Schema formation in a neural population subspace underlies learning-to-learn in flexible sensorimotor problem-solving
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

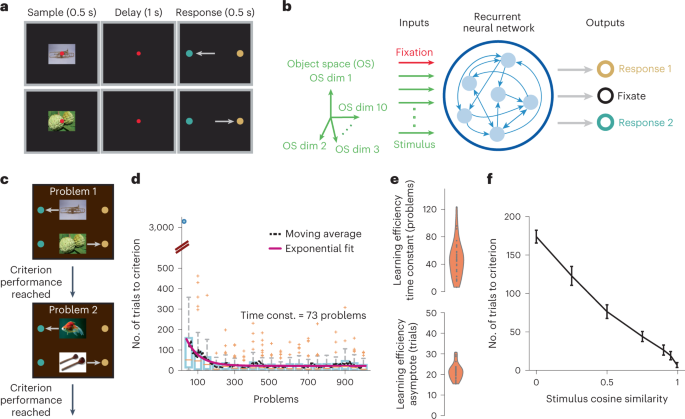
Learning-to-learn, a progressive speedup of learning while solving a series of similar problems, represents a core process of knowledge acquisition that draws attention in both neuroscience
and artificial intelligence. To investigate its underlying brain mechanism, we trained a recurrent neural network model on arbitrary sensorimotor mappings known to depend on the prefrontal
cortex. The network displayed an exponential time course of accelerated learning. The neural substrate of a schema emerges within a low-dimensional subspace of population activity; its reuse
in new problems facilitates learning by limiting connection weight changes. Our work highlights the weight-driven modifications of the vector field, which determines the population
trajectory of a recurrent network and behavior. Such plasticity is especially important for preserving and reusing the learned schema in spite of undesirable changes of the vector field due
to the transition to learning a new problem; the accumulated changes across problems account for the learning-to-learn dynamics.
Data files, including pre-trained networks, are available for further analyses on GitHub (https://github.com/xjwanglab/learning-2-learn) in Python and MATLAB readable formats.
All training and analysis codes are available on GitHub (https://github.com/xjwanglab/learning-2-learn).
We thank A. L. Fairhall, I. Skelin, J. J. Lin, B. Doiron, G. R. Yang, N. Y. Masse, U. P. Obilinovic, L. Y. Tian, D. V. Buonomano, J. Jaramillo, J. E. Fitzgerald and H. Sompolinksy for
fruitful discussions and Y. Liu, K. Berlemont, A. Battista and P. Theodoni for critical comments on the manuscript. This work was supported by National Institute of Health U-19 program grant
5U19NS107609-03 and Office of Naval Research grant N00014-23-1-2040.
Center for Neural Science, New York University, New York, NY, USA
Department of Neurobiology, University of Chicago, Chicago, IL, USA
Department of Physiology and Biophysics, University of Washington School of Medicine, Seattle, WA, USA
Washington National Primate Research Center, Seattle, WA, USA
B.P., D.J.F., E.A.B. and X.-J.W. designed the study. V.G. performed the research. V.G. and X.-J.W. wrote the manuscript.
Nature Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Notes, Supplementary Methods, Supplementary Discussion and Supplementary Figs. 1–10.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author
self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Anyone you share the following link with will be able to read this content: