On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

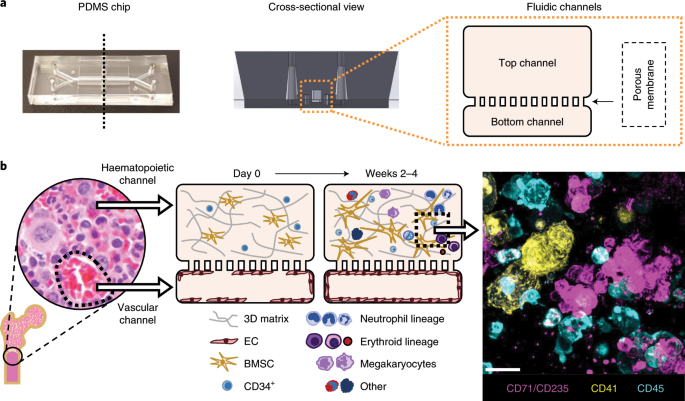
ABSTRACT The inaccessibility of living bone marrow (BM) hampers the study of its pathophysiology under myelotoxic stress induced by drugs, radiation or genetic mutations. Here, we show that
a vascularized human BM-on-a-chip (BM chip) supports the differentiation and maturation of multiple blood cell lineages over 4 weeks while improving CD34+ cell maintenance, and that it
recapitulates aspects of BM injury, including myeloerythroid toxicity after clinically relevant exposures to chemotherapeutic drugs and ionizing radiation, as well as BM recovery after
drug-induced myelosuppression. The chip comprises a fluidic channel filled with a fibrin gel in which CD34+ cells and BM-derived stromal cells are co-cultured, a parallel channel lined by
human vascular endothelium and perfused with culture medium, and a porous membrane separating the two channels. We also show that BM chips containing cells from patients with the rare
genetic disorder Shwachman–Diamond syndrome reproduced key haematopoietic defects and led to the discovery of a neutrophil maturation abnormality. As an in vitro model of haematopoietic
dysfunction, the BM chip may serve as a human-specific alternative to animal testing for the study of BM pathophysiology. Access through your institution Buy or subscribe This is a preview
of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A MICROFLUIDIC BONE MARROW CHIP FOR THE
SAFETY PROFILING OF BIOLOGICS IN PRE-CLINICAL DRUG DEVELOPMENT Article Open access 15 May 2025 GENERATING HUMAN BONE MARROW ORGANOIDS FOR DISEASE MODELING AND DRUG DISCOVERY Article 26 March
2024 BIOENGINEERED NICHES THAT RECREATE PHYSIOLOGICAL EXTRACELLULAR MATRIX ORGANISATION TO SUPPORT LONG-TERM HAEMATOPOIETIC STEM CELLS Article Open access 10 July 2024 DATA AVAILABILITY All
of the data supporting the results in this study are available within the Article and its Supplementary Information. The broad range of raw datasets acquired and analysed (or any subsets of
it), which for reuse would require contextual metadata, are available from the corresponding author on reasonable request. CHANGE HISTORY * _ 12 FEBRUARY 2020 A Correction to this paper has
been published: https://doi.org/10.1038/s41551-020-0529-6 _ REFERENCES * Doulatov, S., Notta, F., Laurenti, E. & Dick, J. E. Hematopoiesis: a human perspective. _Cell Stem Cell_ 10,
120–136 (2012). Article CAS PubMed Google Scholar * Eaves, C. J. Hematopoietic stem cells: concepts, definitions, and the new reality. _Blood_ 125, 2605–2613 (2015). Article CAS PubMed
PubMed Central Google Scholar * Wognum, B., Yuan, N., Lai, B. & Miller, C. L. Colony forming cell assays for human hematopoietic progenitor cells. _Methods Mol. Biol._ 946, 267–283
(2013). Article CAS PubMed Google Scholar * Dexter, T. M., Allen, T. D. & Lajtha, L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. _J. Cell
Physiol._ 91, 335–344 (1977). Article CAS PubMed Google Scholar * Sieber, S. et al. Bone marrow-on-a-chip: long-term culture of human haematopoietic stem cells in a three-dimensional
microfluidic environment. _J. Tissue Eng. Regen. Med._ 12, 479–489 (2018). Article CAS PubMed Google Scholar * Thon, J. N. et al. Platelet bioreactor-on-a-chip. _Blood_ 124, 1857–1867
(2014). Article CAS PubMed PubMed Central Google Scholar * Di Maggio, N. et al. Toward modeling the bone marrow niche using scaffold-based 3D culture systems. _Biomaterials_ 32, 321–329
(2011). Article CAS PubMed Google Scholar * Rodling, L. et al. 3D models of the hematopoietic stem cell niche under steady-state and active conditions. _Sci. Rep._ 7, 4625 (2017).
Article PubMed PubMed Central CAS Google Scholar * Bourgine, P. E. et al. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. _Proc. Natl Acad.
Sci. USA_ 115, E5688–E5695 (2018). Article CAS PubMed PubMed Central Google Scholar * Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. _Nature_
505, 327–334 (2014). Article CAS PubMed PubMed Central Google Scholar * Boitano, A. E. et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem
cells. _Science_ 329, 1345–1348 (2010). Article CAS PubMed PubMed Central Google Scholar * Fares, I. et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human
hematopoietic stem cell self-renewal. _Science_ 345, 1509–1512 (2014). Article CAS PubMed PubMed Central Google Scholar * Ferreira, M. S. et al. Cord blood-hematopoietic stem cell
expansion in 3D fibrin scaffolds with stromal support. _Biomaterials_ 33, 6987–6997 (2012). Article PubMed CAS Google Scholar * Lee, H. Y. et al. PPAR-α and glucocorticoid receptor
synergize to promote erythroid progenitor self-renewal. _Nature_ 522, 474–477 (2015). Article CAS PubMed PubMed Central Google Scholar * Pineault, N. & Abu-Khader, A. Advances in
umbilical cord blood stem cell expansion and clinical translation. _Exp. Hematol._ 43, 498–513 (2015). Article PubMed Google Scholar * Pessina, A. et al. Application of the CFU-GM assay
to predict acute drug-induced neutropenia: an international blind trial to validate a prediction model for the maximum tolerated dose (MTD) of myelosuppressive xenobiotics. _Toxicol. Sci._
75, 355–367 (2003). Article CAS PubMed Google Scholar * Boulais, P. E. & Frenette, P. S. Making sense of hematopoietic stem cell niches. _Blood_ 125, 2621–2629 (2015). Article CAS
PubMed PubMed Central Google Scholar * Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. _Nature_ 466, 829–834 (2010). Article PubMed
PubMed Central CAS Google Scholar * Sacchetti, B. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. _Cell_ 131, 324–336 (2007).
Article CAS PubMed Google Scholar * Rafii, S., Butler, J. M. & Ding, B. S. Angiocrine functions of organ-specific endothelial cells. _Nature_ 529, 316–325 (2016). Article CAS
PubMed PubMed Central Google Scholar * Hassell, B. A. et al. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. _Cell
Rep._ 21, 508–516 (2017). Article CAS PubMed Google Scholar * Huh, D. et al. Reconstituting organ-level lung functions on a chip. _Science_ 328, 1662–1668 (2010). Article CAS PubMed
PubMed Central Google Scholar * Dorrell, C., Gan, O. I., Pereira, D. S., Hawley, R. G. & Dick, J. E. Expansion of human cord blood CD34+CD38− cells in ex vivo culture during retroviral
transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. _Blood_ 95, 102–110 (2000). Article CAS PubMed Google
Scholar * Von Laer, D. et al. Loss of CD38 antigen on CD34+CD38+ cells during short-term culture. _Leukemia_ 14, 947–948 (2000). Article CAS PubMed Google Scholar * Danet, G. H., Lee,
H. W., Luongo, J. L., Simon, M. C. & Bonnet, D. A. Dissociation between stem cell phenotype and NOD/SCID repopulating activity in human peripheral blood CD34+ cells after ex vivo
expansion. _Exp. Hematol._ 29, 1465–1473 (2001). Article CAS PubMed Google Scholar * Csaszar, E. et al. Rapid expansion of human hematopoietic stem cells by automated control of
inhibitory feedback signaling. _Cell Stem Cell_ 10, 218–229 (2012). Article CAS PubMed Google Scholar * Jalili-Firoozinezhad, S. et al. A complex human gut microbiome cultured in an
anaerobic intestine-on-a-chip. _Nat. Biomed. Eng._ 3, 520–531 (2019). Article CAS PubMed PubMed Central Google Scholar * Longley, D. B., Harkin, D. P. & Johnston, P. G.
5-fluorouracil: mechanisms of action and clinical strategies. _Nat. Rev. Cancer_ 3, 330–338 (2003). Article CAS PubMed Google Scholar * Lee, J. J., Beumer, J. H. & Chu, E.
Therapeutic drug monitoring of 5-fluorouracil. _Cancer Chemother. Pharm._ 78, 447–464 (2016). Article CAS Google Scholar * Saif, M. W., Choma, A., Salamone, S. J. & Chu, E.
Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. _J. Natl Cancer Inst._ 101, 1543–1552 (2009). Article CAS PubMed
Google Scholar * Santini, J. et al. 5-FU therapeutic monitoring with dose adjustment leads to an improved therapeutic index in head and neck cancer. _Br. J. Cancer_ 59, 287–290 (1989).
Article CAS PubMed PubMed Central Google Scholar * Trump, D. L. et al. Pharmacokinetic and pharmacodynamic analysis of fluorouracil during 72-hour continuous infusion with and without
dipyridamole. _J. Clin. Oncol._ 9, 2027–2035 (1991). Article CAS PubMed Google Scholar * Malerba, I., Casati, S., Diodovich, C., Parent-Massin, D. & Gribaldo, L. Inhibition of
CFU-E/BFU-E and CFU-GM colony growth by cyclophosphamide, 5-fluorouracil and taxol: development of a high-throughput in vitro method. _Toxicol. Vitr._ 18, 293–300 (2004). Article CAS
Google Scholar * Carmena, M., Wheelock, M., Funabiki, H. & Earnshaw, W. C. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. _Nat. Rev. Mol. Cell
Biol._ 13, 789–803 (2012). Article CAS PubMed PubMed Central Google Scholar * Boss, D. S. et al. Clinical evaluation of AZD1152, an i.v. inhibitor of Aurora B kinase, in patients with
solid malignant tumors. _Ann. Oncol._ 22, 431–437 (2011). Article CAS PubMed Google Scholar * Kantarjian, H. M. et al. Stage I of a phase 2 study assessing the efficacy, safety, and
tolerability of barasertib (AZD1152) versus low-dose cytosine arabinoside in elderly patients with acute myeloid leukemia. _Cancer_ 119, 2611–2619 (2013). Article CAS PubMed Google
Scholar * Schwartz, G. K. et al. Phase I study of barasertib (AZD1152), a selective inhibitor of Aurora B kinase, in patients with advanced solid tumors. _Invest. New Drugs_ 31, 370–380
(2013). Article CAS PubMed Google Scholar * Yang, J. et al. AZD1152, a novel and selective Aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin
depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. _Blood_ 110, 2034–2040 (2007). Article CAS PubMed Google Scholar * Waselenko, J. K.
et al. Medical management of the acute radiation syndrome: recommendations of the strategic national stockpile radiation working group. _Ann. Intern. Med._ 140, 1037–1051 (2004). Article
PubMed Google Scholar * Singh, V. K. & Seed, T. M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation
sub-syndromes, animal models and FDA-approved countermeasures. _Int. J. Radiat. Biol._ 93, 851–869 (2017). Article CAS PubMed Google Scholar * Dror, Y. et al. Draft consensus guidelines
for diagnosis and treatment of Shwachman–Diamond syndrome. _Ann. NY Acad. Sci._ 1242, 40–55 (2011). Article PubMed Google Scholar * Myers, K. C., Davies, S. M. & Shimamura, A.
Clinical and molecular pathophysiology of Shwachman–Diamond syndrome: an update. _Hematol. Oncol. Clin. North Am._ 27, 117–128 (2013). Article PubMed Google Scholar * Bezzerri, V. &
Cipolli, M. Shwachman–Diamond syndrome: molecular mechanisms and current perspectives. _Mol. Diagnosis Ther._ 23, 281–290 (2019). Article CAS Google Scholar * Myers, K. C. et al. Variable
clinical presentation of Shwachman–Diamond syndrome: update from the North American Shwachman–Diamond syndrome registry. _J. Pediatr._ 164, 866–870 (2014). Article PubMed Google Scholar
* Finch, A. J. et al. Uncoupling of GTP hydrolysis from eIF6 release on the ribosome causes Shwachman–Diamond syndrome. _Genes Dev._ 25, 917–929 (2011). Article CAS PubMed PubMed Central
Google Scholar * Raaijmakers, M. H. et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. _Nature_ 464, 852–857 (2010). Article CAS PubMed PubMed Central
Google Scholar * Rawls, A. S., Gregory, A. D., Woloszynek, J. R., Liu, F. & Link, D. C. Lentiviral-mediated RNAi inhibition of _Sbds_ in murine hematopoietic progenitors impairs their
hematopoietic potential. _Blood_ 110, 2414–2422 (2007). Article CAS PubMed PubMed Central Google Scholar * Zambetti, N. A. et al. Deficiency of the ribosome biogenesis gene _Sbds_ in
hematopoietic stem and progenitor cells causes neutropenia in mice by attenuating lineage progression in myelocytes. _Haematologica_ 100, 1285–1293 (2015). Article CAS PubMed PubMed
Central Google Scholar * Zhang, S., Shi, M., Hui, C. C. & Rommens, J. M. Loss of the mouse ortholog of the Shwachman–Diamond syndrome gene (_Sbds_) results in early embryonic
lethality. _Mol. Cell Biol._ 26, 6656–6663 (2006). Article CAS PubMed PubMed Central Google Scholar * Dror, Y. & Freedman, M. H. Shwachman–Diamond syndrome: an inherited preleukemic
bone marrow failure disorder with aberrant hematopoietic progenitors and faulty marrow microenvironment. _Blood_ 94, 3048–3054 (1999). Article CAS PubMed Google Scholar * Kuijpers, T.
W. et al. Hematologic abnormalities in Shwachman Diamond syndrome: lack of genotype–phenotype relationship. _Blood_ 106, 356–361 (2005). Article CAS PubMed Google Scholar * Walasek, M.
A., van Os, R. & de Haan, G. Hematopoietic stem cell expansion: challenges and opportunities. _Ann. NY Acad. Sci._ 1266, 138–150 (2012). Article CAS PubMed Google Scholar * Lis, R.
et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. _Nature_ 545, 439–445 (2017). Article CAS PubMed PubMed Central Google Scholar * Sugimura, R. et al.
Haematopoietic stem and progenitor cells from human pluripotent stem cells. _Nature_ 545, 432–438 (2017). Article CAS PubMed PubMed Central Google Scholar * Kohn, L. A. et al. Lymphoid
priming in human bone marrow begins before expression of CD10 with upregulation of l-selectin. _Nat. Immunol._ 13, 963–971 (2012). Article CAS PubMed PubMed Central Google Scholar *
Rawlings, D. J., Quan, S. G., Kato, R. M. & Witte, O. N. Long-term culture system for selective growth of human B-cell progenitors. _Proc. Natl Acad. Sci. USA_ 92, 1570–1574 (1995).
Article CAS PubMed PubMed Central Google Scholar * Harrison, J. S., Rameshwar, P., Chang, V. & Bandari, P. Oxygen saturation in the bone marrow of healthy volunteers. _Blood_ 99,
394 (2002). Article CAS PubMed Google Scholar * Nombela-Arrieta, C. et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone
marrow microenvironment. _Nat. Cell Biol._ 15, 533–543 (2013). Article CAS PubMed PubMed Central Google Scholar * Spencer, J. A. et al. Direct measurement of local oxygen concentration
in the bone marrow of live animals. _Nature_ 508, 269–273 (2014). Article CAS PubMed PubMed Central Google Scholar * Broxmeyer, H. E., O’Leary, H. A., Huang, X. & Mantel, C. The
importance of hypoxia and extra physiologic oxygen shock/stress for collection and processing of stem and progenitor cells to understand true physiology/pathology of these cells ex vivo.
_Curr. Opin. Hematol._ 22, 273–278 (2015). Article CAS PubMed PubMed Central Google Scholar * Andre, V. et al. Mesenchymal stem cells from Shwachman–Diamond syndrome patients display
normal functions and do not contribute to hematological defects. _Blood Cancer J._ 2, e94 (2012). Article CAS PubMed PubMed Central Google Scholar * Dror, Y. et al. Immune function in
patients with Shwachman–Diamond syndrome. _Br. J. Haematol._ 114, 712–717 (2001). Article CAS PubMed Google Scholar * Orelio, C. & Kuijpers, T. W. Shwachman–Diamond syndrome
neutrophils have altered chemoattractant-induced F-actin polymerization and polarization characteristics. _Haematologica_ 94, 409–413 (2009). Article CAS PubMed PubMed Central Google
Scholar * Novak, R. et al. Scalable fabrication of stretchable, dual channel, microfluidic organ chips. _J. Vis. Exp._ 140, e58151 (2018). Google Scholar * Keizer, R. J., Zandvliet, A. S.,
Beijnen, J. H., Schellens, J. H. & Huitema, A. D. Two-stage model-based design of cancer phase I dose escalation trials: evaluation using the phase I program of barasertib (AZD1152).
_Invest. New Drugs_ 30, 1519–1530 (2012). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This research was sponsored by funding from: the US Food and Drug
Administration (grants HHSF223201310079C and 75F40119C10098), the Defense Advanced Research Projects Agency (under Cooperative Agreement Number W911NF-12-2-0036), AstraZeneca and the Wyss
Institute for Biologically Inspired Engineering (to D.E.I.); the US National Institutes of Health (R24 DK099808 and 5U01HL134812 to A.S., R01 DK102165 to C.D.N. and training grant
5T32CA009216-37 to D.B.C.); and the Department of Defense (W81XWH-14-1-0124 to C.D.N.). Additional funding was provided by the Dana-Farber Cancer Center Claudia Adams Barr Award (to C.E.J.)
and the EPSRC Centre for Innovative Manufacturing in Regenerative Medicine (to A.R.). The authors thank S. Sweeney for helpful discussions, P. Machado and J. Caramanica for machining
expertise, and M. DeLelys, R. Mathews, J. Houston, J. Patel, D. Kingman, A. Shay, J. Graham, S. Chung, T. Spitzer and F. Preffer at the Massachusetts General Hospital, as well as M. Fleming
and M. Armant at Boston Children’s Hospital for invaluable help in relation to working with patient data and samples. AUTHOR INFORMATION Author notes * These authors contributed equally:
David B. Chou, Viktoras Frismantas. AUTHORS AND AFFILIATIONS * Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA, USA David B. Chou, Viktoras Frismantas,
Yuka Milton, Liliana S. Moreira Teixeira, Arianna Rech, Elizabeth Calamari, Sasan Jalili-Firoozinezhad, Brooke A. Furlong, Lucy R. O’Sullivan, Carlos F. Ng, Youngjae Choe, Susan Marquez,
Richard Novak, Oren Levy, Rachelle Prantil-Baun & Donald E. Ingber * Department of Pathology, Massachusetts General Hospital, Boston, MA, USA David B. Chou & Robert P. Hasserjian *
Clinical Pharmacology and Safety Sciences, BioPharmaceuticals R&D, AstraZeneca, Cambridge, UK Rhiannon David & Lorna Ewart * DMPK, Oncology R&D, AstraZeneca, Boston, MA, USA
Petar Pop-Damkov & Douglas Ferguson * Clinical and Quantitative Pharmacology, Clinical Pharmacology and Safety Sciences, Pharmaceutical Sciences, R&D, AstraZeneca, Cambridge, UK
Alexander MacDonald * Dana Farber/Boston Children’s Cancer and Blood Disorders Center, Boston, MA, USA Özge Vargel Bölükbaşı & Akiko Shimamura * Department of Cancer Immunology and
Virology, Dana-Farber Cancer Institute, Boston, MA, USA Cailin E. Joyce & Carl D. Novina * Department of Medicine, Harvard Medical School, Boston, MA, USA Cailin E. Joyce & Carl D.
Novina * Department of Chemical Engineering, Loughborough University, Loughborough, UK Arianna Rech * Vascular Biology Program and Department of Surgery, Boston Children’s Hospital and
Harvard Medical School, Boston, MA, USA Amanda Jiang & Donald E. Ingber * Department of Bioengineering and Institute for Bioengineering and Biosciences (iBB), Instituto Superior Técnico,
Universidade de Lisboa, Lisbon, Portugal Sasan Jalili-Firoozinezhad * Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, USA Kasiani C. Myers * Division
of Bone Marrow Transplantation and Immune Deficiency, Cincinnati Children’s Hospital, Cincinnati, OH, USA Kasiani C. Myers * Department of Pathology, Boston Children’s Hospital, Boston, MA,
USA Olga K. Weinberg * Broad Institute of Harvard and MIT, Cambridge, MA, USA Carl D. Novina * Harvard John A. Paulson School of Engineering and Applied Sciences, Cambridge, MA, USA Donald
E. Ingber Authors * David B. Chou View author publications You can also search for this author inPubMed Google Scholar * Viktoras Frismantas View author publications You can also search for
this author inPubMed Google Scholar * Yuka Milton View author publications You can also search for this author inPubMed Google Scholar * Rhiannon David View author publications You can also
search for this author inPubMed Google Scholar * Petar Pop-Damkov View author publications You can also search for this author inPubMed Google Scholar * Douglas Ferguson View author
publications You can also search for this author inPubMed Google Scholar * Alexander MacDonald View author publications You can also search for this author inPubMed Google Scholar * Özge
Vargel Bölükbaşı View author publications You can also search for this author inPubMed Google Scholar * Cailin E. Joyce View author publications You can also search for this author inPubMed
Google Scholar * Liliana S. Moreira Teixeira View author publications You can also search for this author inPubMed Google Scholar * Arianna Rech View author publications You can also search
for this author inPubMed Google Scholar * Amanda Jiang View author publications You can also search for this author inPubMed Google Scholar * Elizabeth Calamari View author publications You
can also search for this author inPubMed Google Scholar * Sasan Jalili-Firoozinezhad View author publications You can also search for this author inPubMed Google Scholar * Brooke A. Furlong
View author publications You can also search for this author inPubMed Google Scholar * Lucy R. O’Sullivan View author publications You can also search for this author inPubMed Google Scholar
* Carlos F. Ng View author publications You can also search for this author inPubMed Google Scholar * Youngjae Choe View author publications You can also search for this author inPubMed
Google Scholar * Susan Marquez View author publications You can also search for this author inPubMed Google Scholar * Kasiani C. Myers View author publications You can also search for this
author inPubMed Google Scholar * Olga K. Weinberg View author publications You can also search for this author inPubMed Google Scholar * Robert P. Hasserjian View author publications You can
also search for this author inPubMed Google Scholar * Richard Novak View author publications You can also search for this author inPubMed Google Scholar * Oren Levy View author publications
You can also search for this author inPubMed Google Scholar * Rachelle Prantil-Baun View author publications You can also search for this author inPubMed Google Scholar * Carl D. Novina
View author publications You can also search for this author inPubMed Google Scholar * Akiko Shimamura View author publications You can also search for this author inPubMed Google Scholar *
Lorna Ewart View author publications You can also search for this author inPubMed Google Scholar * Donald E. Ingber View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS D.B.C. and V.F. participated in the design and performance of all experiments and analysed the data, alongside D.E.I., who also supervised all of the work. Y.M.
helped design and perform the experiments. R.D., P.P.-D., D.F., A.M. and L.E. helped to design experiments relating to drug testing, performed the mass spectrometry and pharmacokinetics
modelling, analysed the data and helped to write the manuscript. O.V.B. and C.E.J. helped to design, perform and interpret SDS-related studies, with input from and supervision of A.S. and
C.D.N. K.C.M. and O.K.W. provided access to patient data and material for SDS-related studies. L.S.M.T. and A.R. helped to conceive the BM chip design and performed the experiments. A.J.
helped to perform the radiation-related studies. B.A.F. and L.R.O. helped to analyse the data and revise the manuscript. E.C., C.F.N., Y.C. and S.C. fabricated and participated in the design
of the BM chip with input from and supervision of R.N. and D.E.I. E.C., S.J.-F. and S.C. helped to perform the oxygen studies. R.P.H. provided scientific supervision, as well as access to
patient material. O.L. and R.P.-B. helped to design the experiments and interpret the data, and supervised all of the work. D.B.C., V.F. and D.E.I. prepared the manuscript, with input from
all authors. CORRESPONDING AUTHOR Correspondence to Donald E. Ingber. ETHICS DECLARATIONS COMPETING INTERESTS D.E.I. is a founder, and holds equity in, Emulate, Inc., and chairs its
scientific advisory board. D.B.C., V.F., Y.M., L.S.M.T., O.L., R.N. and D.E.I. are co-inventors on a patent application describing the BM chip. R.D., P.P.-D., D.F., A.M. and L.E. are
employed by AstraZeneca, which is developing AZD2811. The remaining authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–7, Table 1 and references.
REPORTING SUMMARY RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chou, D.B., Frismantas, V., Milton, Y. _et al._ On-chip recapitulation of clinical bone
marrow toxicities and patient-specific pathophysiology. _Nat Biomed Eng_ 4, 394–406 (2020). https://doi.org/10.1038/s41551-019-0495-z Download citation * Received: 23 October 2018 *
Accepted: 22 November 2019 * Published: 27 January 2020 * Issue Date: 01 April 2020 * DOI: https://doi.org/10.1038/s41551-019-0495-z SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative