Immune control by amino acid catabolism during tumorigenesis and therapy
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

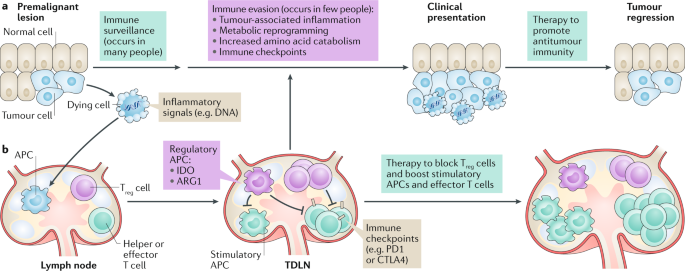
Immune checkpoints arise from physiological changes during tumorigenesis that reprogramme inflammatory, immunological and metabolic processes in malignant lesions and local lymphoid tissues,
which constitute the immunological tumour microenvironment (TME). Improving clinical responses to immune checkpoint blockade will require deeper understanding of factors that impact local
immune balance in the TME. Elevated catabolism of the amino acids tryptophan (Trp) and arginine (Arg) is a common TME hallmark at clinical presentation of cancer. Cells catabolizing Trp and
Arg suppress effector T cells and stabilize regulatory T cells to suppress immunity in chronic inflammatory diseases of clinical importance, including cancers. Processes that induce Trp and
Arg catabolism in the TME remain incompletely defined. Indoleamine 2,3 dioxygenase (IDO) and arginase 1 (ARG1), which catabolize Trp and Arg, respectively, respond to inflammatory cues
including interferons and transforming growth factor-β (TGFβ) cytokines. Dying cells generate inflammatory signals including DNA, which is sensed to stimulate the production of type I
interferons via the stimulator of interferon genes (STING) adaptor. Thus, dying cells help establish local conditions that suppress antitumour immunity to promote tumorigenesis. Here, we
review evidence that Trp and Arg catabolism contributes to inflammatory processes that promote tumorigenesis, impede immune responses to therapy and might promote neurological comorbidities
associated with cancer.
Research in the A.L.M. and L.H. laboratory is supported by US National Institutes of Health (NIH) (AI103347), Cancer Research UK and the Faculty of Medical Sciences at Newcastle University.
Research in the G.C.P. laboratory is supported by NIH (CA191191), the W.W. Smith Trust, the Lankenau Medical Center Foundation and Main Line Health. G.C.P. is the Havens Chair in Biomedical
Research at the Lankenau Institute for Medical Research.
Institute of Cellular Medicine, Faculty of Medical Sciences, Framlington Place, Newcastle University, Newcastle-upon-Tyne, UK
All authors researched data for the article, substantially contributed to the discussion of content and wrote, reviewed and edited the manuscript.
A.L.M. and G.C.P. receive remuneration as scientific consultants for NewLink Genetics Inc. and are also shareholders in this company. G.C.P. also discloses interests in Incyte as a
shareholder and in Kyn Therapeutics as a scientific adviser. A.L.M. also discloses interests as a scientific adviser to Kyn Therapeutics. The other authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mechanisms that suppress local immunity in inflamed tissues such as the tumour microenvironment.
(TME). Primary tumour lesions and local draining lymph nodes where antitumour immunity is controlled.
(ISR). A cellular response to stress that impacts protein translation via effects on the eukaryotic initiation factor eIF2.
(DAMPs). Molecules released by dead and dying cells, which are sensed by innate immune cells.
A subset of macrophages typically associated with wound healing and tissue repair.
(NMDAR signalling). A signalling pathway that has dichotomous effects on neurons such as promoting death or survival of neurons, resistance to trauma and synaptic plasticity and
transmission.
Anyone you share the following link with will be able to read this content: