The pattern of modification, the length of the pEtN cellulose, and the
mode of interaction with curli remain unknown18. Pure, well-defined oligosaccharide standards are essentials to better understand pEtN cellulose and its role in the ECM, in anticipation of
applications. Isolation of pEtN cellulose from natural sources generates ill-defined mixtures and may alter its chemical structure17. Chemical synthesis can provide standards with precise
control over the sequence, length, and substitution pattern24,25. However, to date, the inherent complexity of carbohydrate synthesis has prevented the production of pEtN cellulose oligomers
beyond a disaccharide26. Here, we report the synthesis by automated glycan assembly27 (AGA) of nine pEtN cellulose oligosaccharides with varying chain lengths, degrees and patterns of pEtN
substitution. The interaction of these glycans with a representative amylogenic peptide of curli (R5)28,29 is studied. Co-assembly experiments generate artificial fibers and matrices with
morphologies and mechanical properties depending on the oligosaccharide structure. Unnatural, synthetic oligosaccharides disrupt or modulate the artificial fibers. These results suggest that
selective polysaccharide modification is a valuable approach to generate tunable biofilm-inspired materials. RESULTS SYNTHESIS OF PETN-SUBSTITUTED OLIGOSACCHARIDES The pEtN-substituted
oligosaccharides were prepared by a combination of AGA and post-AGA steps. The cellulose backbone was constructed by AGA, following cycles of glycosylation and Fmoc deprotection on solid
support L1 (Fig. 1b). BB1 allowed for linear chain elongation. BB2 was designed with a levulinoyl (Lev) ester at C-6 that can be selectively hydrolyzed to unmask the hydroxyl group for the
subsequent introduction of pEtN. BB1 and BB2 were strategically assembled to generate oligomers with the desired pattern of hydroxyl groups. BB3 was employed in the last cycle of the
assembly. After Lev removal, the oligosaccharide backbone was cleaved from the solid support and subjected to post-AGA transformations. The available hydroxyl groups were coupled to the
H-phosphonate 4 to give the protected phosphorylated compounds, upon oxidation with aqueous iodine30. Steric hindrance made multi-phosphorylation progressively more difficult, requiring five
equiv. of 4 and pivaloyl chloride (PivCl) per hydroxyl group to reach full conversion. Excess reagents necessitated extensive purifications to avoid interference with the deprotection
steps. Removal of all the remaining protecting groups (PGs) via methanolysis and hydrogenolysis required a careful optimization of the reaction conditions to avoid aggregation/precipitation
of the amphiphilic intermediates31. Nine zwitterionic compounds were prepared; a mono- and a di-saccharide bearing one pEtN group, a trisaccharide carrying two pEtN groups, and six
hexasaccharides substituted with one, two or three pEtN units (Fig. 1c). The neutral cellulose analogue A6 was synthesized as a control. ASSEMBLY OF ARTIFICIAL AMYLOID FIBERS Well-defined
pEtN oligosaccharides provided the bases for exploring the role of the carbohydrate component in biofilm-inspired assemblies. We envisioned an artificial model system consisting of synthetic
molecules representatives of the major components of the _E. coli_ ECM. As epitome for the protein part, we selected R5 (Fig. 1d), the most amyloidogenic repeat of the CsgA unit of curli28
(the detailed solid-phase synthesis is available in the SI). To generate artificial curli fibers, R5 was dissolved in hexafluoroisopropanol (HFIP)32,33. HFIP was then removed under nitrogen
purging followed by evaporation under high vacuum. Addition of water triggered a structural transition from an alpha helix to a beta-sheet conformation, as confirmed by circular dichroism
(CD) spectroscopy (Supplementary Fig. 50). The transition was completed within 20 min (Fig. 2a and Supplementary Fig. 53). Microscopic analysis (AFM, TEM, and SEM, Fig. 2a and Supplementary
Fig. 56) performed after 1 or 5 days of incubation showed the presence of ill-defined aggregates. We then repeated the same assembly process in the presence of the respective oligosaccharide
using a R5:oligosaccharide mass ratio of 6:1 that best resembles the ECM produced by the uropathogenic _E. coli_ strain UTI8916. First, we screened the effect of the oligosaccharide length
on the aggregation of R5. Co-assembly with the shorter analogues P, PA, and PAP did not significantly affect the structural transition rate of R5, while in the presence of longer
hexasaccharides, the secondary structure transition of R5 to the beta-sheet conformation was slower (Fig. 2a and Supplementary Fig. 52). The beta-sheet motif was confirmed by the ThT binding
test34 (Supplementary Fig. 60). Fiber-like structures instead of ill-defined aggregates were detected. We observed a length dependent behavior, with fibers becoming longer with the
oligosaccharide chain length. We then examined seven cellulose hexasaccharides with different degree and pattern of pEtN substitution on the assembly of R5 (Fig. 2b and Supplementary Figs.
52 and 55). CD analysis indicated that differences in pEtN substitution (degree and pattern) affect the secondary structure transition rate of R5, with the sample prepared in the presence of
(PA)3 showing the slowest transition (>6 h) into the beta-sheet conformation (Fig. 2a). Microscopy analysis showed that the R5 sample containing the unsubstituted cellulose oligomer A6
assembled into thin fibrils (Fig. 2b, Day 1) that developed into a fibrous network within 5 days (Fig. 2b). All pEtN substituted hexasaccharides also generated fibrils, albeit with different
growth rates and morphologies (Fig. 2b, and Supplementary Figs. 52 and 55). While the samples containing the three-substituted oligomers (PA)3 and P2APA2 showed long and defined fibrils
already on Day 1 (Fig. 2b), the less substituted analogues formed shorter aggregates (Fig. 2b and Supplementary Fig. 55). Interestingly, the fibers observed for R5 and (PA)3 adopt the
classical curled shape responsible for the name of the natural analogue (Fig. 2b)35. On Day 5, all samples formed fibrous networks (Fig. 2b, Supplementary Fig. 55). The modular approach
allowed us to explore different peptide:carbohydrate ratios to better mimic the ECM produced by other bacteria strains. For example, the _E. coli_ AR3110 strain produces a ECM with a much
higher pEtN cellulose content (3:1 by mass)23. No drastic differences were observed in the fiber morphology, however the fibrils obtained starting from a 3:1 ratio of R5 and (PA)3 or P2APA2
were embedded in a much thicker surrounding matrix (Supplementary Fig. 59b–c). This observation suggests that the pEtN-modified cellulose forms the network connecting the peptide-based
fibers, consistent with the existing descriptions of pEtN-cellulose as the “glue” that provides cohesion8,16. STRUCTURAL ANALYSIS The fibres obtained from the R5 sample in the presence of
A6, (PA)3 or P2APA2 showed a similar z-height of around 0.8 nm (Fig. 3a), suggesting that the fibrils are built on the same peptide core. The sample containing R5 and A6 showed “naked”
fibers together with random aggregates, identified as self-sorted A6 clusters with height of 4 nm (Fig. 2b and Supplementary Fig. 57, indicated with white arrows and corresponding to
Supplementary Fig. 58). In contrast, the fibers generated from the sample containing R5 and (PA)3 or P2APA2 were embedded in a thin matrix (Fig. 3a middle and bottom, highlighted with white
arrows). Non-stained TEM images confirmed the presence of a matrix around the fibrils, showing the fibers brighter than the surrounding36 (Fig. 2b). To gain insights into the molecular
interaction between R5 and the oligosaccharides, we employed solution-state NMR spectroscopy following an approach that revealed key interactions between synthetic heparin oligosaccharides
and amyloid fibers37. 2D 1H-1H total correlated spectroscopy (TOCSY) helped the assignment of the nineteen amidic protons of R5 (Supplementary Fig. 63). This sample suffered from poor
solubility due to aggregation, as shown by the broadening and decreased intensity of the NMR signal with time (Supplementary Fig. 64). The three samples containing both R5 and A6, (PA)3 and
P2APA2 respectively showed higher solubility and chemical shift perturbations for selected amino acids (Fig. 3b). Tyrosine, glutamine, histidine and serine were the most affected amino acids
in all three samples, albeit to a different extent (Fig. 3b, top panels). The 31P-NMR signals of the pEtN groups did not show any significant line broadening or chemical shift perturbation
(Supplementary Fig. 72), indicating that the pEtN groups are not directly involved in the interaction with R538. Taken together, these results indicate that the presence of the
oligosaccharide slow down the R5 transition into the beta-sheet conformation, favoring the formation of long amyloid fibers over ill-defined aggregates39,40. This could be the result of a
direct peptide-oligosaccharide interaction that leaves the ionic pEtN groups exposed to water or of a change in the peptide environment due to the presence of the oligosaccharide. MECHANICAL
PROPERTIES OF ARTIFICIAL BIOFILM-INSPIRED MATRICES The co-assembled samples that generated fibers were drop-casted on a glass slide to prepare artificial biofilm-inspired matrices with a
thickness of around 300 nm. Their mechanical properties were explored using AFM force-distance curve analysis (Fig. 4). A stiffness of around 12 MPa for all the matrices was measured with
AFM nanoindentation experiments, indicating that the peptide fibres are the major structural component of the artificial matrix. The presence of the pEtN-modified oligosaccharides
dramatically enhanced adhesion. The adhesion force for the sample containing R5 and (PA)3 was around 130 nN, six times higher than the value obtained for the sample containing R5 and A6. No
direct correlation between the number of pEtN groups and the adhesion was found. The highest values were measured for compounds with the pEtN moiety coupled to the non-reducing end glucose
(i.e., (PA)3 and PA5). Multiple pEtN substituents in close vicinity (e.g., A2P2A2) resulted in much lower adhesion forces, underscoring the importance of the substitution pattern in
determining the mechanical properties of the film. THE EFFECT OF UNNATURAL OLIGOSACCHARIDE MODIFICATIONS The discovery of the naturally modified pEtN-cellulose opened up opportunities to
generate tuneable materials upon engineering of the carbohydrate components18. It has been shown that carbohydrates can modulate the formation of neurotoxic amylogenic fibrils, with chitosan
oligosaccharides inhibiting aggregation41 and heparan sulfates promoting fiber formation42. To explore the effect of different glycan modifications on R5 aggregation, two hexasaccharides
not present in natural bacterial biofilms were prepared following established protocols43,44 (Fig. 5a). N6 is a neutral analogue of A6 that carries an acetyl amino substituent in position
C-2. (SA)3 is an analogue of (PA)3 in which the pEtN groups are replaced by negatively charged sulfate moieties. In the presence of the _N_-acetyl glucosamine hexasaccharide N6, the
secondary structure transition of R5 into beta-sheet was completed in less than 3 h (Fig. 5b). Fibrils shorter than 1 µm that further aggregated into supramolecular bundles were formed (Fig.
5b and Supplementary Fig. 56). Artificial matrices composed of R5 and N6 were prepared, showing comparable stiffness but higher adhesion than the samples prepared from R5 and A6
(Supplementary Table 17). In contrast, the negatively charged sulfated hexasaccharide (SA)3 interrupted the R5 transition into the beta-sheet conformation (Fig. 5b) and the formation of
fibrils (Fig. 5b and Supplementary Fig. 56). This inhibition might be a consequence of strong columbic interactions between the negatively charged oligosaccharide (SA)3 and the cationic
groups on R5, stressing the importance of the zwitterionic pEtN groups in directing R5 aggregation. The ability of the sulfated hexasaccharide, (SA)3, to inhibit amyloid formation renders
this compound an interesting starting point for novel approaches toward the treatment of neurological diseases or as antibacterial agent45,46. DISCUSSION A collection of pEtN-modified
oligosaccharides was synthesized with full control over chain length, degree and pattern of substitutions, providing essential standards to study complex biological systems. The
oligosaccharides were incubated with a synthetic peptide, R5, representative of curli, to generate a modular model of the _E. coli_ biofilm ECM and break down its complexity. Full control
over the chemical structure of the individual components permitted to explore the role of oligosaccharide length and substitution in peptide aggregation. While shorter oligomers had little
effect, the longer hexasaccharides slowed down the secondary structure transition into beta-sheet of the peptide R5, inducing the growth of extended fibrous structures. The oligosaccharide
fine structure dramatically affected the fiber growth rate and the mechanical properties of the composite. We demonstrated that not only the degree, but also the pattern of pEtN substitution
influences adhesion. In contrast, stiffness remains unchanged for all samples indicating its strong connection to the peptide component. Modifications beyond the natural one were screened,
delivering interesting targets for the future production of engineered biofilm-inspired materials. Metabolic engineering47 and/or directed evolution approaches48,49 may introduce such
modifications in vivo and produce novel cellulosic materials with non-natural modifications. METHODS SYNTHESIS The oligosaccharides were prepared using a home-built synthesizer designed at
the Max Planck Institute of Colloids and Interfaces. The solid-phase peptides synthesis (SPPS) of R5 was performed with a microwave-assisted peptide synthesizer (Liberty Blue, CEM, USA). All
details concerning building block synthesis, AGA modules, post-AGA manipulations, and SPPS can be found in Supplementary Information. ASSEMBLY OF ARTIFICIAL FIBERS AND MATRICES Stock
solutions were prepared dissolving separately R5 and the oligosaccharides in HFIP with a concentration of 200 µM (0.4 mg mL−1) and 0.13 mg mL−1, respectively. The R5 and oligosaccharide
stock solutions were mixed with 2 to 1 (or 1 to 1) volume ratio to reach the final mass ratio with 6 to 1 (or 3 to 1) and sonicated for 10 min. HFIP was removed under gentle nitrogen purging
followed by evaporation under high vacuum. Water was added to the dried films to reach the final peptide concentration of 25 µM for imaging, CD, and ThT binding test, and 200 µM for 2D
TOCSY NMR analysis. The artificial films were prepared by drop-casting of a 25 µM solution of the co-assembled sample on a pre-washed glass substrate to generate films with a thickness of
300 nm. The co-assembled sample were prepared with a 6:1 peptide:oligosaccharide mass ratio and incubated for 5 days before drop-casting. AFM imaging and force measurement were performed in
air in an AFM chamber with a relative humidity of 25%. If not mentioned, the standard ratio between R5 and oligosaccharide is 6 to 1 by mass. All details concerning fibrils’ structural
analysis and films’ mechanical properties can be found in Supplementary Information. DATA AVAILABILITY The authors declare that all data supporting the findings of this study are available
within the paper and in the Supplementary Information files. Data are also available from the corresponding author upon request. REFERENCES * Flemming, H.-C. & Wingender, J. The biofilm
matrix. _Nat. Rev. Microbiol._ 8, 623–633 (2010). Article CAS PubMed Google Scholar * Gilbert, C. et al. Living materials with programmable functionalities grown from engineered
microbial co-cultures. _Nat. Mater._ 20, 691–700 (2021). Article ADS CAS PubMed Google Scholar * Moradali, M. F. & Rehm, B. H. A. Bacterial biopolymers: from pathogenesis to
advanced materials. _Nat. Rev. Microbiol._ 18, 195–210 (2020). Article CAS PubMed PubMed Central Google Scholar * Nguyen, P. Q., Courchesne, N.-M. D., Duraj-Thatte, A., Praveschotinunt,
P. & Joshi, N. S. Engineered living materials: prospects and challenges for using biological systems to direct the assembly of smart materials. _Adv. Mater._ 30, 1704847 (2018). Article
CAS Google Scholar * Hayta, E. N., Ertelt, M. J., Kretschmer, M. & Lieleg, O. Bacterial materials: applications of natural and modified biofilms. _Adv. Mater. Interfac._ 8, 2101024
(2021). Article CAS Google Scholar * Evans, M. L. & Chapman, M. R. Curli biogenesis: order out of disorder. _Biochim. Biophys. Acta_ 1843, 1551–1558 (2014). Article CAS PubMed
Google Scholar * Ke, P. C. et al. Half a century of amyloids: past, present and future. _Chem. Soc. Rev._ 49, 5473–5509 (2020). Article CAS PubMed PubMed Central Google Scholar *
Serra, D. O., Richter, A. M. & Hengge, R. Cellulose as an Architectural Element in Spatially Structured Escherichia coli Biofilms. _J. Bacteriol._ 195, 5540–5554 (2013). Article CAS
PubMed PubMed Central Google Scholar * Zogaj, X., Nimtz, M., Rohde, M., Bokranz, W. & Romling, U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce
cellulose as the second component of the extracellular matrix. _Mol. Microbiol._ 39, 1452–1463 (2001). Article CAS PubMed Google Scholar * Cameron, D. E., Bashor, C. J. & Collins, J.
J. A brief history of synthetic biology. _Nat. Rev. Microbiol._ 12, 381–390 (2014). Article CAS PubMed Google Scholar * Chen, A. Y. et al. Synthesis and patterning of tunable multiscale
materials with engineered cells. _Nat. Mater._ 13, 515–523 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Nguyen, P. Q., Botyanszki, Z., Tay, P. K. R. & Joshi, N.
S. Programmable biofilm-based materials from engineered curli nanofibres. _Nat. Commun._ 5, 4945 (2014). Article ADS CAS PubMed Google Scholar * Duraj-Thatte, A. M. et al.
Water-processable, biodegradable and coatable aquaplastic from engineered biofilms. _Nat. Chem. Biol._ 17, 732–738 (2021). Article CAS PubMed PubMed Central Google Scholar * Florea, M.
et al. Engineering control of bacterial cellulose production using a genetic toolkit and a new cellulose-producing strain. _Proc. Natl Acad. Sci. USA_ 113, E3431–E3440 (2016). Article CAS
PubMed PubMed Central Google Scholar * Zhang, J. & Poh, C. L. Regulating exopolysaccharide gene wcaF allows control of Escherichia coli biofilm formation. _Sci. Rep._ 8, 13127 (2018).
Article ADS PubMed PubMed Central CAS Google Scholar * Thongsomboon, W. et al. Phosphoethanolamine cellulose: a naturally produced chemically modified cellulose. _Science_ 359,
334–338 (2018). Article ADS CAS PubMed Google Scholar * Hollenbeck, E. C. et al. Phosphoethanolamine cellulose enhances curli-mediated adhesion of uropathogenic Escherichia coli to
bladder epithelial cells. _Proc. Natl Acad. Sci. USA_ 115, 10106–10111 (2018). Article ADS CAS PubMed PubMed Central Google Scholar * Jeffries, J., Fuller, G. G. & Cegelski, L.
Unraveling Escherichia coli’s Cloak: Identification of Phosphoethanolamine Cellulose, Its Functions, and Applications. _Microbiol. Insights_ 12, 117863611986523 (2019). Article Google
Scholar * Singh, A., Walker, K. T., Ledesma-Amaro, R. & Ellis, T. Engineering Bacterial Cellulose by Synthetic Biology. _Int. J. Mol. Sci._ 21, 9185 (2020). Article CAS PubMed Central
Google Scholar * Yi, W. et al. Remodeling bacterial polysaccharides by metabolic pathway engineering. _Proc. Natl Acad. Sci. USA_ 106, 4207–4212 (2009). Article ADS CAS PubMed PubMed
Central Google Scholar * Goon, S., Schilling, B., Tullius, M. V., Gibson, B. W. & Bertozzi, C. R. Metabolic incorporation of unnatural sialic acids into Haemophilus ducreyi
lipooligosaccharides. _Proc. Natl Acad. Sci. USA_ 100, 3089–3094 (2003). Article ADS CAS PubMed PubMed Central Google Scholar * Rehm, B. H. A. Bacterial polymers: Biosynthesis,
modifications and applications. _Nat. Rev. Microbiol._ 8, 578–592 (2010). Article CAS PubMed Google Scholar * Jeffries, J. et al. Variation in the ratio of curli and phosphoethanolamine
cellulose associated with biofilm architecture and properties. _Biopolymers_ 112, 1–11 (2021). Article MathSciNet CAS Google Scholar * Yu, Y. et al. Systematic Hydrogen‐Bond
Manipulations To Establish Polysaccharide Structure–Property Correlations. _Angew. Chem. Int. Ed._ 58, 13127–13132 (2019). Article CAS Google Scholar * Fittolani, G., Tyrikos-Ergas, T.,
Vargová, D., Chaube, M. A. & Delbianco, M. Progress and challenges in the synthesis of sequence controlled polysaccharides. _Beilstein J. Org. Chem._ 17, 1981–2025 (2021). Article CAS
PubMed PubMed Central Google Scholar * Nguyen, J. M., Moore, R. E., Spicer, S. K., Gaddy, J. A. & Townsend, S. D. Synthetic Phosphoethanolamine Cellobiose Promotes Escherichia coli
Biofilm Formation and Congo Red Binding. _ChemBioChem_ 22, 2540–2545 (2021). Article CAS PubMed Google Scholar * Guberman, M. & Seeberger, P. H. Automated Glycan assembly: a
perspective. _J. Am. Chem. Soc._ 141, 5581–5592 (2019). Article CAS PubMed PubMed Central Google Scholar * Wang, X., Smith, D. R., Jones, J. W. & Chapman, M. R. In Vitro
Polymerization of a Functional Escherichia coli Amyloid Protein. _J. Biol. Chem._ 282, 3713–3719 (2007). Article CAS PubMed Google Scholar * Wang, X., Hammer, N. D. & Chapman, M. R.
The Molecular Basis of Functional Bacterial Amyloid Polymerization and Nucleation. _J. Biol. Chem._ 283, 21530–21539 (2008). Article CAS PubMed PubMed Central Google Scholar * Tsai,
Y.-H. et al. A general and convergent synthesis of diverse glycosylphosphatidylinositol glycolipids. _Chem. Sci._ 4, 468–481 (2013). Article CAS Google Scholar * Yu, Y. et al.
Oligosaccharides Self-Assemble and Show Intrinsic Optical Properties. _J. Am. Chem. Soc._ 141, 4833–4838 (2019). Article CAS PubMed PubMed Central Google Scholar * Tomaselli, S. et al.
The α-to-β Conformational Transition of Alzheimer’s Aβ-(1-42) Peptide in Aqueous Media is Reversible: A Step by Step Conformational Analysis Suggests the Location of β Conformation Seeding.
_ChemBioChem_ 7, 257–267 (2006). Article CAS PubMed Google Scholar * Hirota, N., Goto, Y. & Mizuno, K. Cooperative α-helix formation of β-lactoglobulin and melittin induced by
hexafluoroisopropanol. _Protein Sci._ 6, 416–421 (1997). Article CAS PubMed PubMed Central Google Scholar * Biancalana, M. & Koide, S. Molecular mechanism of Thioflavin-T binding to
amyloid fibrils. _Biochim. Biophys. Acta_ 1804, 1405–1412 (2010). Article CAS PubMed PubMed Central Google Scholar * Sleutel, M. et al. Nucleation and growth of a bacterial functional
amyloid at single-fiber resolution. _Nat. Chem. Biol._ 13, 902–908 (2017). Article CAS PubMed PubMed Central Google Scholar * Raheja, A., Agarwal, A., Muthuvijayan, V., Chandra, T. S.
& Natarajan, T. S. Studies on Encapsulation of Bovine Serum Albumin, Lysozyme and Insulin Through Coaxial Electrospinning. _J. Biomater. Tissue Eng._ 3, 669–672 (2013). Article CAS
Google Scholar * Wang, P. et al. Probing Amyloid β Interactions with Synthetic Heparan Sulfate Oligosaccharides. _ACS Chem. Biol._ 16, 1894–1899 (2021). Article CAS PubMed Google Scholar
* Manzenrieder, F., Frank, A. O. & Kessler, H. Phosphorus NMR Spectroscopy as a Versatile Tool for Compound Library Screening. _Angew. Chem. Int. Ed._ 47, 2608–2611 (2008). Article
CAS Google Scholar * Wei, G. et al. Self-assembling peptide and protein amyloids: from structure to tailored function in nanotechnology. _Chem. Soc. Rev._ 46, 4661–4708 (2017). Article
CAS PubMed PubMed Central Google Scholar * Ilie, I. M. & Caflisch, A. Simulation Studies of Amyloidogenic Polypeptides and Their Aggregates. _Chem. Rev._ 119, 6956–6993 (2019).
Article CAS PubMed Google Scholar * Dai, X. et al. Chitosan Oligosaccharides Inhibit/Disaggregate Fibrils and Attenuate Amyloid β-Mediated Neurotoxicity. _Int. J. Mol. Sci._ 16,
10526–10536 (2015). Article CAS PubMed PubMed Central Google Scholar * Liu, C.-C. et al. Neuronal heparan sulfates promote amyloid pathology by modulating brain amyloid-β clearance and
aggregation in Alzheimer’s disease. _Sci. Transl. Med._ 8, 332ra44–332ra44 (2016). Article PubMed PubMed Central Google Scholar * Tyrikos-Ergas, T. et al. Systematic Structural
Characterization of Chitooligosaccharides Enabled by Automated Glycan Assembly. _Chem. Eur. J._ 27, 2321–2325 (2021). Article CAS PubMed Google Scholar * Tyrikos-Ergas, T., Sletten, E.
T., Huang, J.-Y., Seeberger, P. H. & Delbianco, M. On resin synthesis of sulfated oligosaccharides. _Chem. Sci._ 13, 2115–2120 (2022). Article CAS PubMed PubMed Central Google
Scholar * Sievers, S. A. et al. Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation. _Nature_ 475, 96–100 (2011). Article CAS PubMed PubMed Central
Google Scholar * Chuang, E., Hori, A. M., Hesketh, C. D. & Shorter, J. Amyloid assembly and disassembly. _J. Cell Sci._ 131, 1–18 (2018). Article CAS Google Scholar * Laughlin, S. T.
& Bertozzi, C. R. Metabolic labeling of glycans with azido sugars and subsequent glycan-profiling and visualization via Staudinger ligation. _Nat. Protoc._ 2, 2930–2944 (2007). Article
CAS PubMed Google Scholar * Romero, P. A. & Arnold, F. H. Exploring protein fitness landscapes by directed evolution. _Nat. Rev. Mol. Cell Biol._ 10, 866–876 (2009). Article CAS
PubMed PubMed Central Google Scholar * Kan, A. & Joshi, N. S. Towards the directed evolution of protein materials. _MRS Commun._ 9, 441–455 (2019). Article CAS PubMed PubMed
Central Google Scholar Download references ACKNOWLEDGEMENTS We thank the Max Planck Society, the MPG-FhG Cooperation Project Glyco3Dysplay, and the German Federal Ministry of Education and
Research (BMBF, grant number 13XP5114) for generous financial support. J.Y.H. acknowledges the International Max Planck Research School on Multiscale Bio-Systems for funding. J.Y.H. and
M.D. acknowledge support from the Max Planck Queensland Centre on the Materials Science for Extracellular Matrices. D.V.S. and S.P.M. thank the RIKEN-Max Planck Joint Center for Systems
Chemical Biology for financial support. We thank Dr. Cécile Bidan for proofreading the paper. FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION Author
notes * Daniel Varón Silva Present address: Institute of Chemistry and Bioanalytics, School of Life Sciences, University of Applied Sciences and Arts Northwestern Switzerland,
Hofackerstrasse 30, 4132, Muttenz, Switzerland * These authors contributed equally: Theodore Tyrikos-Ergas, Soeun Gim, Jhih-Yi Huang. AUTHORS AND AFFILIATIONS * Department of Biomolecular
Systems, Max Planck Institute of Colloids and Interfaces, Am Mühlenberg 1, 14476, Potsdam, Germany Theodore Tyrikos-Ergas, Soeun Gim, Jhih-Yi Huang, Sandra Pinzón Martín, Peter H. Seeberger
& Martina Delbianco * Department of Chemistry and Biochemistry, Freie Universität Berlin, Arnimallee 22, 14195, Berlin, Germany Theodore Tyrikos-Ergas, Soeun Gim, Jhih-Yi Huang, Sandra
Pinzón Martín, Daniel Varón Silva & Peter H. Seeberger Authors * Theodore Tyrikos-Ergas View author publications You can also search for this author inPubMed Google Scholar * Soeun Gim
View author publications You can also search for this author inPubMed Google Scholar * Jhih-Yi Huang View author publications You can also search for this author inPubMed Google Scholar *
Sandra Pinzón Martín View author publications You can also search for this author inPubMed Google Scholar * Daniel Varón Silva View author publications You can also search for this author
inPubMed Google Scholar * Peter H. Seeberger View author publications You can also search for this author inPubMed Google Scholar * Martina Delbianco View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS T.T.E. and M.D. conceived this project. T.T.E. and J.Y.H. performed the synthesis and the NMR analysis. S.G. developed the
assembly methods, performed microscopic measurements and the mechanical property characterization of the films, and analyzed the data. J.Y.H. performed the nano-indentation of the films and
analyzed the data. S.P. assisted with the synthesis of R5. M.D. supervised the project. All authors contributed to and discussed the paper. CORRESPONDING AUTHOR Correspondence to Martina
Delbianco. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewer(s)
for their contribution to the peer review of this work. Peer reviewer reports are available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFO PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tyrikos-Ergas, T., Gim, S., Huang, JY. _et al._
Synthetic phosphoethanolamine-modified oligosaccharides reveal the importance of glycan length and substitution in biofilm-inspired assemblies. _Nat Commun_ 13, 3954 (2022).
https://doi.org/10.1038/s41467-022-31633-5 Download citation * Received: 08 March 2022 * Accepted: 28 June 2022 * Published: 08 July 2022 * DOI: https://doi.org/10.1038/s41467-022-31633-5
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative