Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

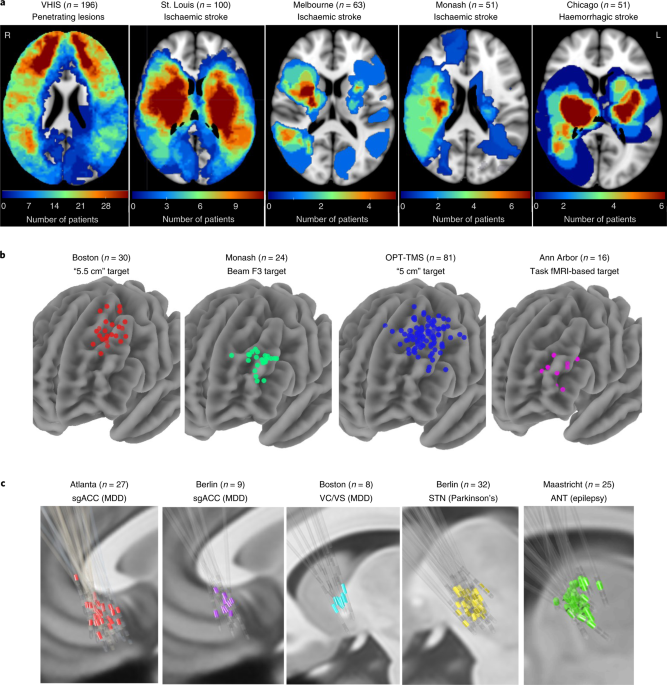
ABSTRACT Damage to specific brain circuits can cause specific neuropsychiatric symptoms. Therapeutic stimulation to these same circuits may modulate these symptoms. To determine whether
these circuits converge, we studied depression severity after brain lesions (_n_ = 461, five datasets), transcranial magnetic stimulation (_n_ = 151, four datasets) and deep brain
stimulation (_n_ = 101, five datasets). Lesions and stimulation sites most associated with depression severity were connected to a similar brain circuit across all 14 datasets (_P_ <
0.001). Circuits derived from lesions, deep brain stimulation and transcranial magnetic stimulation were similar (_P_ < 0.0005), as were circuits derived from patients with major
depression versus other diagnoses (_P_ < 0.001). Connectivity to this circuit predicted out-of-sample antidepressant efficacy of transcranial magnetic stimulation and deep brain
stimulation sites (_P_ < 0.0001). In an independent analysis, 29 lesions and 95 stimulation sites converged on a distinct circuit for motor symptoms of Parkinson’s disease (_P_ <
0.05). We conclude that lesions, transcranial magnetic stimulation and DBS converge on common brain circuitry that may represent improved neurostimulation targets for depression and other
disorders. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access
Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital
issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be
subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR
CONTENT BEING VIEWED BY OTHERS CLOSING THE LOOP IN PSYCHIATRIC DEEP BRAIN STIMULATION: PHYSIOLOGY, PSYCHOMETRICS, AND PLASTICITY Article 06 July 2023 ELECTROCONVULSIVE THERAPY-INDUCED
VOLUMETRIC BRAIN CHANGES CONVERGE ON A COMMON CAUSAL CIRCUIT IN DEPRESSION Article Open access 20 November 2023 TRANSCRANIAL DIRECT CURRENT STIMULATION (TDCS) IN DEPRESSION INDUCES
STRUCTURAL PLASTICITY Article Open access 17 February 2023 DATA AVAILABILITY STATEMENT This paper used de-identified data from 14 different datasets collected by 14 different teams of
investigators at various institutions across four different countries. Each dataset is available upon reasonable request from each respective team of investigators. Data sharing will be
subject to the policies and procedures of the institution where each dataset was collected as well as the laws of the country where each dataset was collected. CODE AVAILABILITY STATEMENT
All custom MATLAB code used in this study is available upon reasonable request from the corresponding author. REFERENCES * Czéh, B., Fuchs, E., Wiborg, O. & Simon, M. Animal models of
major depression and their clinical implications. _Prog. Neuro-Psychopharmacol. Biol. Psychiatry_ 64, 293–310 (2016). Google Scholar * Etkin, A. Mapping causal circuitry in human
depression. _Biol. Psychiatry_ 86, 732–733 (2019). PubMed Google Scholar * Nestler, E. J. & Hyman, S. E. Animal models of neuropsychiatric disorders. _Nat. Neurosci._ 13, 1161–1169
(2010). CAS PubMed PubMed Central Google Scholar * Monteggia, L. M., Heimer, H. & Nestler, E. J. Meeting report: can we make animal models of human mental illness? _Biol. Psychiatry_
84, 542–545 (2018). PubMed PubMed Central Google Scholar * Fox, M. D. Mapping symptoms to brain networks with the human connectome. _N. Engl. J. Med_ 379, 2237–2245 (2018). CAS PubMed
Google Scholar * Siddiqi, S. H. et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. _Am. J. Psychiatry_ 177, 435–446 (2020). PubMed PubMed Central Google
Scholar * Padmanabhan, J. L. et al. A human depression circuit derived from focal brain lesions. _Biol. Psychiatry_ https://doi.org/10.1016/j.biopsych.2019.07.023 (2019). * Weigand, A. et
al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. _Biol. Psychiatry_ 84, 28–37 (2018). CAS PubMed Google
Scholar * Riva-Posse, P. et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. _Biol.
Psychiatry_ 76, 963–969 (2014). PubMed PubMed Central Google Scholar * Etkin, A. Addressing the causality gap in human psychiatric neuroscience. _JAMA Psychiatry_ 75, 3–4 (2018). PubMed
Google Scholar * Ressler, K. J. & Mayberg, H. S. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. _Nat. Neurosci._ 10, 1116–1124
(2007). CAS PubMed PubMed Central Google Scholar * Matthews, P. M. & Hampshire, A. Clinical concepts emerging from fMRI functional connectomics. _Neuron_ 91, 511–528 (2016). CAS
PubMed Google Scholar * Fox, M. D. et al. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. _Proc. Natl Acad.
Sci. USA_ 111, E4367–E4375 (2014). CAS PubMed PubMed Central Google Scholar * Drysdale, A. T. _et al_. Resting-state connectivity biomarkers define neurophysiological subtypes of
depression. _Nat. Med._ https://doi.org/10.1038/nm.4246 (2016). * Koenigs, M. et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. _Nat. Neurosci._
11, 232–237, https://doi.org/10.1038/nn2032 (2008). Article CAS PubMed Google Scholar * Johnson, K. A. et al. Prefrontal rTMS for treating depression: location and intensity results from
the OPT-TMS multi-site clinical trial. _Brain Stimul._ 6, 108–117 (2013). PubMed Google Scholar * Taylor, S. F. et al. Changes in brain connectivity during a sham-controlled, transcranial
magnetic stimulation trial for depression. _J. Affect Disord._ 232, 143–151 (2018). PubMed PubMed Central Google Scholar * Cash, R. F. H. et al. Subgenual functional connectivity
predicts antidepressant treatment response to transcranial magnetic stimulation: independent validation and evaluation of personalization. _Biol. Psychiatry_ 86, e5–e7 (2019). PubMed Google
Scholar * Horn, A. et al. Connectivity predicts deep brain stimulation outcome in Parkinson disease. _Ann. Neurol._ 82, 67–78 (2017). PubMed PubMed Central Google Scholar * Dougherty,
D. D. et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. _Biol. Psychiatry_ 78, 240–248
(2015). PubMed Google Scholar * Irmen, F. _et al_. Left prefrontal impact links subthalamic stimulation with depressive symptoms. _Ann. Neurol._ https://doi.org/10.1002/ana.25734 (2020). *
Merkl, A. et al. Antidepressant effects after short-term and chronic stimulation of the subgenual cingulate gyrus in treatment-resistant depression. _Exp. Neurol._ 249, 160–168 (2013).
PubMed Google Scholar * Schaper, F. L. W. V. J. et al. Deep brain stimulation in epilepsy: a role for modulation of the mammillothalamic tract in seizure control? _Neurosurgery_ 87,
602–610 (2020). PubMed PubMed Central Google Scholar * Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. _J. Neurophysiol._
106, 1125–1165 (2011). PubMed Google Scholar * Bates, E. et al. Voxel-based lesion–symptom mapping. _Nat. Neurosci._ 6, 448–450 (2003). CAS PubMed Google Scholar * Gourisankar, A. et
al. Mapping movement, mood, motivation and mentation in the subthalamic nucleus. _R. Soc. Open Sci._ 5, 171177 (2018). PubMed PubMed Central Google Scholar * Choi, K. S., Riva-Posse, P.,
Gross, R. E. & Mayberg, H. S. Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. _JAMA Neurol._ 72, 1252–1260 (2015). PubMed
PubMed Central Google Scholar * Joutsa, J., Horn, A., Hsu, J. & Fox, M. D. Localizing parkinsonism based on focal brain lesions. _Brain_ 141, 2445–2456 (2018). PubMed PubMed Central
Google Scholar * Yang, C. et al. Repetitive transcranial magnetic stimulation therapy for motor recovery in Parkinson’s disease: a meta-analysis. _Brain Behav._ 8, e01132 (2018). PubMed
PubMed Central Google Scholar * James, G. A. et al. Exploratory structural equation modeling of resting-state fMRI: applicability of group models to individual subjects. _NeuroImage_ 45,
778–787 (2009). PubMed Google Scholar * Mayberg, H. S. et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. _Am. J.
Psychiatry_ 156, 675–682 (1999). CAS PubMed Google Scholar * Drevets, W. C. et al. Subgenual prefrontal cortex abnormalities in mood disorders. _Nature_ 386, 824–827 (1997). CAS PubMed
Google Scholar * Müller, V. I. et al. Altered brain activity in unipolar depression revisited: meta-analyses of neuroimaging studies. _JAMA Psychiatry_ 74, 47–55 (2017). PubMed PubMed
Central Google Scholar * Gray, J. P., Müller, V. I., Eickhoff, S. B. & Fox, P. T. Multimodal abnormalities of brain structure and function in najor depressive disorder: a meta-analysis
of neuroimaging studies. _Am. J. Psychiatry_ 177, 422–434 (2020). PubMed PubMed Central Google Scholar * Williams, L. M. Defining biotypes for depression and anxiety based on large-scale
circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. _Depress. Anxiety_ 34, 9–24 (2017). PubMed Google Scholar * Holtzheimer, P. E. et
al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. _Lancet Psychiatry_ 4, 839–849 (2017). PubMed Google
Scholar * Yesavage, J. A. et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. _JAMA
Psychiatry_ 75, 884–893 (2018). PubMed PubMed Central Google Scholar * Kozak, M. J. & Cuthbert, B. N. The NIMH Research Domain Criteria initiative: background, issues, and pragmatics.
_Psychophysiology_ 53, 286–297 (2016). PubMed Google Scholar * Poldrack, R. A. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. _Neuron_ 72,
692–697 (2011). CAS PubMed PubMed Central Google Scholar * Cole, EleanorJ. et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. _Am. J.
Psychiatry_ https://doi.org/10.1176/appi.ajp.2019.19070720 (2020). * Blumberger, D. M. et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation
in patients with depression (THREE-D): a randomised non-inferiority trial. _Lancet_ 391, 1683–1692 (2018). PubMed Google Scholar * Cash, R. F. H. _et al_. Using brain imaging to improve
spatial targeting of transcranial magnetic stimulation for depression. _Biol. Psychiatry_ https://doi.org/10.1016/j.biopsych.2020.05.033 (2020). * Cash, R. F. H., Cocchi, L., Lv, J.,
Fitzgerald, P. B. & Zalesky, A. Functional magnetic resonance imaging-guided personalization of transcranial magnetic stimulation treatment for depression. _JAMA Psychiatry_,
https://doi.org/10.1001/jamapsychiatry.2020.3794 (2020). * Siddiqi, S. H., Weigand, A., Pascual-Leone, A. & Fox, M. D. Identification of personalized TMS targets based on subgenual
cingulate connectivity: an independent replication. _Biol. Psychiatry_ https://doi.org/10.1016/j.biopsych.2021.02.015 (2021). * Riva-Posse, P. et al. A connectomic approach for subcallosal
cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. _Mol. Psychiatry_ 23, 843–849 (2018). CAS PubMed Google Scholar * Fox, M. D., Liu, H.
& Pascual-Leone, A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. _Neuroimage_ 66, 151–160 (2013). PubMed
Google Scholar * Opitz, A., Fox, M. D., Craddock, R. C., Colcombe, S. & Milham, M. P. An integrated framework for targeting functional networks via transcranial magnetic stimulation.
_Neuroimage_ 127, 86–96 (2016). PubMed Google Scholar * Rouder, J. N. & Morey, R. D. Default Bayes factors for model selection in regression. _Multivar. Behav. Res._ 47, 877–903
(2012). Google Scholar * Wagenmakers, E.-J., Verhagen, J. & Ly, A. How to quantify the evidence for the absence of a correlation. _Behav. Res. Methods_ 48, 413–426 (2016). PubMed
Google Scholar * Turner, J. A. et al. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. _Front Neurosci._ 7, 137 (2013). PubMed PubMed
Central Google Scholar * Slotnick, S. D. Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. _Cogn. Neurosci._ 8, 150–155 (2017). PubMed Google
Scholar Download references ACKNOWLEDGEMENTS The authors thank all research participants, funding bodies, allied health staff and other research staff that made this work possible. The
present work was supported by the Sidney R. Baer Foundation (S.H.S., J.L.P., M.D.F.), the Brain & Behavior Research Foundation (SHS) and the National Institute of Mental Health (grant
no. K23MH121657 to S.H.S.; grant nos. R01MH113929 and R01MH115949 to M.D.F.). The funders were not directly involved in the conceptualization, design, data collection, analysis, decision to
publish or preparation of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Center for Brain Circuit Therapeutics, Brigham and Women’s Hospital, Boston, MA, USA Shan H. Siddiqi,
Frederic L. W. V. J. Schaper, Andreas Horn, Jaya L. Padmanabhan & Michael D. Fox * Department of Psychiatry, Harvard Medical School, Boston, MA, USA Shan H. Siddiqi & Darin D.
Dougherty * Department of Neurology, Harvard Medical School, Boston, MA, USA Frederic L. W. V. J. Schaper, Andreas Horn, Alvaro Pascual-Leone & Michael D. Fox * Department of Neurology,
Maastricht University Medical Center, Maastricht, the Netherlands Frederic L. W. V. J. Schaper & Rob P. W. Rouhl * Movement Disorders and Neuromodulation Unit, Department for Neurology,
Charité University Medicine Berlin, Berlin, Germany Andreas Horn & Andrea A. Kuhn * University of Pittsburgh School of Medicine, Pittsburgh, PA, USA Joey Hsu * Melbourne Neuropsychiatry
Centre, The University of Melbourne, Melbourne, Victoria, Australia Amy Brodtmann, Robin F. H. Cash & Andrew Zalesky * Department of Biomedical Engineering, The University of Melbourne,
Melbourne, Victoria, Australia Robin F. H. Cash & Andrew Zalesky * Department of Neuroscience, Padova Neuroscience Center (PNC), Venetian Institute of Molecular Medicine (VIMM),
University of Padova, Padova, Italy Maurizio Corbetta * Departments of Neurology, Radiology, Bioengineering, and Neuroscience, Washington University, St. Louis, MO, USA Maurizio Corbetta *
Nash Family Center for Advanced Circuit Therapeutics, Icahn School of Medicine at Mount Sinai, New York, NY, USA Ki Sueng Choi & Helen S. Mayberg * Department of Psychiatry,
Massachusetts General Hospital, Boston, MA, USA Darin D. Dougherty * Melbourne School of Psychological Sciences, University of Melbourne, Melbourne, Victoria, Australia Natalia Egorova * The
Florey Institute of Neuroscience and Mental Health, Melbourne, Victoria, Australia Natalia Egorova * Epworth Centre for Innovation in Mental Health, Epworth Healthcare and Monash University
Department of Psychiatry, Camberwell, Victoria, Australia Paul B. Fitzgerald * Brain Stimulation Laboratory, Psychiatry Department, Medical University of South Carolina, Charleston, SC, USA
Mark S. George * Ralph H. Johnson VA Medical Center, Charleston, SC, USA Mark S. George * Department of Neurology, Monash Health and Department of Medicine, School of Clinical Sciences,
Monash University, Clayton, Victoria, Australia Sophia A. Gozzi & Thanh G. Phan * Department of Neurology, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität
Berlin, Humboldt-Universität zu Berlin and Berlin Institute of Health, Berlin, Germany Frederike Irmen * School of Medicine, Florida State University, Tallahassee, FL, USA Kevin A. Johnson *
Feinberg School of Medicine, Northwestern University, Chicago, IL, USA Andrew M. Naidech, Joel L. Voss & Jordan H. Grafman * Hinda and Arthur Marcus Institute for Aging Research and
Center for Memory Health, Hebrew SeniorLife, Boston, MA, USA Alvaro Pascual-Leone * Guttmann Brain Health Institut, Universitat Autonoma, Barcelona, Spain Alvaro Pascual-Leone * School for
Mental Health and Neuroscience, Maastricht University, Maastricht, the Netherlands Rob P. W. Rouhl * Academic Center for Epileptology Kempenhaeghe/Maastricht University Medical Center,
Maastricht, the Netherlands Rob P. W. Rouhl * Department of Psychiatry, University of Michigan School of Medicine, Ann Arbor, MI, USA Stephan F. Taylor * Shirley Ryan AbilityLab, Chicago,
IL, USA Jordan H. Grafman Authors * Shan H. Siddiqi View author publications You can also search for this author inPubMed Google Scholar * Frederic L. W. V. J. Schaper View author
publications You can also search for this author inPubMed Google Scholar * Andreas Horn View author publications You can also search for this author inPubMed Google Scholar * Joey Hsu View
author publications You can also search for this author inPubMed Google Scholar * Jaya L. Padmanabhan View author publications You can also search for this author inPubMed Google Scholar *
Amy Brodtmann View author publications You can also search for this author inPubMed Google Scholar * Robin F. H. Cash View author publications You can also search for this author inPubMed
Google Scholar * Maurizio Corbetta View author publications You can also search for this author inPubMed Google Scholar * Ki Sueng Choi View author publications You can also search for this
author inPubMed Google Scholar * Darin D. Dougherty View author publications You can also search for this author inPubMed Google Scholar * Natalia Egorova View author publications You can
also search for this author inPubMed Google Scholar * Paul B. Fitzgerald View author publications You can also search for this author inPubMed Google Scholar * Mark S. George View author
publications You can also search for this author inPubMed Google Scholar * Sophia A. Gozzi View author publications You can also search for this author inPubMed Google Scholar * Frederike
Irmen View author publications You can also search for this author inPubMed Google Scholar * Andrea A. Kuhn View author publications You can also search for this author inPubMed Google
Scholar * Kevin A. Johnson View author publications You can also search for this author inPubMed Google Scholar * Andrew M. Naidech View author publications You can also search for this
author inPubMed Google Scholar * Alvaro Pascual-Leone View author publications You can also search for this author inPubMed Google Scholar * Thanh G. Phan View author publications You can
also search for this author inPubMed Google Scholar * Rob P. W. Rouhl View author publications You can also search for this author inPubMed Google Scholar * Stephan F. Taylor View author
publications You can also search for this author inPubMed Google Scholar * Joel L. Voss View author publications You can also search for this author inPubMed Google Scholar * Andrew Zalesky
View author publications You can also search for this author inPubMed Google Scholar * Jordan H. Grafman View author publications You can also search for this author inPubMed Google Scholar
* Helen S. Mayberg View author publications You can also search for this author inPubMed Google Scholar * Michael D. Fox View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS Conception and design of study: S.H.S., A.H. and M.D.F. Design of analytical procedures: S.H.S. and M.D.F. Neuroimaging analyses and statistical analyses:
S.H.S. Preprocessing and preparation of data for analysis: S.H.S., A.H., J.H., J.L.P. and F.S. Contribution of data: A.H., F.S., R.F.H.C., A.B., K.A.J., N.E., A.M.N., S.G., T.G.P., K.S.C.,
F.I., A.K., P.B.F., M.S.G., R.P.W.R., S.F.T., A.Z., J.L.V., M.C., D.D.D., A.P.-L., J.H.G., H.S.M. and M.D.F. Writing of manuscript: S.H.S. and M.D.F. with input from all authors.
CORRESPONDING AUTHOR Correspondence to Shan H. Siddiqi. ETHICS DECLARATIONS COMPETING INTERESTS S.H.S. serves as a clinical consultant for Kaizen Brain Center. S.H.S. and M.D.F. have jointly
received investigator-initiated research support from Neuronetics. None of these organizations were involved in the present work. S.H.S. and M.D.F. each own independent intellectual
property on the use of brain network mapping to target neuromodulation. The present work did not utilize any of this intellectual property. The authors report no other conflicts of interest
related to the present work. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Human Behaviour_ thanks Nolan Williams and the other, anonymous, reviewer(s) for their contribution to the
peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION Supplementary Tables 1 and 2 and Supplementary Figs. 1–5. REPORTING SUMMARY RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Siddiqi, S.H., Schaper, F.L.W.V.J., Horn, A. _et al._ Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. _Nat Hum Behav_ 5, 1707–1716 (2021).
https://doi.org/10.1038/s41562-021-01161-1 Download citation * Received: 30 December 2020 * Accepted: 11 June 2021 * Published: 08 July 2021 * Issue Date: December 2021 * DOI:
https://doi.org/10.1038/s41562-021-01161-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative