Fucosylation of glycoproteins and glycolipids: opposing roles in cholera intoxication
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

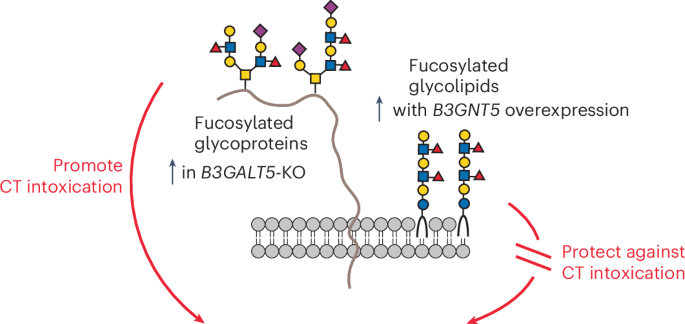
ABSTRACT Cholera toxin (CT) is the etiological agent of cholera. Here we report that multiple classes of fucosylated glycoconjugates function in CT binding and intoxication of intestinal
epithelial cells. In Colo205 cells, knockout (KO) of _B3GNT5_, which encodes an enzyme required for synthesis of lacto and neolacto series glycosphingolipids (GSLs), reduces CT binding but
sensitizes cells to intoxication. Overexpressing _B3GNT5_ to generate more fucosylated GSLs confers protection against intoxication, indicating that fucosylated GSLs act as decoy receptors
for CT. KO of _B3GALT5_ causes increased production of fucosylated _O_-linked and _N_-linked glycoproteins and leads to increased CT binding and intoxication. KO of _B3GNT5_ in _B3GALT5_-KO
cells eliminates production of fucosylated GSLs but increases intoxication, identifying fucosylated glycoproteins as functional receptors for CT. These findings provide insight into the
molecular determinants regulating CT sensitivity of host cells. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn
more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to
full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS _PAENICLOSTRIDIUM SORDELLII_ HEMORRHAGIC TOXIN TARGETS TMPRSS2 TO INDUCE COLONIC EPITHELIAL LESIONS Article Open access
26 July 2022 CHARACTERIZATION AND UTILITY OF TWO MONOCLONAL ANTIBODIES TO CHOLERA TOXIN B SUBUNIT Article Open access 15 March 2023 CLIC AND MEMBRANE WOUND REPAIR PATHWAYS ENABLE PANDEMIC
NOROVIRUS ENTRY AND INFECTION Article Open access 28 February 2023 DATA AVAILABILITY Data used to generate Fig. 1b,d are available from the Gene Expression Omnibus under accession number
GSE242156. MS data used to generate Fig. 3a, Extended Data Fig. 4a–d, Fig. 4a, Extended Data Fig. 7a–c, Extended Data Fig. 8a–c and Extended Data Fig. 6a are available from GlycoPOST under
accession number GPST000467. MS data used to generate Extended Data Fig. 6b,c are available from GlycoPOST under accession number GPST000465. Replicate immunoblot and lectin blot data are
available from the Texas Data Repository (https://doi.org/10.18738/T8/N9NF8B). Source data are provided with this paper. REFERENCES * Grant, T. A., Balasubramanian, D. & Almagro-Moreno,
S. JMM profile: _Vibrio cholerae_: an opportunist of human crises. _J. Med. Microbiol._ 70, 001423 (2021). Article Google Scholar * White, C., Bader, C. & Teter, K. The manipulation of
cell signaling and host cell biology by cholera toxin. _Cell Signal._ 100, 110489 (2022). Article CAS PubMed PubMed Central Google Scholar * Cuatrecasas, P. Gangliosides and membrane
receptors for cholera toxin. _Biochemistry_ 12, 3558–3566 (1973). Article CAS PubMed Google Scholar * Cuatrecasas, P., Parikh, I. & Hollenberg, M. D. Affinity chromatography and
structural analysis of _Vibrio cholerae_ enterotoxin–ganglioside agarose and the biological effects of ganglioside-containing soluble polymers. _Biochemistry_ 12, 4253–4264 (1973). Article
CAS PubMed Google Scholar * Heyningen, S. V. Cholera toxin: interaction of subunits with ganglioside GM1. _Science_ 183, 656–657 (1974). Article Google Scholar * Holmgren, J., Lonnroth,
I., Mansson, J. & Svennerholm, L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. _Proc. Natl Acad. Sci. USA_ 72, 2520–2524 (1975). Article CAS PubMed
PubMed Central Google Scholar * King, C. A. & Van Heyningen, W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. _J. Infect. Dis._ 127, 639–647
(1973). Article CAS PubMed Google Scholar * Holmgren, J., Lönnroth, I. & Svennerholm, L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside
and related glycolipids. _Infect. Immun._ 8, 208–214 (1973). Article CAS PubMed PubMed Central Google Scholar * Merritt, E. A. et al. Structural studies of receptor binding by cholera
toxin mutants. _Protein Sci._ 6, 1516–1528 (1997). Article CAS PubMed PubMed Central Google Scholar * Merritt, E. A. et al. Crystal structure of cholera toxin B-pentamer bound to
receptor GM1 pentasaccharide. _Protein Sci._ 3, 166–175 (1994). Article CAS PubMed PubMed Central Google Scholar * Turnbull, W. B., Precious, B. L. & Homans, S. W. Dissecting the
cholera toxin–ganglioside GM1 interaction by isothermal titration calorimetry. _J. Am. Chem. Soc._ 126, 1047–1054 (2004). Article CAS PubMed Google Scholar * Cervin, J. et al. GM1
ganglioside-independent intoxication by cholera toxin. _PLoS Pathog._ 14, e1006862 (2018). Article PubMed PubMed Central Google Scholar * Breimer, M. E., Hansson, G. C., Karlsson, K. A.,
Larson, G. & Leffler, H. Glycosphingolipid composition of epithelial cells isolated along the villus axis of small intestine of a single human individual. _Glycobiology_ 22, 1721–1730
(2012). Article CAS PubMed Google Scholar * Alisson-Silva, F. et al. Human evolutionary loss of epithelial Neu5Gc expression and species-specific susceptibility to cholera. _PLoS
Pathog._ 14, e1007133 (2018). Article PubMed PubMed Central Google Scholar * Glass, R. I. et al. Predisposition for cholera of individuals with O blood group. Possible evolutionary
significance. _Am. J. Epidemiol._ 121, 791–796 (1985). Article CAS PubMed Google Scholar * Barua, D. & Paguio, A. S. ABO blood groups and cholera. _Ann. Hum. Biol._ 4, 489–492
(1977). Article CAS PubMed Google Scholar * Chaudhuri, A. & De, S. Cholera and blood-groups. _Lancet_ 2, 404 (1977). Article CAS PubMed Google Scholar * Swerdlow, D. L. et al.
Severe life-threatening cholera associated with blood group O in Peru: implications for the Latin American epidemic. _J. Infect. Dis._ 170, 468–472 (1994). Article CAS PubMed Google
Scholar * Harris, J. B. et al. Blood group, immunity, and risk of infection with _Vibrio cholerae_ in an area of endemicity. _Infect. Immun._ 73, 7422–7427 (2005). Article CAS PubMed
PubMed Central Google Scholar * Harris, J. B. et al. Susceptibility to _Vibrio cholerae_ infection in a cohort of household contacts of patients with cholera in Bangladesh. _PLoS Negl.
Trop. Dis._ 2, e221 (2008). Article PubMed PubMed Central Google Scholar * Heggelund, J. E. et al. High-resolution crystal structures elucidate the molecular basis of cholera blood group
dependence. _PLoS Pathog._ 12, e1005567 (2016). Article PubMed PubMed Central Google Scholar * Heggelund, J. E. et al. Both El Tor and classical cholera toxin bind blood group
determinants. _Biochem. Biophys. Res. Commun._ 418, 731–735 (2012). Article CAS PubMed Google Scholar * Holmner, A. et al. Novel binding site identified in a hybrid between cholera toxin
and heat-labile enterotoxin: 1.9 Å crystal structure reveals the details. _Structure_ 12, 1655–1667 (2004). Article CAS PubMed Google Scholar * Bennun, F. R., Roth, G. A., Monferran, C.
G. & Cumar, F. A. Binding of cholera toxin to pig intestinal mucosa glycosphingolipids: relationship with the ABO blood group system. _Infect. Immun._ 57, 969–974 (1989). Article CAS
PubMed PubMed Central Google Scholar * Wands, A. M. et al. Fucosylated molecules competitively interfere with cholera toxin binding to host cells. _ACS Infect. Dis._ 4, 758–770 (2018).
Article CAS PubMed PubMed Central Google Scholar * Prudden, A. R. et al. Synthesis of asymmetrical multiantennary human milk oligosaccharides. _Proc. Natl Acad. Sci. USA_ 114, 6954–6959
(2017). Article CAS PubMed PubMed Central Google Scholar * Heim, J. B., Hodnik, V., Heggelund, J. E., Anderluh, G. & Krengel, U. Crystal structures of cholera toxin in complex with
fucosylated receptors point to importance of secondary binding site. _Sci. Rep._ 9, 12243 (2019). Article PubMed PubMed Central Google Scholar * Garber, J. M., Hennet, T. &
Szymanski, C. M. Significance of fucose in intestinal health and disease. _Mol. Microbiol._ 115, 1086–1093 (2021). Article CAS PubMed Google Scholar * Wands, A. M. et al. Fucosylation
and protein glycosylation create functional receptors for cholera toxin. _eLife_ 4, e09545 (2015). Article PubMed PubMed Central Google Scholar * Blanco, L. P. & DiRita, V. J.
Bacterial-associated cholera toxin and GM1 binding are required for transcytosis of classical biotype _Vibrio cholerae_ through an in vitro M cell model system. _Cell Microbiol_ 8, 982–998
(2006). Article CAS PubMed Google Scholar * Sethi, A. et al. Cell type and receptor identity regulate cholera toxin subunit B (CTB) internalization. _Interface Focus_ 9, 20180076 (2019).
Article PubMed PubMed Central Google Scholar * Sojitra, M. et al. Genetically encoded multivalent liquid glycan array displayed on M13 bacteriophage. _Nat. Chem. Biol._ 17, 806–816
(2021). Article CAS PubMed PubMed Central Google Scholar * Yanagisawa, M., Ariga, T. & Yu, R. K. Cholera toxin B subunit binding does not correlate with GM1 expression: a study
using mouse embryonic neural precursor cells. _Glycobiology_ 16, 19g–22g (2006). Article CAS PubMed Google Scholar * Bakker, H., Ashikov, A., Routier, F. H. & Gerardy-Schahn, R. in
_Handbook of Glycosyltransferases and Related Genes_ Ch. 124 (eds Taniguchi, N. et al.) 1403–1412 (Springer, 2014). * Foulquier, F. et al. TMEM165 deficiency causes a congenital disorder of
glycosylation. _Am. J. Hum. Genet_ 91, 15–26 (2012). Article CAS PubMed PubMed Central Google Scholar * Togayachi, A. & Narimatsu, H. in _Handbook of Glycosyltransferases and
Related Genes_ Ch. 29 (eds Taniguchi, N. et al.) 311–320 (Springer, 2014). * Togayachi, A. & Narimatsu, H. in _Handbook of Glycosyltransferases and Related Genes_ Ch. 9 (eds Taniguchi,
N. et al.) 89–100 (Springer, 2014). * Lin, C. H. et al. Enhanced expression of β3-galactosyltransferase 5 activity is sufficient to induce in vivo synthesis of extended type 1 chains on
lactosylceramides of selected human colonic carcinoma cell lines. _Glycobiology_ 19, 418–427 (2009). Article CAS PubMed Google Scholar * Lin, R. J. et al. _B3GALT5_ knockout alters
gycosphingolipid profile and facilitates transition to human naive pluripotency. _Proc. Natl Acad. Sci. USA_ 117, 27435–27444 (2020). Article CAS PubMed PubMed Central Google Scholar *
Isshiki, S. et al. Cloning, expression, and characterization of a novel UDP-galactose: β-_N_-acetylglucosamine β-galactosyltransferase (β3Gal-T5) responsible for synthesis of type 1 chain in
colorectal and pancreatic epithelia and tumor cells derived therefrom. _J. Biol. Chem._ 274, 12499–12507 (1999). Article CAS PubMed Google Scholar * Groth, T., Diehl, A. D., Gunawan, R.
& Neelamegham, S. GlycoEnzOnto: a glycoenzyme pathway and molecular function ontology. _Bioinformatics_ 38, 5413–5420 (2022). Article CAS PubMed PubMed Central Google Scholar *
Ancheta, L. R., Shramm, P. A., Bouajram, R., Higgins, D. & Lappi, D. A. Streptavidin–saporin: converting biotinylated materials into targeted toxins. _Toxins_ 15, 181 (2023). Article
CAS PubMed PubMed Central Google Scholar * Stirpe, F. et al. Ribosome-inactivating proteins from the seeds of _Saponaria officinalis_ L. (soapwort), of _Agrostemma githago_ L. (corn
cockle) and of _Asparagus officinalis_ L. (asparagus), and from the latex of _Hura crepitans_ L. (sandbox tree). _Biochem. J._ 216, 617–625 (1983). Article CAS PubMed PubMed Central
Google Scholar * Lee, L., Abe, A. & Shayman, J. A. Improved inhibitors of glucosylceramide synthase. _J. Biol. Chem._ 274, 14662–14669 (1999). Article CAS PubMed Google Scholar *
Singla, A. et al. Cholera intoxication of human enteroids reveals interplay between decoy and functional glycoconjugate ligands. _Glycobiology_ 33, 801–816 (2023). Article PubMed PubMed
Central Google Scholar * Moss, J., Fishman, P. H., Manganiello, V. C., Vaughan, M. & Brady, R. O. Functional incorporation of ganglioside into intact cells—induction of choleragen
responsiveness. _Proc. Natl Acad. Sci. USA_ 73, 1034–1037 (1976). Article CAS PubMed PubMed Central Google Scholar * Gilbert, L. A. et al. Genome-scale CRISPR-mediated control of gene
repression and activation. _Cell_ 159, 647–661 (2014). Article CAS PubMed PubMed Central Google Scholar * Tringali, C. et al. Modification of sialidase levels and sialoglycoconjugate
pattern during erythroid and erytroleukemic cell differentiation. _Glycoconj. J._ 24, 67–79 (2007). Article CAS PubMed Google Scholar * Arumugam, S. et al. Ceramide structure dictates
glycosphingolipid nanodomain assembly and function. _Nat. Commun._ 12, 3675 (2021). Article CAS PubMed PubMed Central Google Scholar * Chinnapen, D. J. et al. Lipid sorting by ceramide
structure from plasma membrane to ER for the cholera toxin receptor ganglioside GM1. _Dev. Cell_ 23, 573–586 (2012). Article CAS PubMed PubMed Central Google Scholar * Kenworthy, A. K.
et al. Cholera toxin as a probe for membrane biology. _Toxins_ 13, 543 (2021). Article CAS PubMed PubMed Central Google Scholar * Madunic, K. et al. Specific (sialyl-)Lewis core 2
_O_-glycans differentiate colorectal cancer from healthy colon epithelium. _Theranostics_ 12, 4498–4512 (2022). Article CAS PubMed PubMed Central Google Scholar * Robbe, C., Capon, C.,
Coddeville, B. & Michalski, J. C. Structural diversity and specific distribution of _O_-glycans in normal human mucins along the intestinal tract. _Biochem. J._ 384, 307–316 (2004).
Article CAS PubMed PubMed Central Google Scholar * Goto, Y. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. _Science_ 345, 1254009 (2014). Article
PubMed PubMed Central Google Scholar * Carroll, D. J. et al. Interleukin-22 regulates _B3GNT7_ expression to induce fucosylation of glycoproteins in intestinal epithelial cells. _J. Biol.
Chem._ 298, 101463 (2022). Article CAS PubMed Google Scholar * Pham, T. A. et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an
opportunistic pathogen. _Cell Host Microbe_ 16, 504–516 (2014). Article CAS PubMed PubMed Central Google Scholar * Pickard, J. M. et al. Rapid fucosylation of intestinal epithelium
sustains host–commensal symbiosis in sickness. _Nature_ 514, 638–641 (2014). Article CAS PubMed PubMed Central Google Scholar * Pickard, J. M. & Chervonsky, A. V. Intestinal fucose
as a mediator of host–microbe symbiosis. _J. Immunol._ 194, 5588–5593 (2015). Article CAS PubMed Google Scholar * Meng, D. et al. Bacterial symbionts induce a FUT2-dependent fucosylated
niche on colonic epithelium via ERK and JNK signaling. _Am. J. Physiol. Gastrointest. Liver Physiol._ 293, G780–G787 (2007). Article CAS PubMed Google Scholar * Muraoka, W. T. &
Zhang, Q. Phenotypic and genotypic evidence for l-fucose utilization by _Campylobacter jejuni_. _J. Bacteriol._ 193, 1065–1075 (2011). Article CAS PubMed Google Scholar * Stahl, M. et
al. l-Fucose utilization provides _Campylobacter jejuni_ with a competitive advantage. _Proc. Natl Acad. Sci. USA_ 108, 7194–7199 (2011). Article CAS PubMed PubMed Central Google Scholar
* Staib, L. & Fuchs, T. M. Regulation of fucose and 1,2-propanediol utilization by _Salmonella enterica_ serovar Typhimurium. _Front. Microbiol_ 6, 1116 (2015). Article PubMed PubMed
Central Google Scholar * Ruiz-Palacios, G. M., Cervantes, L. E., Ramos, P., Chavez-Munguia, B. & Newburg, D. S. _Campylobacter jejuni_ binds intestinal H(O) antigen (Fucα1, 2Galβ1,
4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. _J. Biol. Chem._ 278, 14112–14120 (2003). Article CAS PubMed Google Scholar * Chessa, D., Winter,
M. G., Jakomin, M. & Bäumler, A. J. _Salmonella enterica_ serotype Typhimurium Std fimbriae bind terminal α(1,2)fucose residues in the cecal mucosa. _Mol. Microbiol._ 71, 864–875 (2009).
Article CAS PubMed Google Scholar * Barra, J. L., Monferran, C. G., Balanzino, L. E. & Cumar, F. A. _Escherichia coli_ heat-labile enterotoxin preferentially interacts with blood
group A-active glycolipids from pig intestinal mucosa and A- and B-active glycolipids from human red cells compared to H-active glycolipids. _Mol. Cell. Biochem._ 115, 63–70 (1992). Article
CAS PubMed Google Scholar * Balanzino, L. E., Barra, J. L., Galván, E. M., Roth, G. A. & Monferran, C. G. Interaction of cholera toxin and _Escherichia coli_ heat-labile enterotoxin
with glycoconjugates from rabbit intestinal brush border membranes: relationship with ABH blood group determinants. _Mol. Cell. Biochem._ 194, 53–62 (1999). Article CAS PubMed Google
Scholar * Galván, E. M., Roth, G. A. & Monferran, C. G. Participation of ABH glycoconjugates in the secretory response to _Escherichia coli_ heat-labile toxin in rabbit intestine. _J.
Infect. Dis._ 180, 419–425 (1999). Article PubMed Google Scholar * Galván, E. M., Diema, C. D., Roth, G. A. & Monferran, C. G. Ability of blood group A-active glycosphingolipids to
act as _Escherichia coli_ heat-labile enterotoxin receptors in HT-29 cells. _J. Infect. Dis._ 189, 1556–1564 (2004). Article PubMed Google Scholar * Galván, E. M., Roth, G. A. &
Monferran, C. G. Functional interaction of _Escherichia coli_ heat-labile enterotoxin with blood group A-active glycoconjugates from differentiated HT29 cells. _FEBS J._ 273, 3444–3453
(2006). Article PubMed Google Scholar * Holmner, A., Askarieh, G., Okvist, M. & Krengel, U. Blood group antigen recognition by _Escherichia coli_ heat-labile enterotoxin. _J. Mol.
Biol._ 371, 754–764 (2007). Article CAS PubMed Google Scholar * Marionneau, S. et al. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of
secretor individuals. _Gastroenterology_ 122, 1967–1977 (2002). Article CAS PubMed Google Scholar * Huang, P. et al. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group
antigens: identification of 4 distinct strain-specific patterns. _J. Infect. Dis._ 188, 19–31 (2003). Article CAS PubMed Google Scholar * Lindesmith, L. et al. Human susceptibility and
resistance to Norwalk virus infection. _Nat. Med._ 9, 548–553 (2003). Article CAS PubMed Google Scholar * Harrington, P. R., Vinjé, J., Moe, C. L. & Baric, R. S. Norovirus capture
with histo-blood group antigens reveals novel virus–ligand interactions. _J. Virol._ 78, 3035–3045 (2004). Article CAS PubMed PubMed Central Google Scholar * Huang, P. et al. Norovirus
and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. _J. Virol._ 79,
6714–6722 (2005). Article CAS PubMed PubMed Central Google Scholar * Borén, T., Falk, P., Roth, K. A., Larson, G. & Normark, S. Attachment of _Helicobacter pylori_ to human gastric
epithelium mediated by blood group antigens. _Science_ 262, 1892–1895 (1993). Article PubMed Google Scholar * Ilver, D. et al. _Helicobacter pylori_ adhesin binding fucosylated
histo-blood group antigens revealed by retagging. _Science_ 279, 373–377 (1998). Article CAS PubMed Google Scholar * Ikehara, Y. et al. Polymorphisms of two fucosyltransferase genes
(Lewis and Secretor genes) involving type I Lewis antigens are associated with the presence of anti-_Helicobacter pylori_ IgG antibody. _Cancer Epidemiol. Biomark. Prev._ 10, 971–977 (2001).
CAS Google Scholar * Aspholm-Hurtig, M. et al. Functional adaptation of BabA, the _H. pylori_ ABO blood group antigen binding adhesin. _Science_ 305, 519–522 (2004). Article CAS PubMed
Google Scholar * Hage, N. et al. Structural basis of Lewisb antigen binding by the _Helicobacter pylori_ adhesin BabA. _Sci. Adv._ 1, e1500315 (2015). Article PubMed PubMed Central
Google Scholar * Chakraberty, R., Reiz, B. & Cairo, C. W. Profiling of glycosphingolipids with SCDase digestion and HPLC-FLD–MS. _Anal. Biochem_ 631, 114361 (2021). Article CAS PubMed
Google Scholar * Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. _Nat. Biotechnol._ 34, 184–191 (2016). Article CAS
PubMed PubMed Central Google Scholar * Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. _Genome Biol._ 15, 554 (2014).
Article PubMed PubMed Central Google Scholar * Liao, Y., Wang, J., Jaehnig, E. J., Shi, Z. & Zhang, B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs.
_Nucleic Acids Res._ 47, W199–W205 (2019). Article CAS PubMed PubMed Central Google Scholar * Turner, S. D. qqman: an R package for visualizing GWAS results using Q–Q and Manhattan
plots. _J. Open Source Softw._ 3, 731 (2018). Article Google Scholar * Varadi, M. et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of
protein-sequence space with high-accuracy models. _Nucleic Acids Res._ 50, D439–D444 (2022). Article CAS PubMed Google Scholar * Kadirvelraj, R. et al. Comparison of human
poly-_N_-acetyl-lactosamine synthase structure with GT-A fold glycosyltransferases supports a modular assembly of catalytic subsites. _J. Biol. Chem._ 296, 100110 (2021). Article CAS
PubMed Google Scholar * Miller, J. J. et al. α-Galactosidase A-deficient rats accumulate glycosphingolipids and develop cardiorenal phenotypes of Fabry disease. _FASEB J._ 33, 418–429
(2019). Article CAS PubMed Google Scholar * Barone, A., Benktander, J., Teneberg, S. & Breimer, M. E. Characterization of acid and non-acid glycosphingolipids of porcine heart valve
cusps as potential immune targets in biological heart valve grafts. _Xenotransplantation_ 21, 510–522 (2014). Article PubMed Google Scholar * Karlsson, H., Halim, A. & Teneberg, S.
Differentiation of glycosphingolipid-derived glycan structural isomers by liquid chromatography/mass spectrometry. _Glycobiology_ 20, 1103–1116 (2010). Article CAS PubMed Google Scholar
* Shajahan, A., Heiss, C., Ishihara, M. & Azadi, P. Glycomic and glycoproteomic analysis of glycoproteins—a tutorial. _Anal. Bioanal. Chem._ 409, 4483–4505 (2017). Article CAS PubMed
PubMed Central Google Scholar * Shajahan, A. et al. Comprehensive characterization of _N_- and _O_-glycosylation of SARS-CoV-2 human receptor angiotensin converting enzyme 2.
_Glycobiology_ 31, 410–424 (2021). Article CAS PubMed Google Scholar * Shajahan, A., Supekar, N. T., Gleinich, A. S. & Azadi, P. Deducing the _N_- and _O_-glycosylation profile of
the spike protein of novel coronavirus SARS-CoV-2. _Glycobiology_ 30, 981–988 (2020). Article CAS PubMed PubMed Central Google Scholar * Chai, W. et al. Negative-ion electrospray mass
spectrometry of neutral underivatized oligosaccharides. _Anal. Chem._ 73, 651–657 (2001). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank A. Singla, D.
Andrade Silva and M. Burns for comments on the manuscript. We thank E. Capota and D. Andrade Silva for technical support. We thank A. Wands, D. Carroll, H. Wu, H. Khan and M. Shiloh for
advice and reagents. We thank the UT Southwestern Proteomics Core Facility and its director, A. Lemoff. We acknowledge support from the National Institutes of Health (R01GM090271 and
R35GM145599 to J.J.K. and R24GM137782 to P.A.), the Swedish Cancer Foundation (22 2079 Pj to S.T.) and the Welch Foundation (I-1686 to J.J.K.). M.T.G. received support from the NIH
(T32GM145467). We thank C. Cairo (University of Alberta) for the RhtrECI plasmid and R. Schnaar (Johns Hopkins) for the P4 inhibitor. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department
of Biochemistry, UT Southwestern Medical Center, Dallas, TX, USA Atossa C. Ghorashi, Rohit Sai Reddy Konada & Jennifer J. Kohler * Department of Microbiology and Immunology, Institute
of Biomedicine, University of Gothenburg, Gothenburg, Sweden Andrew Boucher & Ulf Yrlid * Complex Carbohydrate Research Center, The University of Georgia, Athens, GA, USA Stephanie A.
Archer-Hartmann, Mehrnoush Taherzadeh Ghahfarrokhi, Nathan B. Murray & Parastoo Azadi * Department of Medical Biochemistry and Cell Biology, Institute of Biomedicine, University of
Gothenburg, Gothenburg, Sweden Dani Zalem & Susann Teneberg * McDermott Center for Human Growth and Development, UT Southwestern Medical Center, Dallas, TX, USA Xunzhi Zhang & Chao
Xing * Department of Bioinformatics, UT Southwestern Medical Center, Dallas, TX, USA Chao Xing Authors * Atossa C. Ghorashi View author publications You can also search for this author
inPubMed Google Scholar * Andrew Boucher View author publications You can also search for this author inPubMed Google Scholar * Stephanie A. Archer-Hartmann View author publications You can
also search for this author inPubMed Google Scholar * Dani Zalem View author publications You can also search for this author inPubMed Google Scholar * Mehrnoush Taherzadeh Ghahfarrokhi View
author publications You can also search for this author inPubMed Google Scholar * Nathan B. Murray View author publications You can also search for this author inPubMed Google Scholar *
Rohit Sai Reddy Konada View author publications You can also search for this author inPubMed Google Scholar * Xunzhi Zhang View author publications You can also search for this author
inPubMed Google Scholar * Chao Xing View author publications You can also search for this author inPubMed Google Scholar * Susann Teneberg View author publications You can also search for
this author inPubMed Google Scholar * Parastoo Azadi View author publications You can also search for this author inPubMed Google Scholar * Ulf Yrlid View author publications You can also
search for this author inPubMed Google Scholar * Jennifer J. Kohler View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.C.G. and J.J.K.
conceptualized the project and experimental approach, with input from A.B., R.S.R.K. and U.Y. A.C.G. conducted all cell culture experiments, flow cytometry experiments, functional assays and
immunoblot experiments. A.B. prepared biotinylated CTB and CTB mutants with supervision from U.Y. S.A.A.-H. conducted MS analysis of intact glycolipids with supervision from P.A. M.T.G.
performed MS analysis of procainamide-labeled glycans from glycolipids with supervision from P.A. D.Z. isolated neutral glycolipids and conducted MS analysis of their glycans with
supervision from S.T. N.B.M. and S.A.A.-H. conducted _N_-linked and _O_-linked glycomic analyses with supervision from P.A. R.S.R.K. prepared EGCase and conducted preliminary analyses of
glycolipids. X.Z. performed bioinformatics analyses with supervision from C.X. A.C.G. and J.J.K. wrote the manuscript with input from all authors. CORRESPONDING AUTHOR Correspondence to
Jennifer J. Kohler. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Chemical Biology_ thanks the anonymous
reviewers for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 CHARACTERIZATION OF _B3GALT5-_KO AND _B3GALT5-_KO + OE CELLS. (A, B) Representative histograms (left panel) from the
flow cytometry analyses of cell surface binding of Lewis a antibody (A) or Lewis x antibody (B) to indicated cell lines. Bar graphs (right panel) show quantification from 3 independent
trials. Error bars indicate mean ± SD. (C) Quantification of gMFI from flow cytometry analyses of cells treated with increasing concentrations of CTB. Data shown are from 3 independent
trials and normalized to the maximum APC signal in WT cells. Error bars indicate mean ± SD. Indicated cell lines were incubated for 72 h with increasing concentrations of CTB-Saporin (D) or
unconjugated saporin (E). Cell survival upon internalization of CTB-saporin was measured using the Cell Titer-Glo 2.0 assay. Data shown are luminescence values normalized to the signal from
the untreated condition for each cell type. Each datapoint is a biological replicate consisting of 3 averaged technical replicates. Error bars indicate mean ± SD of 3 biological replicates.
(F) Cells pretreated with BFA or vehicle control for 0.5 h were incubated for 1.5 h with CT (1 nM) or buffer alone. Accumulation of cAMP was measured. Data shown are inverse of luminescence
values normalized first to the total amount of cells plated for each cell line, then to the signal in CT-treated control cells. Each datapoint is a biological replicate consisting of 3
averaged technical replicates. Error bars indicate mean ± SD of 3 biological replicates. (G) Control, _B3GALT5-_KO m1, m2 and KO + OE cells were treated with forskolin (10 µM) for 0.5 h and
then analyzed as in panel F. Statistical analyses for panels A and B were performed by one-way ANOVA with Tukey correction and for panels C, F, and G by two-way ANOVA with Tukey
correction.‘ns’ indicates not significant, **** indicates adjusted _P_-value < 0.0001. Exact _P_-values are as follows: 0.0004 and 0.0412 for control versus _B3GALT5-_KO m2 and
_B3GALT5-_KO + OE; 0.0041 and 0.0024 for _B3GALT5-_KO m1 versus _B3GALT5-_KO m2 and _B3GALT5-_KO + OE, respectively (panel B); 0.0390 for control versus _B3GALT5-_KO m2 treated with 0.0375
μg/mL; 0.0002 and 0.0014 for control versus _B3GALT5-_KO m2 or m1 treated with 0.075 μg/mL, respectively (panel C). Source data EXTENDED DATA FIG. 2 CHARACTERIZATION OF _B3GNT5_-KO AND
_B3GNT5_-KO + OE CELLS. (A) Quantification of gMFI from flow cytometry analyses of cells treated with increasing concentrations of CTB. Data shown are from 3 independent trials and
normalized to the maximum APC signal in WT cells. Error bars indicate mean ± SD. (B, C) Representative histograms (left panel) from the flow cytometry analyses of cell surface binding of
Lewis x antibody (B) or Lewis a antibody (C) to control, _B3GNT5_-KO m1, m2 and KO + OE cells. Bar graphs (right panel) show quantification from 3 independent trials. Control, _B3GNT5_-KO m1
and KO + OE cells were incubated for 72 h with increasing concentrations of CTB-Saporin (D) or unconjugated saporin (E). Cell survival upon internalization of CTB-saporin was measured using
the Cell Titer-Glo 2.0 assay. Data shown are luminescence values normalized to the signal from the untreated condition for each cell type. Each datapoint is a biological replicate
consisting of 3 averaged technical replicates. Error bars indicate mean ± SD of 3 biological replicates. (F) Cells pretreated with BFA or a vehicle control for 0.5 h were incubated for 1.5 h
with CT (1 nM) or buffer alone. Accumulation of cAMP was measured. Data shown are inverse of luminescence values normalized first to the total amount of cells plated for each cell line,
then to the signal in CT-treated control cells. Each datapoint is a biological replicate consisting of 3 averaged technical replicates. Error bars indicate mean ± SD of 3 biological
replicates. (G) Control, _B3GNT5_-KO m1, m2 and KO + OE cells were treated with forskolin (10 µM) for 0.5 h and then analyzed as in panel F. Statistical analyses for panels B and C were
performed by one-way ANOVA with Tukey correction and for panels A, D, E, F, and G by two-way ANOVA with Tukey correction. ‘ns’ indicates not significant, **** indicates adjusted _P_-value
< 0.0001. Exact _P_-values are as follows: 0.0219 for control versus _B3GNT5_-KO m1 treated with 1.25 μg/mL; 0.0010 and 0.0077 for control versus _B3GNT5_-KO m1 or m2 treated with 2.5
μg/mL, respectively (panel A); 0.0059 and 0.0036 for control versus _B3GNT5_-KO + OE treated with 2.5 or 12.5 μg/mL CTB-saporin (panel D). Source data EXTENDED DATA FIG. 3 CHARACTERIZATION
OF _B3GALT5_ + _B3GNT5_-DKO CELLS. (A) Representative histograms (left panel) from the flow cytometry analyses of cell surface binding of Lewis x antibody to control, _B3GALT5_ -KO m1,
_B3GNT5_-KO m1, and _B3GALT5_ + _B3GNT5_-dKO cells. Bar graph (right panel) shows quantification from 3 independent trials. (B, C) Control, _B3GALT5-_KO m1, and _B3GALT5_ + _B3GNT5_-dKO
cells were incubated for 72 h with increasing concentrations of CTB-Saporin (B) or unconjugated saporin (C). Cell survival upon internalization of CTB-saporin measured using the Cell
Titer-Glo 2.0 assay. Data shown are luminescence values normalized to the signal from the untreated condition for each cell type. Each datapoint is a biological replicate consisting of 3
averaged technical replicates. Error bars indicate mean ± SD of 3 biological replicates. (D) Cells pretreated with BFA or a vehicle control for 0.5 h were incubated for 1.5 h with CT (1 nM)
or buffer alone. Accumulation of cAMP was measured. Data shown are inverse of luminescence values normalized first to the total amount of cells plated for each cell line, then to the signal
in CT-treated control cells. Each datapoint is a biological replicate (n = 2) consisting of 3 averaged technical replicates. (E) Control, _B3GALT5-_KO m1, m2 and KO + OE cells were treated
with forskolin (10 µM) for 0.5 h and then analyzed as in panel D. Each datapoint is a biological replicate consisting of 3 averaged technical replicates. Error bars indicate mean ± SD of 3
biological replicates. Statistical analyses for panel A were performed by one-way ANOVA with Tukey correction and for panels B, C, D, and E by two-way ANOVA with Tukey correction. ****
indicates adjusted _P_-value < 0.0001. Exact _P_-values are as follows: 0.0003 and 0.0048 for _B3GALT5_ + _B3GNT5_-dKO versus control or _B3GALT5-_KO m1, respectively (panel A); 0.0002
and 0.0171 for control versus _B3GALT5_ + _B3GNT5_-dKO treated with 2.5 μg/mL CTB saporin or 12.5 μg/mL saporin (panels C and D, respectively). Source data EXTENDED DATA FIG. 4 FUCOSYLATED
LACTO-SERIES GSLS DETECTED IN CONTROL BUT NOT KO CELL LINES. MS analysis of GSLs from control (A), _B3GALT5-_KO m1 (B), and _B3GNT5_-KO m1 (C) cells. (D) Example MS/MS spectrum of a
fucosylated lacto-series glycolipid detected in control cells, confirming structure. EXTENDED DATA FIG. 5 VALIDATION OF GSLS AS DECOY RECEPTORS FOR CT. (A) Lectin blot with CTB-biotin of
control and _B3GALT5_-KO m1 cell lysates, and pure GM1. Samples were treated for 16 h with endoglycoceramidase or a vehicle control. Data shown are a single representative trial of 3
independent biological replicates. (B-G) Control, _B3GNT5_-KO + OE, and _B3GALT5-_KO m1 cells were treated with P4 inhibitor of glycosphingolipid biosynthesis for 72 h then lysed for lectin
blot analysis (B) or treated with BFA or a vehicle control for 0.5 h prior to incubation with CT (1 nM) for analysis of cAMP accumulation (C, D, and E). Alternately, cells were treated with
forskolin (10 µM) for 0.5 h (F and G). Accumulation of cAMP was measured. Data shown are inverse of luminescence values normalized first to the total amount of cells plated for each cell
line, then to the signal in CT-treated control cells. Each datapoint is a biological replicate (n = 2 in panel E, n = 3 in panels C, D, F, and G) consisting of 3 averaged technical
replicates. Error bars indicate mean ± SD of 3 biological replicates. Statistical analyses were performed by two-way ANOVA with Tukey correction. Exact _P_-values are 0.0002 for control
versus inhibitor-treated _B3GALT5-_KO m1 as well as inhibitor-treated control versus untreated _B3GALT5-_KO m1; for inhibitor-treated control versus inhibitor-treated _B3GALT5-_KO m1, the
exact _P_-value is 0.0052 (panel C). Source data EXTENDED DATA FIG. 6 ANALYSIS OF GSLS FROM _B3GNT5_-KO + OE CELLS. (A) Glycolipids were isolated from _B3GALT5_-KO m1 or _B3GNT5_-KO + OE
cells. Glycans were released with endoglycoceramidase, labeled with procainamide, and analyzed by mass spectrometry with HILIC-FL separation. (B, C) LC-ESI/MS analysis of oligosaccharides
obtained by digestion of neutral GSLs from _B3GNT5_-KO + OE cells with endoglycoceramidase I. Diagnostic ions indicate carbohydrate linkage positions. (C) An MS spectrum displaying a series
of C type fragment ions (C2α at _m/z_ 528, C3α at _m/z_ 690, C4α at _m/z_ 1039, C5α at _m/z_ 1201, C6 at _m/z_ 1550, and C7 at _m/z_ 1712), which identified an oligosaccharide with
Hex-(Fuc-)HexNAc-Hex-(Fuc-)HexNAc-Hex-(Fuc-)HexNAc-Hex-Hex sequence. The ion at _m/z_ 364 is obtained by double glycosidic cleavage of the 3-linked branch (C2/Z3β), and characteristic for an
internal 4-linked GlcNAc substituted with a Fuc at 3-position that is a terminal Lex (refs. 90,94). Taken together this indicated an undecasaccharide with a terminal Lex determinant. (D, E)
_B3GALT5_ + _B3GNT5_-dKO cells were incubated with GSLs extracted from _B3GNT5_-KO + OE cells, a commercial mixture of neutral GSLs, or purified GM1. Cells were then treated with BFA or a
vehicle control for 0.5 h prior to incubation with CT (1 nM) for analysis of cAMP accumulation (D). Alternately, cells were treated with forskolin (10 µM) for 0.5 h (n = 2) (E). Accumulation
of cAMP was measured. Data shown are inverse of luminescence values normalized first to the total amount of cells plated for each cell line, then to the signal in CT-treated control cells.
Each datapoint is a biological replicate (n = 3 for panel D, n = 2 for panel E) consisting of 2 averaged technical replicates. Error bars indicate mean ± SD of 3 biological replicates (panel
D). Statistical analyses were performed by two-way ANOVA with Tukey correction. No significant differences were observed. Source data EXTENDED DATA FIG. 7 _B3GALT5_-KO CELLS EXHIBIT
INCREASED FUCOSYLATION ON _N_-LINKED GLYCOPROTEINS. (A) _N_-linked glycoforms detected in control and _B3GALT5-_KO m1 cells by LC-MS/MS analysis. Quantification is based on triplicate
analysis. (B) Example MS/MS of a complex, fucosylated _N_-linked glycan detected in _B3GALT5_-KO m1 cells confirming placement of fucose. (C) Bar graphs showing the relative enrichment of
mono- vs di- vs tri-fucosylated _N_-linked glycans detected by LC-MS/MS analysis of control and _B3GALT5-_KO m1 cells. Statistical analysis was performed by two-tailed t-test with Holm-Šídák
correction. Exact adjusted _P_-value = 0.013508 for mono-fucosylated _N_-linked glycans detected in control versus _B3GALT5-_KO m1. Exact adjusted _P_-value = 0.014105 for both di- and
tri-fucosylated _N_-linked glycans detected in _B3GALT5-_KO m1 versus control. EXTENDED DATA FIG. 8 _B3GALT5-_KO CELLS EXHIBIT INCREASED FUCOSYLATION ON _O_-LINKED GLYCOPROTEINS. (A)
_O_-linked glycoforms detected in control and _B3GALT5-_KO m1 cells by LC-MS/MS analysis. Quantification is based on triplicate analysis. (B) Example MS/MS of a fucosylated _O_-linked glycan
detected in _B3GALT5_-KO m1 cells. (C) Example MS/MS of core 2 _O_-linked glycan detected in _B3GALT5-_KO m1 cells confirming structure. (D, E) _B3GALT5-_KO m1 cells were rescued with
either WT or catalytically dead (mut) _B3GALT5-_OE. Rescued cells were treated with BFA or a vehicle control for 0.5 h prior to incubation with CT (1 nM) for analysis of cAMP accumulation
(D). Alternately, cells were treated with forskolin (10 µM) for 0.5 h (E). Accumulation of cAMP was measured. Data shown are inverse of luminescence values normalized first to the total
amount of cells plated for each cell line, then to the signal in CT-treated control cells. Each datapoint is a biological replicate consisting of 2 averaged technical replicates. Error bars
indicate mean ± SD of 3 biological replicates. Statistical analyses were performed by two-way ANOVA with Tukey correction. No significant differences were observed. Source data EXTENDED DATA
FIG. 9 VALIDATION OF _SLC35C1-_KO CELL LINES. (A) GM1 was treated with EGCase or vehicle, then detected by lectin blot using WT CTB-biotin, W88K CTB-biotin, and H18L CTB-biotin. Data
presented are from 3 distinct membranes processed simultaneously (separated by vertical lines) and are a single representative trial from 3 biological replicates. (B, C) Representative
histograms (left panel) from flow cytometry analyses of anti-Lex antibody (B) and AAL (C) binding to surfaces of control, _B3GALT5-_KO m1, _SLC35C1-_KO, and _B3GALT5_ + _SLC35C1-_dKO cells.
Quantification of gMFIs (right panel) from 3 biological replicates are normalized to the maximum signal in control cells. Lysates from control, _B3GALT5-_KO m1, _SLC35C1-_KO and _B3GALT5_ +
_SLC35C1_-dKO cells were analyzed by immunoblot probing with anti-Lex antibody or lectin blot probing with AAL (D). Data shown are a single representative trial from 3 biological replicates.
(E) Control, _B3GALT5-_KO m1, _SLC35C1-_KO and _B3GALT5_ + _SLC35C1-_dKO cells were incubated for 72 h with unconjugated saporin (12.5 μg/mL). Survival data shown are luminescence values
normalized to the signal from the untreated condition for each cell type. Each datapoint indicates the mean of 2 biological replicates, each consisting of 3 averaged technical replicates.
(F) Cells pretreated with BFA or a vehicle control for 0.5 h were incubated for 1.5 h with CT (1 nM) or buffer alone. Accumulation of cAMP was measured. Data shown are inverse of
luminescence values normalized first to the total amount of cells plated for each cell line, then to the signal in CT-treated control cells. Each datapoint is a biological replicate (n = 2)
consisting of 3 averaged technical replicates. (G) Cells were treated with forskolin (10 µM) for 0.5 h and then analyzed as in panel F. Each datapoint is a biological replicate consisting of
3 averaged technical replicates. Error bars indicate mean ± SD of 3 biological replicates. Statistical analyses were performed by one-way (panels B and C) or two-way (panels F and G) ANOVA
with Tukey correction. **** indicates adjusted _P_-value < 0.0001. Exact _P_-value for control versus _B3GALT5-_KO m1 treated with AAL is 0.0018. Source data EXTENDED DATA FIG. 10
CTB-INTERACTING GLYCOPROTEINS. (A) Top 50 proteins identified from CTB-biotin pulldown from _B3GALT5_ + _B3GNT5_-dKO versus _B3GALT5_ + _SLC35C1-_dKO cell lysates. Fold-change is the
abundance in the _B3GALT5_ + _B3GNT5_-dKO pulldown as compared to the _B3GALT5_ + _SLC35C1-_dKO pulldown. Unadjusted _P_-values are derived from two-tailed student’s t-test. (B) Gene
ontology (GO) analysis using Fisher’s exact test with Bonferroni correction to identify pathways enriched in CTB-biotin pulldown from _B3GALT5_ + _B3GNT5-_dKO cell lysates as compared to
_B3GALT5_ + _SLC35C1-_dKO cell lysates. Top 25 pathways are shown. (C) Two hits (FLOT1 and MUC1, boldface font in panel A) were selected for confirmation. Total lysates and material from
CTB-biotin pulldowns were analyzed by immunoblot using antibodies against FLOT1 and MUC1. Source data SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Fig. 1. REPORTING
SUMMARY SUPPLEMENTARY TABLE 1 Reagents list. SUPPLEMENTARY TABLE 2 Oligonucleotide list. SUPPLEMENTARY TABLE 3 CRISPR KO sequence validation. SOURCE DATA SOURCE DATA FIG. 1 Statistical
source data. SOURCE DATA FIG. 2 Statistical source data. SOURCE DATA FIG. 3 Statistical source data. SOURCE DATA FIG. 4 Statistical source data. SOURCE DATA FIG. 5 Statistical source data.
SOURCE DATA FIG. 6 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 1 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 2 Statistical source data. SOURCE DATA EXTENDED DATA FIG.
3 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 5 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 6 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 8 Statistical
source data. SOURCE DATA EXTENDED DATA FIG. 9 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 9 Uncropped western blot. SOURCE DATA EXTENDED DATA FIG. 10 Uncropped western blot.
RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other
rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ghorashi, A.C., Boucher, A., Archer-Hartmann, S.A. _et al._ Fucosylation of glycoproteins and glycolipids: opposing roles in cholera
intoxication. _Nat Chem Biol_ 21, 555–566 (2025). https://doi.org/10.1038/s41589-024-01748-5 Download citation * Received: 08 August 2023 * Accepted: 13 September 2024 * Published: 16
October 2024 * Issue Date: April 2025 * DOI: https://doi.org/10.1038/s41589-024-01748-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative