Chromosome-level genome assembly and annotation of a potential model organism gossypium arboreum zb-1
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Recent advancements in plant regeneration and synthetic polyploid creation have been documented in _Gossypium arboreum_ ZB-1. These developments make ZB-1 a potential model within
the _Gossypium_ genus for investigating gene function and polyploidy. This work generated the sequence and annotation of the ZB-1 genome. The contig-level genome was constructed using the
PacBio high-fidelity reads, encompassing 81 contigs with an N50 length of 112.12 Mb. The Hi-C data assisted the construction of the chromosome-level genome, which consists of 13
_pseudo_-chromosomes and 39 un-anchored contigs, with a total length of about 1.67 Gb. Repetitive sequences accounted for about 69.7% of the genome in length. Based on _ab initio_ and
evidence-based prediction, we have identified 48,021 protein-coding genes in the ZB-1 genome. Comparative genomics analysis revealed conserved gene content and arrangement between ZB-1 and
_G. arboreum_ SXY1. The single nucleotide polymorphism occurrence rate between ZB-1 and SXY1 was about 0.54 per 1,000 nucleotides. This study enriched the genomic resources for further
exploration into cotton regeneration and polyploidy mechanisms. SIMILAR CONTENT BEING VIEWED BY OTHERS CHROMOSOME-LEVEL GENOME ASSEMBLY AND ANNOTATION OF THE PRICKLY NIGHTSHADE _SOLANUM
ROSTRATUM_ DUNAL Article Open access 01 June 2023 CHROMOSOME-LEVEL GENOME ASSEMBLY OF COTTON THRIPS _THRIPS TABACI_ (THYSANOPTERA: THRIPIDAE) Article Open access 16 September 2024 A
CHROMOSOME-LEVEL GENOME ASSEMBLY OF THE _KNOXIA ROXBURGHII_ (RUBIACEAE) Article Open access 15 November 2023 BACKGROUND & SUMMARY Cotton (_Gossypium spp_.) is one of the most crucial
fiber and oilseed crops worldwide. The _Gossypium_ genus comprises about 50 species, which include 45 diploids categorized into eight genome groups (A-G and K) and seven tetraploids denoted
as AD1-AD71,2. The tetraploids originated from a single hybridization event between the A- and D-genome progenitors, followed by polyploidization3. Historically, four of these species have
undergone independent domestication, _i.e_., the diploids _G. arboreum_ (A2) and _G. herbaceum_ (A1)4, as well as the tetraploids _G. hirsutum_ (AD1) and _G. barbadence_ (AD2)5. Notably, AD1
has emerged as the predominant species in contemporary cotton cultivation. A2 is critical for investigating the mechanisms underlying natural polyploidy in the genus _Gossypium_. For an
extended period, one of the major concerns in cotton biology is the origin of the A-genome in the natural tetraploids, with the controversy over whether it is from A1 or A2. In the last
decade, the genome sequences of A1, A2, and several tetraploids have been successively generated6. With the insights gained from comparative genomics, researchers are now inclined to
conclude that the ancestor (termed A0) of A1 and A2 is the A-genome donor7,8,9. However, as A0 no longer exists, A1 and A2 are still necessary substitutes for assessing the evolution of the
natural cotton polyploids upon genome merge and doubling. Due to its broader distribution and, more significantly, the more profound understanding of its biology in comparison to A1,
researchers often opt for A2 as the substitute progenitor when studying the evolution of natural tetraploids. Besides, A2 has also frequently been used to generate synthetic cotton
polyploids to investigate issues such as gene expression modulation in the early stage of polyploidy. For example, hybridization between A2 and _G. thurberi_ (D1), _G. raimondii_ (D5), and
_G. bickii_ (G1) gave rise to several hybrids and synthetic tetraploids10,11. Gene expression analysis of these synthetic hybrids or polyploids revealed that the subgenome expression pattern
was rapidly established in the early generations of the progenies, which would then be reconciled during long-term evolution. Likewise, Ke _et al_. generated a novel cotton tetraploid
between A2 (accession ZB-1) and _G. stocksii_ (E1) by somatic hybridization12. As meiosis does not occur during somatic hybridization, the novel polyploid 2(A2E1) might be an ideal model for
further exploring the transcriptional and epigenomic alteration upon genome doubling. In addition, A2-derived new cotton polyploids provide valuable germplasm resources for improving
agronomically important traits in cotton cultivars. For example, A2 and G1-derived tetraploids have glandular vegetative tissue and glandless seeds (without toxic gossypol)13, which provide
novel germplasm to breed cultivars with the seeds could be more widely utilized in industrial production14. Likewise, by hybridizing A2 with E1 and following genome doubling, Nie and
colleagues generated novel tetraploids 2(A2E1), of which the high generations produce high-strength fiber15. Moreover, Chen _et al_. generated a novel cotton hexaploid by hybridization
between A2 (SXY1 or Shixiya 1) and AD1 (TM-1), which could be further utilized to develop the _G. arboreum_-introgressed lines to transfer the drought and _Verticillium_ resistance to
cultivars16. In addition to serving as the progenitor for synthetic cotton polyploids in polyploidy research and breeding, A2 is also a potential model organism for gene function exploration
in cotton species. Currently, the creation of transgenic cotton plants predominantly relies on AD16,17. Nevertheless, ascribed to the doubled genomes in AD1, each allele is associated with
four haplotypes that are similar in sequence. The complexity of the genetic background poses a challenge in gene manipulation in AD1, such as the risk of off-target effects in
CRISPR/Cas9-mediated genome editing. Furthermore, the gene duplication resulting from polyploidy also adds to the complexity of evaluating gene functions in AD1. For instance, several
studies have highlighted that subfunctionalization and neofunctionalization of duplicated genes frequently occur after cotton polyploidization11,18,19. Consequently, diploids such as A2 are
better models for characterizing gene functions in cotton species. However, the method of regenerating plants by somatic embryogenesis has yet to be established for most cotton species,
which significantly hindered the transgenic study in cotton cells. Recently, we have achieved plant regeneration using A2 (ZB-1) with alternative solid-liquid culture method20. The callus
was able to be induced from different explants (hypocotyl, root, and cotyledon), and the regenerated plants could be regularly grown to maturity in soil or be grafted onto A2 seedlings. Upon
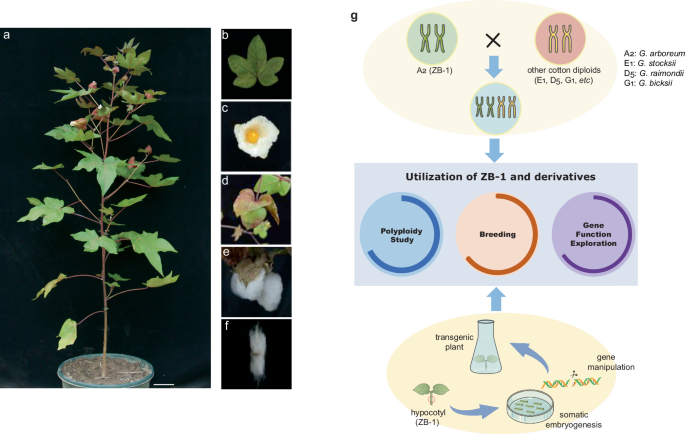
further optimization, ZB-1 will become a readily available tool for the functional analysis of cotton genes. Taken together, the accession ZB-1 with A2 genome is an essential material for
the studies on cotton polyploidy and gene function exploration (Fig. 1). At the same time, we have noticed that although some genome assemblies of A2 are available now and tens of A2
accessions have been resequenced, the chromosome-level A2 genomes were almost exclusively generated from the accession SXY17,21,22,23,24. Therefore, a high-quality genome assembly of ZB-1 is
necessary to provide additional genomic information for studies on polyploidy, gene manipulation, and the mechanisms underlying the regeneration of cotton plants. In this work, we
constructed a chromosome-level genome assembly of _G. arboreum_ ZB-1. The contig-level genome assembly was generated using PacBio HiFi reads. The Hi-C data was used to scaffold the contigs
into _pseudo_-chromosomes. Four transcriptomes of ZB-1 (boll, flower, sepal, and stem) were generated to assist the gene identification. The final gene set was generated by integrating the
results from _ab initio_ prediction, homologs-based gene annotation, and transcripts-derived gene identification using the PASA pipeline. The completeness of the genome assembly was
evaluated by BUSCO assessment and the mapping back of the short reads, which showed the high quality of the provided genome assembly of ZB-1. Moreover, a comparative analysis was carried out
between the assemblies of ZB-1 and SXY1, revealing a large number of single nucleotide polymorphisms (SNPs), suggesting this genome assembly is necessary for further studies using ZB-1 as a
test material. METHODS PLANT MATERIAL COLLECTION _G. arboreum_ ZB-1 was planted in the experimental field on the campus of Zhejiang Sci-Tech University in Hangzhou, Zhejiang Province,
China, in May 2022. The tender leaves (the third to fifth), bolls (10 days post anthesis), flowers (bloom day, including petals, stamens and pistils), sepals, and stems were harvested from
at least five adult plants and pooled. All the tissues were snap-frozen in liquid nitrogen and stored at −80 °C until use. DNA LIBRARY CONSTRUCTION AND GENOME SEQUENCING The genomic DNA
(gDNA) was extracted from the tender leaves using the CTAB (cetyltrimethylammonium bromide) method. After chilling in liquid nitrogen, 20 mg leaves were ground with steel balls in a 2 mL
polypropylene tube. 600 μL pre-heated (65 °C) CTAB buffer (2% w/v CTAB, 100 mM Tris-HCl, 20 mM EDTA, 1.5 M NaCl, pH 8) was added into the tube and thoroughly vortexed. The tube was placed in
a 65 °C water bath for 1 h. The homogenate was then centrifuged for 10 min at 12,000 rpm. The supernatant was transferred to a new tube, and 5 μL of RNase A solution was added. After
incubating at 37 °C for 20 min, an equal volume of phenol/chloroform/isoamyl alcohol (25:24:1) was added and mixed. The sample was centrifuged for 10 min at 12,000 rpm. The upper aqueous
phase was transferred to a new tube, and repeat this extraction once. Cold isopropanol was added with a volume of about two-thirds of the upper phase. The sample was gently mixed and
incubated at −20 °C for 30 min. Next, the sample was centrifuged for 10 min at 12,000 rpm at room temperature, and the pellet was washed using 500 μL ice-cold 70% ethanol. Decant the ethanol
and naturally dry the pellet to remove the ethanol residual. Finally, about 20 uL TE buffer (10 mM Tris, pH 8, 1 mM EDTA) was added to dissolve the DNA pellet. ILLUMINA SEQUENCING For
Illumina short-read sequencing, the purified gDNA was randomly sheared into fragments by a Covaris M220 Ultrasonicator (Covaris, USA). Subsequently, a library with an average length of about
350 bp for the inserted fragments was constructed according to the recommended protocols of the Illumina TruSeq DNA Sample Prep Kits. Qubit 2.0 Fluorometer (ThermoFisher Scientific, USA)
was used to quantify the dsDNA of the library, which was then diluted to 1 ng/μL. Agilent 2100 Bioanalyzer (Agilent, USA) was used to assess the integrity and size of the inserted fragments.
A final concentration of the library was determined with QuantStudio 3 Real-Time PCR Instrument (Thermo Fisher Scientific, USA) (>2 nM). The library was sequenced using the Novaseq 6000
platform (Illumina, USA) and a program of pair-end 150 bp. Quality control of the raw data was performed using Fastp (v0.21.0)25. The read pairs were removed if the ambiguous base (N)
exceeds 10% or the low-quality bases (Q < 5) exceed 20% of a single read in length. The generated raw data contained about 101.03 Gb paired short reads, with 99.91% retained after quality
control (Table 1). PACBIO SEQUENCING A HiFi library was constructed using the SMRTbell Template Prep Kit (Pacific Biosciences, USA). The high molecular weight DNA fragments (_ca_. 20 Kb)
were isolated and purified from the g-TUBE (Covaris, USA) sheared gDNA sample using the AMPure beads (Beckman Coulter, USA), followed by damage repair, end-repair/A-tailing and hairpin
adapter ligation according to the manufacturer’s instructions. The SMRTbell library was sequenced on the Pacbio Sequel II platform with the Circular Consensus Sequencing (CCS) model. The
HiFi reads were generated using SMRT Link software (v12.0) with default settings. About 4.01 M HiFi reads were finally acquired, with a total length and average length of about 71 Gb and
17.7 kb, respectively (Table 1). HI-C LIBRARY CONSTRUCTION AND SEQUENCING The Hi-C library was constructed according to the method previously described by Belton _et al_. with certain
modifications26. Tender leaves of ZB-1 were ground with liquid nitrogen and crosslinked by 4% formaldehyde at room temperature for 30 mins. 2. 5 M glycine was added to quench the
crosslinking reaction. The pellet was centrifuged at 2,500 rpm at 4 °C and resuspended with the lysis buffer (1 M Tris-HCl, pH 8, 1 M NaCl, 10% CA-630, and 13 units protease inhibitor). The
supernatant was centrifuged at 5,000 rpm at room temperature. The pellet was washed twice using the ice-cold NEB buffer and centrifuged again. Then, the nuclei were resuspended by the NEB
buffer, solubilized with dilute sodium dodecyl sulphate (SDS), and incubated at 65 °C for 10 mins. Triton X-100 quenched SDS, and the crosslinked chromatin was digested overnight by the
restriction enzyme _DPN II_ (400 units, 37 °C). The 5’ overhangs were filled in with biotinylated residues (biotin-14-dCTP), and the proximate blunted ends were ligated. Biotin was removed
from the un-ligated ends using T4 DNA polymerase. The ligated DNA was then sheared with the Covaris instrument, and the fragments with an average size of 350 bp were purified via agarose gel
electrophoresis and gel elution. After end repairing by the mixture of T4 DNA polymerase, T4 polynucleotide kinase, and Klenow DNA polymerase, the biotin-labelled DNA fragments were pulled
down using the streptavidin C1 magnetic beads. The library was then constructed with a standard protocol and sequenced on the Illumina Novaseq 6000 platform with 2 × 150 bp paired-end reads
(PE150). About 3.80 Gb short reads were generated from Hi-C library sequencing, with 3.78 Gb (99.6%) retained after removing the low-quality reads (Table 1). Further quality control was
mainly carried out with HICUP27. The truncated sequences were identified and mapped with hicup_truncater and hicup_mapper. The re-ligated and same circularised sequences were removed by
hicup_filter, and the PCR-raised duplication was filtered using hicup_deduplicator. As a result, 2,494,732 unique di-Tags were obtained, with the effect rate of Hi-C sequencing data about
23.32%. RNA LIBRARY CONSTRUCTION AND SEQUENCING Total RNA was isolated from the tissue samples (boll, flower, sepal, and stem) using the RNAprep Pure Plant Plus Kit (DP441, TIANGEN) and
purified with RNase-free DNase I. The sequencing libraries were constructed using NEB Next Ultra RNA Library Prep Kit for Illumina (New England Biolabs, USA). The mRNAs were enriched using
the oligo(dT) attached beads, and the cDNAs were synthesized using Superscript II reverse transcriptase. Fragmented cDNAs with a length of around 350 bp were used for sequencing library
construction with standard protocol. The constructed libraries were sequenced on Novaseq 6000 with the PE150 strategy. About 6.75 Gb, 6.16 Gb, 6.82 Gb, and 6.36 Gb raw data were generated
from sequencing of the libraries of the boll, flower, sepal, and stem, respectively. After quality control, about 6.1 to 6.7 Gb clean data were retained for further analysis, with their
effective ratio ranging from 98.21% to 98.93% (Table 1). GENOME ASSEMBLY The Pacbio sequencing generated HiFi reads were used to construct the contig-level genome assembly with Hifiasm
(v0.13.0-R307)28. 81 contigs were generated with a total length of 1,667.63 Mb. The length of the contigs ranged from 3.8 kb to 149.26 Mb, with the N50 and N90 lengths of 112.12 Mb and 41.97
Mb, respectively. The Hi-C data was then used to construct the chromosome-level assembly with the ALLHiC pipeline29. The tool juicebox (v2.20.00) was used for a manual check and accuracy of
the generated assembly (Fig. 2)30. 52 scaffolds were finally acquired, with the N50 and N90 lengths of 137.59 Mb and 102.94 Mb, respectively (Table 2). The total length of 13
_pseudo_-chromosomes was 1,663.66 Mb, accounting for 99.77% of the entire assembly in length (Table 3). GENOME ANNOTATION AND GENE FUNCTION PREDICTION ANNOTATION OF REPETITIVE SEQUENCES A
hybrid strategy was used to identify the repetitive sequences in the ZB-1 genome. The tandem repeats were predicted using TRF (v4.09) (Match = 2, Mismatch = 7, Delta = 7, PM = 80, PI = 10,
MaxPeriod = 2000)31. The homology-based prediction was carried out using the Repbase database (v202101) and the software RepeatMasker (v4.1.0) (-a -nolow -no_is -norna) and RepeatProteinmask
(v4.1.0) (-noLowSimple -pvalue 0.0001 -engine ncbi) (http://www.repeatmasker.org/)32. The _de novo_ repetitive elements database was built with LTR_FINDER (v1.06) (-C -w 2)33, RepeatScout
(v1.0.5)34, and RepeatModeler (v2.0.1) (-engine ncbi), then all repeat sequences with lengths greater than 100 bp and gap ‘N’ less than 5% constituted the raw transposable element (TE)
library. This TE library was combined with the results from the homology search against Repbase to construct the custom library using Uclust (-id 80)35, and supplied to RepeatMasker for
DNA-level repeat identification. A total of 1,161.62 Mb of repetitive sequences were identified in the ZB-1 genome, representing 69.7% of the entire assembly in length. Apart from the tandem
repeats (61.4 Mb, 3.68%), the other repetitive sequences were mainly classified into long terminal repeats (LTR), DNA, and long interspersed nuclear elements (LINE) (Table 4). ANNOTATION OF
CODING GENES The coding genes were identified by integrating the results from _ab initio_ prediction, homology-based prediction, and cDNA-assisted prediction. Augustus (v3.2.3) (--genemodel
= complete,--noInFrameStop = true)36, GlimmerHMM (v3.0.4)37, SNAP (v2013.11.29)38, Geneid (v1.4), and Genscan (v1.0) were used for _ab initio_ prediction39. The predicted proteomes of
closely related species, _i.e_., A2 (‘SXY1’ genome WHU-updated v1)7, E1 (ZSTU_v1)40, D5 (JGI_v2_a2.1)41, AD1 (‘TM-1’ genome NBI_v1.1)42, and _Theobroma cacao_43, were downloaded from
cottongen (https://www.cottongen.org/) and Ensembl Genomes (http://ensemblgenomes.org/) and used for homology-based gene prediction. These protein sequences were aligned to the ZB-1 genome
assembly using TBLASTN (v2.2.26) (-F T -e 1e-5), and the matching ones were further aligned to the genome with GeneWise (v2.4.1) for an accurate prediction of the gene structures44,45. In
addition, RNA-seq data of the ZB-1 tissues (leaf, boll, flower, sepal, and stem) were used to assist the gene prediction. Notably, the RNA-seq data of ZB-1 seedling leaves were generated and
submitted to the public database by our previous work40, including SRR13933598, SRR13933601, and SRR13933602. Trinity (v2.1.1) (--normalize_reads --full_cleanup –min_glue 2 –min_kmer_cov 2
--KMER_SIZE 25) was used to generate _de novo_ transcripts46. In addition, the short reads from RNA-seq were aligned to the ZB-1 genome using Hisat2 (v2.2.1), and the resulting bam files
were used for re-constructing the gene models using Stringtie (v1.3.3)47,48. The non-redundant gene annotation was then generated by merging the results derived from three methods with
EvidenceModeler (EVM, v1.1.1) (--segmentSize 200000 --overlapSize 20000 --min_intron_length 20) and was improved by using PASA pipeline and manual check49. This study finally identified
50,058 loci of coding genes, including 48,236 genes of high confidence and 1,822 pseudogenes (Table 5). The majority of coding genes (48,021) were found within the constructed chromosomes,
in contrast to a small number (215) of genes in the unanchored contigs. The high confidential genes spanned an average of 2,620 bp in the genome, with an average exon number of 4.3 and an
average coding sequence (CDS) length of 1,047 bp. FUNCTIONAL ANNOTATION Functional annotation of the coding genes was performed by homology search against the non-redundant protein database
(NR) in NCBI using BLASTP (cutoff evalue 1e-4 and identity 25%). The domain architectures and Gene Ontology annotations of the predicted proteins were characterized by Interproscan
(v5.35–74.0) (-appl ProDom, SMART, ProSiteProfiles, PRINTS, Pfam, Panther -iprlookup -dp -goterms) and online service of eggNOG (http://eggnog-mapper.embl.de/)(--evalue 0.001 --score 60
--pident 35)50,51. The combined analysis assigned tentative functions for about 99% of the predicted coding genes. 17,700 genes were mapped to the KEGG pathways, and a similar number of
genes (17,774) were assigned GO terms. ANNOTATION OF NONCODING RNAS The tRNAs in the ZB-1 genome were identified using tRNAscan-SE (v2.0.12)52, and the rRNA genes were identified via
homology search with BLASTN (v2.2.26) (-e 1e-10). In addition, Rfam (v14.1) and INFERNAL (v1.1.3) (http://infernal.janelia.org/) were used to predict the miRNAs and snRNAs53,54. 274 miRNA
genes and 1,369 tRNA genes were identified, as well as 14,383 rRNA and 7,467 snRNA loci (Table 6). COMPARATIVE GENOMICS ANALYSES GENOMIC SYNTENY The predicted proteomes of ZB-1 and SXY1
(WHU-updated v1) were compared using BLASTP (-evalue 1e-5, cutoff identity 50%, coverage 50%). Based on the homology analysis, MCScanX (-s 10) was then used to identify the collinear
blocks55, with the result visualized using the R package RIdeogram (0.2.2)56. Extensive genomic synteny was observed between the genomes of these two A2 accessions (Fig. 3), i_.e_., 99.1% of
the coding genes (including pseudogenes) were encoded by the collinear blocks. SNP CALLING Discovery of the SNPs between ZB-1 and SXY1 was carried out using the MUMmer tools (v4.0.0)57. The
script _nucmer_ was used to align the two genome sequences, and the script _delta-filter_ was subsequently used to remove the redundancy in the alignments (−1 -q -r). The structural
variations were then called using the script _show-snaps_ (-C), and the result was displayed using the R package of CMplot (v4.5.0)58. The overall rate of the SNP occurrence was about 0.53
in every 1,000 nucleotides (Fig. 4). Specifically, 19,017 SNPs were identified in the coding regions (cSNPs), with the occurrence rate of about 0.38 in 1,000 nucleotides. DATA RECORDS All
the high-throughput sequencing data generated in this work were available through NCBI with the project PRJNA935667. The clean data generated by sequencing the genome libraries were
deposited in the Sequence Read Archive (SRA) under the accession numbers SRR27009933 (Illumina PE150) and SRR27009931 (Pacbio CCS)59,60. The data generated from sequencing of the Hi-C
library was deposited under SRR2700993261. The RNA-seq data of the boll, flower, sepal, and stem could be retrieved from the SRA with the accession numbers
SRR27009934-SRR2700993762,63,64,65. The assembled genome sequence of _G. arboreum_ ZB-1, together with the information on the coding gene structures, has been deposited in GenBank under the
accession number JARKNE00000000066. The annotation of noncoding genes and repeat sequence, as well as the gene function prediction, were available in the Figshare database
(https://doi.org/10.6084/m9.figshare.24736338)67. TECHNICAL VALIDATION EVALUATION OF THE COMPLETENESS AND QUALITY OF THE GENOME ASSEMBLY MAPPING THE SHORT READS TO THE GENOME The short reads
generated from Illumina sequencing of the genome library were aligned to the assembly using BWA (v0.7.17)68, which showed that 99.22% of the reads were mapped to 99.95% of the genome
sequence. The average depth of short reads mapping was about 50, with 99.73% of the genomic regions covered by at least four reads, suggesting high concordance and completeness of the ZB-1
genome assembly (Table 7). Based on the result of short reads alignment, SNP calling was performed using samtools (v1.7)69, which showed an extremely low rate of heterozygosis SNP
(0.000563%) and rare homology SNP (<10). In addition, the _k_-mer based method Merqury was also used to estimate the quality of the genome assembly70, which showed high base accuracy of
the genome assembly (Q-value about 50). MAPPING OF RNA-SEQ DATA The RNA-seq data generated by this work, _i.e_., for the ZB-1 samples of flower, sepal, stem, and boll, were aligned to the
genome assembly using HiSat2 (v2.2.1) with default settings71. According to the statistics of the alignment, 94.5% (boll), 96.2% (flower), 91.9% (sepal), and 94.4% (stem) of the properly
paired short reads from RNA-seq could be mapped to the _pseudo_-chromosomes. BUSCO ASSESSMENT The BUSCO (Benchmarking Universal Single-Copy Orthologs) method was also used to evaluate the
completeness of the genome assembly72,73. The ZB-1 genome assembly captured 99.4% of the BUSCO 1614 reference gene set (embryophyta_odb10), indicating the completeness of the ZB-1 genome
assembly (Table 8). EVALUATION OF THE GENOME ANNOTATION The genomic features of the coding genes were compared between _G. arboreum_ ZB-1 and closely related species within the genus
_Gossypium_, including _G. arboreum_ SXY1, _G. hirsutum_, _G. raimondii_, and _G. stocksii_, with the versions of their genome assemblies as aforementioned. According to the distribution of
the exon number and the length of CDS, exon, gene, and intron, the gene features of ZB-1 were highly consistent with other cotton species (Fig. 5), suggesting the overall high accuracy of
the gene annotation. The predicted proteome of ZB-1 was also subjected to a search against the BUSCO 1614 reference gene set (embryophyta_odb10) using HMMER (v3.4) (http://hmmer.org/). With
the recommended cutoff by BUSCO, this assessment showed that 98.4% of the reference genes have been annotated in this study. EVALUATION OF THE SNP CALLING About 95.1 Gb of short reads from
sequencing the genome library of SXY1 were downloaded from SRA with the accession number SRR1306194374. About 97.5% of the reads were retained after quality control using Trimmomatic (v0.33)
(SLIDINGWINDOW:5:20 LEADING:5 TRAILING:5 MINLEN:50)75. 97.1% of the clean data were aligned to the ZB-1 genome sequence using BWA (v0.7.17) with default settings. GATK (v4.0.5.1) tools
_MarkDuplicates_ and _HaplotypeCaller_ were used to remove the redundancy raised by PCR amplification and identify the structure variations (default parameters), respectively76. According to
the result, 88.1% (16,752/19,017) of the cSNPs identified from genome sequence alignment (by MUMMER) were validated by the short reads, suggesting high accuracy of the estimation of the SNP
occurrence rate between SXY1 and ZB-1 and also high quality of the ZB-1 genome assembly. CODE AVAILABILITY All the bioinformatics tools were used according to the users’ manuals. Otherwise
specified in the context, default settings were used during data processing. No specific custom code has been developed in this study. REFERENCES * Huang, G., Huang, J. Q., Chen, X. Y. &
Zhu, Y. X. Recent Advances and Future Perspectives in Cotton Research. _Annu Rev Plant Biol_ 72, 437–462 (2021). Article CAS PubMed Google Scholar * Wendel, J. F., Brubaker, C. L. &
Seelanan, T. The Origin and Evolution of _Gossypium_. in _Physiology of Cotton_ (eds. Stewart, J. M., Oosterhuis, D. M., Heitholt, J. J. & Mauney, J. R.) 1-18 (Springer Netherlands,
Dordrecht, 2010). * Chen, Z. J. _et al_. Genomic diversifications of five _Gossypium_ allopolyploid species and their impact on cotton improvement. _Nat Genet_ 52, 525–533 (2020). Article
CAS PubMed PubMed Central Google Scholar * Grover, C. E. _et al_. Dual Domestication, Diversity, and Differential Introgression in Old World Cotton Diploids. _Genome Biology and
Evolution_ 14, evac170 (2022). Article PubMed PubMed Central Google Scholar * Yuan, D. _et al_. Parallel and Intertwining Threads of Domestication in Allopolyploid Cotton. _Adv Sci
(Weinh)_ 8, 2003634 (2021). Article PubMed Google Scholar * Wen, X. _et al_. A comprehensive overview of cotton genomics, biotechnology and molecular biological studies. _Sci China Life
Sci_ 66, 2214–2256 (2023). Article PubMed Google Scholar * Huang, G. _et al_. Genome sequence of _Gossypium herbaceum_ and genome updates of _Gossypium arboreum_ and _Gossypium hirsutum_
provide insights into cotton A-genome evolution. _Nat Genet_ 52, 516–524 (2020). Article CAS PubMed PubMed Central Google Scholar * Feng, Y. L. _et al_. Assembly and phylogenomic
analysis of cotton mitochondrial genomes provide insights into the history of cotton evolution. _Crop Journal_ 11, 1782–1792 (2023). Article Google Scholar * Wu, Y. _et al_. An insight
into the gene expression evolution in _Gossypium_ species based on the leaf transcriptomes. _BMC Genomics_ 25, 179 (2024). Article CAS PubMed PubMed Central Google Scholar * Flagel, L.
E. & Wendel, J. F. Evolutionary rate variation, genomic dominance and duplicate gene expression evolution during allotetraploid cotton speciation. _New Phytol_ 186, 184–193 (2010).
Article CAS PubMed Google Scholar * Rapp, R. A., Udall, J. A. & Wendel, J. F. Genomic expression dominance in allopolyploids. _BMC Biol_ 7, 18 (2009). Article PubMed PubMed Central
Google Scholar * Ke, L. _et al_. Differential transcript profiling alters regulatory gene expression during the development of _Gossypium arboreum_, _G.stocksii_ and somatic hybrids. _Sci
Rep_ 7, 3120 (2017). Article ADS PubMed PubMed Central Google Scholar * Li, B., Zhu, S., Wang, H. & Zhang, B. Bred and studied of a new allotetraploid cotton germplasm with
glandless seeds/glanded plant trait. _Acta Gossypii Sinica_ 3, 27–32 (1991). Google Scholar * Gao, W. _et al_. Development of the engineered “glanded plant and glandless seed” cotton. _Food
Chem (Oxf)_ 5, 100130 (2022). CAS PubMed Google Scholar * Nie, Y. & Liu, J. The botanical and agronomic characters of new allotetraploid germplasm of _Gossypium arboreum_ x _G.
stocksii_. _Journal of Huazhong Agricultural University_ 14, 333–337 (1995). Google Scholar * Chen, Y. _et al_. A new synthetic amphiploid (AADDAA) between _Gossypium hirsutum_ and _G.
arboreum_ lays the foundation for transferring resistances to _Verticillium_ and drought. _PLoS One_ 10, e0128981 (2015). Article PubMed PubMed Central Google Scholar * Khan, Z. _et al_.
Genome editing in cotton: challenges and opportunities. _Journal of Cotton Research_ 6 (2023). * Dong, Y. _et al_. Parental legacy versus regulatory innovation in salt stress responsiveness
of allopolyploid cotton (_Gossypium_) species. _Plant J_ 111, 872–887 (2022). Article CAS PubMed PubMed Central Google Scholar * Peng, Z. _et al_. Expression patterns and functional
divergence of homologous genes accompanied by polyploidization in cotton (_Gossypium hirsutum_ L.). _Sci China Life Sci_ 63, 1565–1579 (2020). Article CAS PubMed Google Scholar * Ke, L.,
Jiang, Q., Wang, R., Yu, D. & Sun, Y. Plant regeneration via somatic embryogenesis in diploid cultivated cotton (_Gossypium arboreum_ L.). _Plant Cell, Tissue and Organ Culture (PCTOC)_
148, 177–188 (2022). Article CAS Google Scholar * Li, F. _et al_. Genome sequence of the cultivated cotton _Gossypium arboreum_. _Nat Genet_ 46, 567–572 (2014). Article CAS PubMed
Google Scholar * Du, X. _et al_. Resequencing of 243 diploid cotton accessions based on an updated A genome identifies the genetic basis of key agronomic traits. _Nat Genet_ 50, 796–802
(2018). Article CAS PubMed Google Scholar * Wang, M. _et al_. Genomic innovation and regulatory rewiring during evolution of the cotton genus _Gossypium_. _Nat Genet_ 54, 1959–1971
(2022). Article CAS PubMed Google Scholar * Wang, M. _et al_. Comparative Genome Analyses Highlight Transposon-Mediated Genome Expansion and the Evolutionary Architecture of 3D Genomic
Folding in Cotton. _Mol Biol Evol_ 38, 3621–3636 (2021). Article CAS PubMed PubMed Central Google Scholar * Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one
FASTQ preprocessor. _Bioinformatics_ 34, 884–890 (2018). Article Google Scholar * Belton, J. M. _et al_. Hi-C: a comprehensive technique to capture the conformation of genomes. _Methods_
58, 268–276 (2012). Article CAS PubMed Google Scholar * Wingett, S. _et al_. HiCUP: pipeline for mapping and processing Hi-C data. _F1000Res_ 4, 1310 (2015). Article PubMed PubMed
Central Google Scholar * Cheng, H., Concepcion, G. T., Feng, X., Zhang, H. & Li, H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. _Nat Methods_ 18,
170–175 (2021). Article CAS PubMed PubMed Central Google Scholar * Zhang, X., Zhang, S., Zhao, Q., Ming, R. & Tang, H. Assembly of allele-aware, chromosomal-scale autopolyploid
genomes based on Hi-C data. _Nat Plants_ 5, 833–845 (2019). Article CAS PubMed Google Scholar * Durand, N. C. _et al_. Juicebox Provides a Visualization System for Hi-C Contact Maps with
Unlimited Zoom. _Cell Syst_ 3, 99–101 (2016). Article CAS PubMed PubMed Central Google Scholar * Benson, G. Tandem repeats finder: a program to analyze DNA sequences. _Nucleic Acids
Res_ 27, 573–580 (1999). Article CAS PubMed PubMed Central Google Scholar * Bao, W., Kojima, K. K. & Kohany, O. Repbase update, a database of repetitive elements in eukaryotic
genomes. _Mob DNA_ 6, 11 (2015). Article PubMed PubMed Central Google Scholar * Xu, Z. & Wang, H. LTR_FINDER: an efficient tool for the prediction of full-length LTR
retrotransposons. _Nucleic Acids Res_ 35, 265–268 (2007). Article Google Scholar * Price, A. L., Jones, N. C. & Pevzner, P. A. _De novo_ identification of repeat families in large
genomes. _Bioinformatics_ 21(Suppl 1), 351–358 (2005). Article Google Scholar * Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. _Bioinformatics_ 26, 2460–2461
(2010). Article CAS PubMed Google Scholar * Stanke, M. & Waack, S. Gene prediction with a hidden Markov model and a new intron submodel. _Bioinformatics_ 19(Suppl 2), 215–225 (2003).
Article Google Scholar * Majoros, W. H., Pertea, M. & Salzberg, S. L. TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-finders. _Bioinformatics_ 20, 2878–2879
(2004). Article CAS PubMed Google Scholar * Korf, I. Gene finding in novel genomes. _BMC Bioinformatics_ 5, 59 (2004). Article PubMed PubMed Central Google Scholar * Parra, G.,
Blanco, E. & Guigo, R. GeneID in _Drosophila_. _Genome Res_ 10, 511–515 (2000). Article CAS PubMed PubMed Central Google Scholar * Yu, D. _et al_. Multi-omics assisted
identification of the key and species-specific regulatory components of drought-tolerant mechanisms in _Gossypium stocksii_. _Plant Biotechnol J_ 19, 1690–1692 (2021). Article PubMed
PubMed Central Google Scholar * Paterson, A. H. _et al_. Repeated polyploidization of _Gossypium_ genomes and the evolution of spinnable cotton fibres. _Nature_ 492, 423–427 (2012).
Article ADS CAS PubMed Google Scholar * Zhang, T. _et al_. Sequencing of allotetraploid cotton (_Gossypium hirsutum_ L. acc. TM-1) provides a resource for fiber improvement. _Nat
Biotechnol_ 33, 531–537 (2015). Article CAS PubMed Google Scholar * Argout, X. _et al_. The genome of _Theobroma cacao_. _Nat Genet_ 43, 101–108 (2011). Article CAS PubMed Google
Scholar * Altschul, S. F. _et al_. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. _Nucleic Acids Res_ 25, 3389–3402 (1997). Article CAS PubMed PubMed
Central Google Scholar * Birney, E., Clamp, M. & Durbin, R. GeneWise and Genomewise. _Genome Res_ 14, 988–995 (2004). Article CAS PubMed PubMed Central Google Scholar * Grabherr,
M. G. _et al_. Full-length transcriptome assembly from RNA-Seq data without a reference genome. _Nat Biotechnol_ 29, 644–652 (2011). Article CAS PubMed PubMed Central Google Scholar *
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. _Nat Biotechnol_ 37, 907–915 (2019). Article
CAS PubMed PubMed Central Google Scholar * Kovaka, S. _et al_. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. _Genome Biol_ 20, 278 (2019). Article CAS
PubMed PubMed Central Google Scholar * Haas, B. J., Zeng, Q., Pearson, M. D., Cuomo, C. A. & Wortman, J. R. Approaches to Fungal Genome Annotation. _Mycology_ 2, 118–141 (2011).
Article CAS PubMed Google Scholar * Huerta-Cepas, J. _et al_. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502
viruses. _Nucleic Acids Res_ 47, D309–D314 (2019). Article CAS PubMed Google Scholar * Paysan-Lafosse, T. _et al_. InterPro in 2022. _Nucleic Acids Res_ 51, D418–D427 (2023). Article
CAS PubMed Google Scholar * Chan, P. P., Lin, B. Y., Mak, A. J. & Lowe, T. M. tRNAscan-SE 2.0: improved detection and functional classification of transfer RNA genes. _Nucleic Acids
Res_ 49, 9077–9096 (2021). Article CAS PubMed PubMed Central Google Scholar * Griffiths-Jones, S. _et al_. Rfam: annotating non-coding RNAs in complete genomes. _Nucleic Acids Res_ 33,
D121–D124 (2005). Article CAS PubMed Google Scholar * Nawrocki, E. P. & Eddy, S. R. Infernal 1.1: 100-fold faster RNA homology searches. _Bioinformatics_ 29, 2933–2935 (2013).
Article CAS PubMed PubMed Central Google Scholar * Wang, Y. _et al_. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. _Nucleic Acids Res_ 40,
e49 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Hao, Z. _et al_. RIdeogram: drawing SVG graphics to visualize and map genome-wide data on the idiograms. _PeerJ
Comput Sci_ 6, e251 (2020). Article PubMed PubMed Central Google Scholar * Marcais, G. _et al_. MUMmer4: A fast and versatile genome alignment system. _PLoS Comput Biol_ 14, e1005944
(2018). Article PubMed PubMed Central Google Scholar * Yin, L. _et al_. rMVP: A Memory-efficient, Visualization-enhanced, and Parallel-accelerated Tool for Genome-wide Association Study.
_Genomics Proteomics Bioinformatics_ 19, 619–628 (2021). Article PubMed PubMed Central Google Scholar * _NCBI Sequence Read Archive_ https://identifiers.org/ncbi/insdc.sra:SRR27009933
(2023). * _NCBI Sequence Read Archive_ https://identifiers.org/ncbi/insdc.sra:SRR27009931 (2023). * _NCBI Sequence Read Archive_ https://identifiers.org/ncbi/insdc.sra:SRR27009932 (2023). *
_NCBI Sequence Read Archive_ https://identifiers.org/ncbi/insdc.sra:SRR27009934 (2023). * _NCBI Sequence Read Archive_ https://identifiers.org/ncbi/insdc.sra:SRR27009935 (2023). * _NCBI
Sequence Read Archive_ https://identifiers.org/ncbi/insdc.sra:SRR27009936 (2023). * _NCBI Sequence Read Archive_ https://identifiers.org/ncbi/insdc.sra:SRR27009937 (2023). * Yu, D. _ENA_
https://identifiers.org/insdc.gca:GCA_036320975.1 (2024). * Sun, R. _et al_. Chromosome-level genome assembly and annotation of a potential model organism _Gossypium arboreum_ ZB-1.
_Figshare_ https://doi.org/10.6084/m9.figshare.24736338 (2024). * Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. _Bioinformatics_ 25,
1754–1760 (2009). Article CAS PubMed PubMed Central Google Scholar * Li, H. _et al_. The sequence alignment/map format and SAMtools. _Bioinformatics_ 25, 2078–2079 (2009). Article
PubMed PubMed Central Google Scholar * Rhie, A., Walenz, B. P., Koren, S. & Phillippy, A. M. Merqury: reference-free quality, completeness, and phasing assessment for genome
assemblies. _Genome Biol_ 21, 245 (2020). Article CAS PubMed PubMed Central Google Scholar * Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory
requirements. _Nat Methods_ 12, 357–60 (2015). Article CAS PubMed PubMed Central Google Scholar * Manni, M., Berkeley, M. R., Seppey, M., Simao, F. A. & Zdobnov, E. M. BUSCO Update:
Novel and Streamlined Workflows along with Broader and Deeper Phylogenetic Coverage for Scoring of Eukaryotic, Prokaryotic, and Viral Genomes. _Mol Biol Evol_ 38, 4647–4654 (2021). Article
CAS PubMed PubMed Central Google Scholar * Manni, M., Berkeley, M. R., Seppey, M. & Zdobnov, E. M. BUSCO: Assessing Genomic Data Quality and Beyond. _Curr Protoc_ 1, e323 (2021).
Article PubMed Google Scholar * _NCBI Sequence Read Archive_ https://identifers.org/ncbi/insdc.sra:SRR13061943 (2020). * Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible
trimmer for Illumina sequence data. _Bioinformatics_ 30, 2114–20 (2014). Article CAS PubMed PubMed Central Google Scholar * Van der Auwera, G. A. _et al_. From FastQ data to high
confidence variant calls: the Genome Analysis Toolkit best practices pipeline. _Curr Protoc Bioinformatics_ 43, 11.10.1–11.10.33 (2013). PubMed Google Scholar Download references
ACKNOWLEDGEMENTS The authors would like to thank Dr. Wang Tingzhang and Dr. Hua Xuejun for their assistance during the gene annotation and manuscript writing processes. This work was
supported by the National the National Natural Science Foundation of China (32170623 and U190320) and the Fundamental Research Funds of Zhejiang Sci-Tech University (19042398-Y). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Plant Genomics & Molecular Improvement of Colored Fiber Laboratory, College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou,
310008, China Rongnan Sun, Yuqing Wu, Xinyu Zhang, Minghua Lv, Dongliang Yu & Yuqiang Sun Authors * Rongnan Sun View author publications You can also search for this author inPubMed
Google Scholar * Yuqing Wu View author publications You can also search for this author inPubMed Google Scholar * Xinyu Zhang View author publications You can also search for this author
inPubMed Google Scholar * Minghua Lv View author publications You can also search for this author inPubMed Google Scholar * Dongliang Yu View author publications You can also search for this
author inPubMed Google Scholar * Yuqiang Sun View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.Y. and Y.S. conceived and designed the
study; R.S., Y.W. and D.Y. analysed the sequencing data; X.Z. and M.L. collected the samples; R.S. and D.Y. wrote the draft manuscript; Y.S. and D.Y. modified the manuscript. All authors
have read and approved the final version for submission. CORRESPONDING AUTHORS Correspondence to Dongliang Yu or Yuqiang Sun. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sun, R., Wu, Y.,
Zhang, X. _et al._ Chromosome-level genome assembly and annotation of a potential model organism _Gossypium arboreum_ ZB-1. _Sci Data_ 11, 620 (2024).
https://doi.org/10.1038/s41597-024-03481-z Download citation * Received: 15 December 2023 * Accepted: 06 June 2024 * Published: 12 June 2024 * DOI: https://doi.org/10.1038/s41597-024-03481-z
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy
to clipboard Provided by the Springer Nature SharedIt content-sharing initiative