Relationship between body mass index and renal function deterioration among the taiwanese chronic kidney disease population
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT This study investigated the characteristics of patients with different chronic kidney disease (CKD) stages according to various body mass index (BMI) categories and determined the
influence of BMI in renal function deterioration. We conducted a multicenter, longitudinal cohort study based on the Epidemiology and Risk Factors Surveillance of CKD project (2008–2013) and
National Health Insurance Research Database (2001–2013). A total of 7357 patients with CKD aged 20–85 years from 14 hospitals were included in the study. A higher male sex, diabetes
mellitus (DM) and hypertension were noted among overweight and obese CKD patients, while more cancer prevalence was noted among underweight CKD patients. Charlson comorbidity index was
significantly higher and correlated with BMI among late CKD patients. Patients with BMI < 18.5 kg/m2 exhibited non-significantly higher events of eGFR decline events in both early and
late CKD stages than other BMI groups. BMI alone is not a determinant of CKD progression among our Taiwanese CKD patients. Obesity should be re-defined and body weight manipulation should be
individualized in CKD patients. SIMILAR CONTENT BEING VIEWED BY OTHERS ASSOCIATION OF TRIGLYCERIDE-GLUCOSE-BODY MASS INDEX WITH ALL-CAUSE AND CARDIOVASCULAR MORTALITY AMONG INDIVIDUALS WITH
CHRONIC KIDNEY DISEASE Article Open access 04 September 2024 POPULATION CHARACTERISTICS AND DIAGNOSIS RATE OF CHRONIC KIDNEY DISEASE BY EGFR AND PROTEINURIA IN JAPANESE CLINICAL PRACTICE:
AN OBSERVATIONAL DATABASE STUDY Article Open access 02 March 2024 PREDICTORS OF CHRONIC KIDNEY DISEASE SURVIVAL IN TYPE 2 DIABETES: A 12-YEAR RETROSPECTIVE COHORT STUDY UTILIZING ESTIMATED
GLOMERULAR FILTRATION RATE Article Open access 19 April 2024 INTRODUCTION Obesity, a global pandemic problem, is associated with various metabolic disorders and results in a shortened life
span related to adverse health consequences. In Taiwan, the prevalence of overweight and obesity among adults was reported to be 44.1%, of whom 50.8% were men and 36.9% were women, according
to 2005–2008 data1. Moreover, in a survey performed in 2002 and 2007 (Taiwanese Survey on Hypertension, Hyperglycemia, and Hyperlipidemia), the prevalence of obesity increased from 19.2% to
23.3% among men and from 13.4% to 19.0% among women2. The overweight and obesity rates in Taiwan are substantially higher than those in Japan, Singapore, and other neighboring Asian
countries. On the other hand, the prevalence of chronic kidney disease (CKD) in Taiwan is also increasing, with nearly 6 million people undergoing dialysis and approximately 2,000 people
newly diagnosed as having end-stage renal disease (ESRD) annually (http://www.tsn.org.tw/UI/K/K008.aspx). Since the numbers of obese patients with CKD and those undergoing dialysis are also
increasing in parallel, the diagnosis and precise management of obesity have become critical among these patients. Many studies have demonstrated that obesity is an important risk factor for
incident CKD3,4,5,6,7 and increased risk of ESRD8,9,10,11,12. Paradoxically, obesity itself in CKD and ESRD has been found to be associated with more favorable outcomes13,14. A reverse
obesity–mortality association has been consistently observed in patients with ESRD15,16,17; however, conflicting results have been observed among patients with CKD13,18,19,20. Body mass
index (BMI) is a globally accepted anthropometric measure for obesity classification. Recently, many studies have questioned the accuracy of BMI in obesity and excess body fat
assessment21,22,23. Whether BMI can influence the CKD progression among all stages of CKD in the Taiwanese population remains unclear. We conducted a multicenter, longitudinal cohort study
to investigate the characteristics of patients at all CKD stages (CKD stages 1–5 nondialysis [ND]) according to various BMI categories and to determine the influence of BMI in renal function
deterioration by using the data from the Epidemiology and Risk Factors Surveillance of CKD project (2008–2013) and National Health Insurance Research Database (NHIRD) (2001–2013). RESULTS
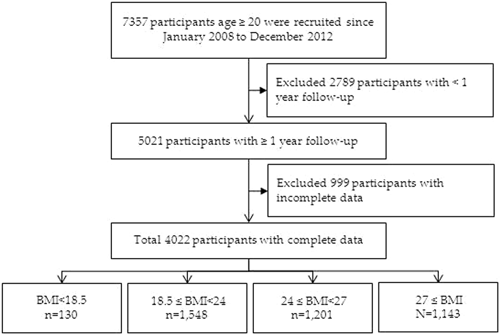
DEMOGRAPHIC CHARACTERISTICS OF THE PATIENTS A total of 7357 patients with CKD aged 20–85 years from 14 hospitals were included in the study. Patients with ESRD, defined as either receiving
maintenance dialysis during this period or having a kidney transplant, were excluded. After the exclusion of patients with less than 1 year of follow-up (n = 2789) and those with missing or
incomplete data (n = 999), 4022 patients with CKD were finally enrolled in this study (Fig. 1). Among these patients, 2008 had early-stage CKD (CKD stages 1, 2, and 3a) and 2014 had
late-stage CKD (CKD stages 3b, 4, and 5ND). The patients were continually traced from the baseline date to the end of the study period (June 18, 2015). The mean age of the cohort was 62.86 ±
14.93 years, and 56.9% of the patients were men. The mean baseline eGFR was 51.5 ± 36.15 mL/min per 1.73 m2. The baseline characteristics of patients according to baseline BMI are presented
in Table 1. For further analysis, we also present the baseline characteristics of patients with early-stage CKD (CKD stages 1–3a) in Table 2 and late-stage CKD (CKD stages 3b–5) in Table 3.
The highest prevalence rates of overweight (24 ≤ BMI <27 kg/m2) and obesity (BMI ≥ 27 kg/m2) were observed among patients aged 45–64 years, with the values being 45% and 39.38%
respectively. We also noted a male predominance in BMI (62.7% for overweight and 56.43% for obesity). Patients with higher BMI exhibited significantly higher baseline DM and hypertension
than those with lower BMI (16.15% in the underweight group, compared with 36.05%, 47.29%, and 52.23% in the normal, overweight, and obesity groups, respectively). Furthermore, patients with
BMI < 18.5 kg/m2 exhibited a characteristically higher cancer prevalence rate (18.46%) than the other groups; the prevalence rates were 9.75%, 9.16%, and 8.22% in the normal, overweight,
and obesity groups, respectively. Baseline coronary artery disease (CAD) and stroke did not differ between the groups. Similarly, the baseline Charlson comorbidity index was significantly
lower in the low and normal BMI groups (3.38 ± 2.50 and 3.53 ± 2.39, respectively), compared with the overweight and obesity groups (3.81 ± 2.46 and 3.73 ± 2.41, respectively). Furthermore,
the higher BMI groups had higher medication use and more smoking, alcohol consumption, and betel nut chewing habits. The biochemical analysis revealed significantly higher hemoglobin and
hematocrit levels, AC sugar, triglyceride, and uric acid levels in the higher BMI groups. No significant difference was observed in baseline cholesterol, electrolytes, albumin, UPCR, and
eGFR between the groups (Table 1). We subgrouped our cohort into early- and late-stage CKD (CKD stages 1–3a and 3b–5) and studied their characteristics according to different BMI categories
(Tables 2 and 3). Similar age and sex prevalence rates were observed after subgrouping. Among patients with early-stage CKD, those in the lower BMI group exhibited significantly higher
cancer prevalence (16.67% in with the underweight group) than those in the higher BMI groups (8.64% in the normal, 8.95% in the overweight, and 6.76% in the obesity groups; p = 0.032).
However, among patients with late-stage CKD, those in the lower BMI group had nonsignificantly higher cancer prevalence than those in the higher BMI groups (20% in the underweight group vs.
10.8% in the normal group, 11.39% in the overweight group, and 9.8% in the obesity group; p = 0.08). Higher DM and hypertension rates were observed in overweight and obese patients with
early- and late-stage CKD. The Charlson comorbidity index did not differ significantly with BMI among patients with early-stage CKD (2.57 ± 2.31 in the underweight group vs. 2.69 ± 2.12 in
the normal group, 2.92 ± 2.19 in the overweight group, and 2.86 ± 2.12 in the obesity group; p = 0.1773). However, a significantly higher Charlson comorbidity index was observed among
overweight and obese patients with late-stage CKD (4.07 ± 2.47 in the underweight group vs. 4.32 ± 2.37 in the normal group, 4.71 ± 2.39 in the overweight group, and 4.67 ± 2.35 in the
obesity group; p = 0.003). The initial stages of CKD did not differ significantly among patients with early-stage CKD; by contrast, they differed significantly among patients with late-stage
CKD according to BMI (p = 0.0004). More prevalent CKD stage 3b (18.57% in the underweight group vs. 26.51% in the normal group, 33.3% in the overweight group, and 31.58% in the obesity
group) and stage 4 (28.5% in the underweight group vs. 36.43% in the normal group, 36.35% in the overweight group, and 38.11% in the obesity group) were observed among patients in the higher
BMI group; by contrast, 52.8% of patients in the underweight group had CKD stage 5 compared with 37.06%, 30.32%, 30.31% in the normal, overweight, and obesity groups, respectively. We
analyzed the proportion of eGFR progression events during the follow-up period among patients with CKD stages 1–5 in different BMI groups. We also executed further subgroup analysis of the
proportion of eGFR progression among patients with early-stage CKD (stages 1–3a) and late-stage CKD (stages 3b–5) in different BMI groups. CORRELATION BETWEEN BMI AND CKD PROGRESSION AMONG
PATIENTS WITH CKD STAGES 1–5 Table 4 presents the proportion of eGFR progression events in patients with CKD stages 1–5. The study outcomes are presented as ORs, and the normal group was
used as the reference group to calculate the OR for each group. The underweight group exhibited the highest proportion of events (25%) compared with the normal (19%), overweight (19%), and
obesity (18%) groups. The ORs of eGFR progression events were 1.44 (0.95, 2.18), 0.99 (0.81, 1.2), and 0.95 (0.78, 1.16) in the underweight, overweight, and obesity groups, respectively.
After adjusting for age, sex, previous diabetes, CAD, stroke, cancer, high blood pressure, Charlson score, TB, COPD, ACEI, ARB, fibrate, smoking, alcohol consumption, betel nut chewing,
baseline UPCR, and baseline eGFR, we observed that the OR was 1.35 (0.87, 2.10) in the underweight group compared with 1.02 (0.83, 1.25) and 0.95 (0.77, 1.18) in the overweight and obesity
groups, respectively (Fig. 2). CORRELATION BETWEEN BMI AND CKD PROGRESSION AMONG PATIENTS WITH EARLY-STAGE CKD (STAGES 1–3A) Table 5 presents the proportion of eGFR deterioration events in
patients with CKD stages 1–3a. The study outcomes are presented as ORs, and the normal group was used as the reference group to calculate the OR for each group. The underweight group
exhibited the highest proportion of eGFR deterioration events (20%) compared with the normal (13%), overweight (13%), and obesity (12%) groups. The ORs of eGFR progression events were 1.67
(0.86, 3.25), 0.98 (0.71, 1.34), and 0.91 (0.66, 1.26) in the underweight, overweight, and obesity groups, respectively. After adjusting for age, sex, previous diabetes, CAD, stroke, cancer,
high blood pressure, Charlson score, TB, COPD, ACEI, ARB, fibrate, smoking, alcohol consumption, betel nut chewing, baseline UPCR, and baseline eGFR, we determined that the ORs were 1.42
(0.70, 2.88), 1.06 (0.76, 1.47), and 0.92 (0.66, 1.30) in the underweight, overweight, and obesity groups, respectively (Fig. 3). CORRELATION BETWEEN BMI AND CKD PROGRESSION AMONG PATIENTS
WITH LATE-STAGE CKD (STAGE 3B–5) Table 6 presents the proportion of eGFR deterioration events in patients with CKD stages 3b–5. The study outcomes are presented as ORs, and the normal group
was used as the reference group to calculate the OR for each group. The underweight group exhibited the highest proportion of eGFR deterioration events (30%) compared with the normal (25%),
overweight (25%), and obesity (25%) groups. The ORs of the eGFR progression events were 1.29 (0.76, 2.21), 1.01 (0.79, 1.30), and 1.02 (0.79, 1.31) in the underweight, overweight, and
obesity groups, respectively. After adjusting for age, sex, previous diabetes, CAD, stroke, cancer, high blood pressure, Charlson score, TB, COPD, ACEI, ARB, fibrate, smoking, alcohol
consumption, betel nut chewing, baseline UPCR, and baseline eGFR, we observed that the ORs were 1.33 (0.74, 2.39), 1.04 (0.79, 1.36), and 1.04 (0.79, 1.39) in the underweight, overweight,
and obesity groups, respectively (Fig. 4). DISCUSSION In this prospective cohort study, we evaluated the characteristics of a CKD cohort according to various BMI categories. Subsequently, we
investigated the association between BMI and the risk of eGFR decline among patients with different CKD stages. The highest prevalence of overweight and obesity was observed among men and
the working age group (45–64 years old) in both early- and late-stage CKD. Previous studies conducted on the Japanese24,25 and Malay populations26 have demonstrated a male sex-specific
association between BMI and kidney disease; similarly, from our baseline data, we observed a higher male prevalence among overweight and obese CKD patients. The mechanism underlying the male
sex-specific association between higher BMI and CKD remains unclear; however, several studies have identified that BMI reflects visceral fat more efficiently in men than in women27,28.
Generally, men exhibit a higher risk of kidney disease and develop the disease earlier in life than women because of hormonal and lifestyle influences29,30,31,32. We observed a significantly
higher prevalence of DM and hypertension among overweight and obese patients with CKD (both early- and late-stage CKD). This observational association might not represent cause and effect;
since obesity itself is associated with various adverse sequelae from metabolic syndrome, as well as from comorbidities including DM and hypertension33,34, and all these conditions are
associated with CKD. Patients with early-stage CKD with a lower BMI exhibited significantly higher cancer prevalence; however, non-significantly higher prevalence was observed in patients
with late-stage CKD. This might also not represent a causal relation; nevertheless, many studies have revealed a bidirectional association between CKD and cancer35,36. Cancer patients with
lower BMI exhibited associated nutritional disturbances and tended to have reduced renal function status from nutritional and specified therapies. A Korean study reported a significantly
higher risk of CKD and proteinuria among cancer survivors37. Furthermore, both CKD and ESRD are higher risks from a number of malignancies38,39. We calculated the Charlson scores at
different CKD stages, which did not differ significantly among patients with early-stage CKD in different BMI categories. However, the median Charlson score increased significantly among
overweight and obese patients with late-stage CKD (Fig. 5), which demonstrates the presence of more comorbidity among these patients. The initial CKD stages did not differ significantly with
BMI among patients with early-stage CKD (Table 2). However, in patients with late-stage CKD, the prevalence of the initial stages of CKD differed significantly according to BMI (Table 6),
with a higher prevalence of CKD stages 3b and 4 observed in overweight and obese patients. We observed a significantly higher number of patients with CKD stage 5 to be underweight (BMI <
18.5). The reason for this finding is unknown, and additional studies are required to confirm whether any nutritional and concurrent comorbidity might play a role in such lower BMI
prevalence among these patients. An obesity paradox was supposed for stage 5 CKD, because low BMI represents more uremia-associated inflammatory cachexia and high BMI represents fewer uremic
consequences and more favorable health40,41,42. More emphasis on improved and adequate nutrition is required for these patients with advanced-stage CKD compared with control obesity in
healthy patients. A follow-up analysis revealed non-significantly increased CKD progression events among underweight patients compared with overweight and obese patients in both early and
late CKD stages. This observed association persisted after adjustment for age, sex, previous diabetes, CAD, stroke, cancer, high blood pressure, Charlson score, TB, COPD, ACEI, ARB, fibrate,
smoking, alcohol consumption, betel nut chewing, baseline UPCR, and baseline eGFR, and it was consistently present in the subgroup analysis among all patients with CKD. Our results are
consistent with the so-called obesity paradox43 among patients with CKD; we found non-significantly higher kidney disease progression events among underweight patients compared with
overweight and obese patients in all stages of CKD. Although several mechanisms have been proposed for patients with late-stage CKD42, we observed the same paradox among patients with
early-stage CKD, which might be explained by the older age and higher cancer prevalence among patients with lower BMI. In patients with late-stage CKD, lower BMI was associated with poor
nutritional status42 or higher prevalence of metabolically obese normal-weight individuals with a higher comorbidity burden44. Our study results are consistent with those of other population
studies. Data from a nationally representative cohort of US veterans with eGFR < 60 mL/min indicated a U-shaped association between BMI and the risk of renal progression20, with
deteriorating outcomes observed in individuals with BMI < 25 kg/m2 and BMI ≥ 35 kg/m2; these data demonstrate that overweight or mild obesity (30–35 kg/m2) results in the most favorable
outcomes and that in advanced CKD stages (eGFR < 30 mL/min), even morbid obesity (BMI ≥ 35 kg/m2) is not associated with adverse outcomes. A similar U-shaped association between an
increased risk of progressive CKD and lowest BMI levels was noted in a large population-based cohort study in Israel45. A retrospective study in the Taiwanese general population reported
that waist-to-height ratio (WHtR), rather than BMI, increased as the prevalence of CKD increased46. Other studies have reported that WHtR and waist circumference, but not BMI, were
associated with mortality in patients with CKD and ESRD47,48. Although the recent global definition uses BMI as a standard measure of obesity, obesity is affected by muscle mass, peripheral
and abdominal adipose tissue mass, and bone; thus, the results should be considered with the condition49. Central obesity has been proved to be more vulnerable to metabolic syndrome and
obesity-related diseases, whereas peripheral obesity and higher muscle mass appear protective50,51,52. BMI failed to represent central obesity because of the variation in individual body
composition and contribution. This explains the nonsignificant association between BMI and CKD progression through all stages of CKD in our study. Our study has several limitations. Because
we studied a prevalent cohort of patients with CKD, we could not determine the effects of obesity on incident CKD. We used the study participants’ personal identities to link health care
databases, and because the NHI database is based on the reporting data system and does not include the population not under medical health care, the study result may not represent the whole
population; however, the missing population is negligible. We used only BMI to determine obesity, which may not be an ideal marker of obesity among our cohort; nevertheless, because BMI is
generally accepted as a predominant index to establish obesity in clinical practice, our results have direct clinical relevance. Because the blood and urine samples of study participants
were collected from individual hospitals and sent to the research center, the use of different equipment and personnel of individual hospitals may have resulted in measurement errors.
Furthermore, we did not determine the influence of low and high BMI on mortality outcomes. MATERIALS AND METHODS ETHICS STATEMENT The study was reviewed and approved by the institutional
ethical committee of Taipei Medical University - Shuang Ho Hospital (TMU-JIRB 201204036), Tri-Service General Hospital (TSGHIRB100-05-197), Cardinal Tien Hospital (TMU-JIRB 201204035),
Changhua Christian Hospital (CCHIRB 20405), Kaohsiung Medical University Chung-Ho Memorial Hospital (KMUHIRB 20120019), Kaohsiung Chang Gung Memorial Hospital (101-1096B), National Cheng
Kung University Hospital (A-ER-101-117) and China Medical University Hospital (DMR101-IRB2-273(CR-1)). After a complete explanation of the study, written informed consent was obtained from
all participants. All clinical and biological samples were collected after patient consent. All the study methods were in accordance with the guidelines approved by the joint institutional
review board and aforementioned governmental regulations. STUDY POPULATION We conducted a multicenter, longitudinal cohort study using data from the Epidemiology and Risk Factors
Surveillance of CKD database (2008–2013) managed by the Bureau of Health Promotion, Ministry of Health and Welfare, Taiwan. After excluding patients with incomplete or missing data, we
linked the biochemical laboratory data to the NHIRD from 2001 to 2013. The same medical laboratory criteria and protocol have been used in our study hospitals, and the serum creatinine
levels derived from different hospitals can be compared and standardized with each other. In this study, we measured CKD progression at the individual level. In addition, the patients were
reexamined in the same hospital to control the individual variation. All patients provided informed consent before data collection. MEASUREMENTS AND VARIABLE DEFINITIONS The patients’
demographic, clinical, and health-related behavior data were collected using a structured questionnaire. The questionnaire collected data on age, sex, cigarette smoking, alcohol consumption,
betel nut chewing, personal and family comorbid conditions, and medication use. Physical examination included anthropometry, blood pressure measurement, pulse rate measurement, and systemic
examination. Height was measured in centimeters by using a wall-mounted measuring tape, and weight was measured in kilograms by using a digital scale (SECA, model 782 2321009; Vogel &
Halke, Germany). BMI was classified into the following groups: <18.5 kg/m2 (underweight), 18.5–23.9 kg/m2 (normal), 24–26.9 kg/m2 (overweight), and ≥27 kg/m2 (obesity). Glycemia, blood
pressure, and lipid control conditions were classified as intensive and poor. Proteinuria status was determined using the urine protein-to-creatinine ratio (UPCR). CKD was defined according
to the Kidney Disease Outcomes Quality Initiative guidelines53 and was evaluated using the estimated glomerular filtration rate (eGFR), which was calculated using the Chronic Kidney
Disease-Epidemiology Collaboration equation: eGFR (mL/min/1.73 m2) = 141 × min (SCr/ƙ, 1)α × max (serum creatinine/ƙ, 1) − 1.209 × 0.993Age × 1.018 (if female) and × 1.159 (if black), where
SCr denotes the serum creatinine level (mg/dL), ƙ = 0.7 (for women) and 0.9 (for men), α = −0.329 (for women) and −0.411(for men), min denotes the minimum of SCr/ƙ or 1, and max denotes the
maximum of SCr/ƙ or 154. CKD was classified as follows: CKD stage 1, eGFR ≥ 90 mL/min/1.73 m2 and the presence of kidney damage (i.e., proteinuria dipsticks ≥1+, UPCR ≥ 150, or urine
albumin-to-creatinine ratio [UACR] ≥30); CKD stage 2, eGFR = 60–89 mL/min/1.73 m2 and the presence of kidney damage (i.e., proteinuria dipsticks ≥1+, UPCR ≥ 150, or UACR ≥ 30); CKD stage 3a,
eGFR = 45–59 mL/min/1.73 m2; CKD stage 3b, eGFR = 30–44 mL/min/1.73 m2; CKD stage 4, eGFR = 15–29 mL/min/1.73 m2; and CKD stage 5, eGFR < 15 mL/min/1.73 m2 55. Renal progression was
defined as an average eGFR decline by more than 5 mL/min/1.73 m2 per year or into the dialysis stage56. STATISTICAL ANALYSIS Consistent with the study hypothesis, all analyses were
stratified according to BMI. We examined BMI as quartiles: <18.5, 18.5–23.9, 24–26.9, and ≥27 kg/m2. The characteristics of different BMI groups were compared using the chi-squared test
for categorical variables and ANOVA for continuous variables. The odds ratio (OR) (95% confidence interval) of CKD was calculated for each BMI category. Next, we explored the data for
confounding and effect modification in stratified analyses. After adjusting for all covariates, we used the multivariate logistic model with stepwise variable selection models to evaluate
the association between BMI and eGFR decline. In our subsequent multivariate modeling, we considered covariates including age; sex; comorbid conditions such as diabetes mellitus (DM),
stroke, and cancer; the Charlson comorbidity index; use of antihypertensive medications (e.g., ACEI/ARB and loop diuretics) within the previous 1 year; and baseline CKD stage. The SAS
statistical package (Version 9.3, SAS Institute Inc., Cary, NC, USA) was used for all statistical tests. Results with P < 0.05 were considered statistically significant. CONCLUSIONS In
conclusion, the definition and classification of obesity among patients with CKD should be intensively re-determined, because misdiagnosis can lead to inappropriate clinical decisions and
might deteriorate patients’ prognosis. The anthropomorphic measures alternate to BMI should be established from randomized controlled clinical trials among the CKD population. CHANGE HISTORY
* _ 13 FEBRUARY 2020 An amendment to this paper has been published and can be accessed via a link at the top of the paper. _ REFERENCES * Yeh, C. J., Chang, H. Y. & Pan, W. H. Time
trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: from NAHSIT 1993–1996 to NAHSIT 2005–2008. _Asia Pac J Clin Nutr_ 20, 292–300 (2011). PubMed Google Scholar
* HPA. Taiwanese Survey on Hypertension, Hyperglycemia, and Hyperlipidemia. (Health Promotion Administration, Ministry of Health and Welfare, 2007). * Foster, M. C. _et al_. Overweight,
obesity, and the development of stage 3 CKD: the Framingham Heart Study. _Am J Kidney Dis_ 52, 39–48, https://doi.org/10.1053/j.ajkd.2008.03.003 (2008). Article PubMed PubMed Central
Google Scholar * Ejerblad, E. _et al_. Obesity and risk for chronic renal failure. _J Am Soc Nephrol_ 17, 1695–1702, https://doi.org/10.1681/ASN.2005060638 (2006). Article CAS PubMed
Google Scholar * Fox, C. S. _et al_. Predictors of new-onset kidney disease in a community-based population. _JAMA_ 291, 844–850, https://doi.org/10.1001/jama.291.7.844 (2004). Article CAS
PubMed Google Scholar * Kramer, H. _et al_. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. _Am J Kidney Dis_ 46, 587–594,
https://doi.org/10.1053/j.ajkd.2005.06.007 (2005). Article PubMed Google Scholar * Pinto-Sietsma, S. J. _et al_. A central body fat distribution is related to renal function impairment,
even in lean subjects. _Am J Kidney Dis_ 41, 733–741 (2003). Article PubMed Google Scholar * Pscheidt, C. _et al_. Sex- and Time-Dependent Patterns in Risk Factors of End-Stage Renal
Disease: A Large Austrian Cohort with up to 20 Years of Follow-Up. _PLoS One_ 10, e0135052, https://doi.org/10.1371/journal.pone.0135052 (2015). Article CAS PubMed PubMed Central Google
Scholar * Hsu, C. Y., McCulloch, C. E., Iribarren, C., Darbinian, J. & Go, A. S. Body mass index and risk for end-stage renal disease. _Ann Intern Med_ 144, 21–28 (2006). Article
PubMed Google Scholar * Franceschini, N. _et al_. Adiposity patterns and the risk for ESRD in postmenopausal women. _Clin J Am Soc Nephrol_ 10, 241–250,
https://doi.org/10.2215/CJN.02860314 (2015). Article PubMed Google Scholar * Panwar, B. _et al_. Obesity, metabolic health, and the risk of end-stage renal disease. _Kidney Int_ 87,
1216–1222, https://doi.org/10.1038/ki.2014.384 (2015). Article PubMed Google Scholar * Kramer, H. _et al_. Waist Circumference, Body Mass Index, and ESRD in the REGARDS (Reasons for
Geographic and Racial Differences in Stroke) Study. _Am J Kidney Dis_ 67, 62–69, https://doi.org/10.1053/j.ajkd.2015.05.023 (2016). Article PubMed Google Scholar * Kovesdy, C. P.,
Anderson, J. E. & Kalantar-Zadeh, K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. _Am J Kidney Dis_ 49, 581–591,
https://doi.org/10.1053/j.ajkd.2007.02.277 (2007). Article PubMed Google Scholar * Kalantar-Zadeh, K., Abbott, K. C., Salahudeen, A. K., Kilpatrick, R. D. & Horwich, T. B. Survival
advantages of obesity in dialysis patients. _Am J Clin Nutr_ 81, 543–554 (2005). Article CAS PubMed Google Scholar * Kakiya, R. _et al_. Body fat mass and lean mass as predictors of
survival in hemodialysis patients. _Kidney Int_ 70, 549–556, https://doi.org/10.1038/sj.ki.5000331 (2006). Article CAS PubMed Google Scholar * Glanton, C. W. _et al_. Factors associated
with improved short term survival in obese end stage renal disease patients. _Ann Epidemiol_ 13, 136–143 (2003). Article PubMed Google Scholar * Kalantar-Zadeh, K. _et al_. Association of
morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. _Am J Kidney Dis_ 46, 489–500, https://doi.org/10.1053/j.ajkd.2005.05.020 (2005). Article
PubMed Google Scholar * Madero, M. _et al_. Body mass index and mortality in CKD. _Am J Kidney Dis_ 50, 404–411, https://doi.org/10.1053/j.ajkd.2007.06.004 (2007). Article PubMed
Google Scholar * Kwan, B. C., Murtaugh, M. A. & Beddhu, S. Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. _Clin J Am Soc Nephrol_ 2,
992–998, https://doi.org/10.2215/CJN.04221206 (2007). Article PubMed Google Scholar * Lu, J. L., Kalantar-Zadeh, K., Ma, J. Z., Quarles, L. D. & Kovesdy, C. P. Association of body
mass index with outcomes in patients with CKD. _J Am Soc Nephrol_ 25, 2088–2096, https://doi.org/10.1681/ASN.2013070754 (2014). Article PubMed PubMed Central Google Scholar * Tomiyama,
A. J., Hunger, J. M., Nguyen-Cuu, J. & Wells, C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005–2012. _Int J Obes (Lond)_ 40, 883–886,
https://doi.org/10.1038/ijo.2016.17 (2016). Article CAS Google Scholar * Peterson, M. D., Al Snih, S., Stoddard, J., Shekar, A. & Hurvitz, E. A. Obesity misclassification and the
metabolic syndrome in adults with functional mobility impairments: Nutrition Examination Survey 2003-2006. _Prev Med_ 60, 71–76, https://doi.org/10.1016/j.ypmed.2013.12.014 (2014). Article
PubMed Google Scholar * Caleyachetty, R., Meunnig, P. & Kengne, A. P. Misclassification of cardiometabolic health when using body mass index categories. _Int J Obes (Lond)_ 40, 1332,
https://doi.org/10.1038/ijo.2016.65 (2016). Article CAS Google Scholar * Iseki, K. Body mass index and the risk of chronic renal failure: the Asian experience. _Contrib Nephrol_ 151,
42–56, https://doi.org/10.1159/000095318 (2006). Article PubMed Google Scholar * Iseki, K. _et al_. Body mass index and the risk of development of end-stage renal disease in a screened
cohort. _Kidney Int_ 65, 1870–1876, https://doi.org/10.1111/j.1523-1755.2004.00582.x (2004). Article PubMed Google Scholar * Shankar, A. _et al_. Association between body mass index and
chronic kidney disease in men and women: population-based study of Malay adults in Singapore. _Nephrol Dial Transplant_ 23, 1910–1918, https://doi.org/10.1093/ndt/gfm878 (2008). Article
PubMed Google Scholar * Horber, F. F., Gruber, B., Thomi, F., Jensen, E. X. & Jaeger, P. Effect of sex and age on bone mass, body composition and fuel metabolism in humans. _Nutrition_
13, 524–534 (1997). Article CAS PubMed Google Scholar * Kuk, J. L., Lee, S., Heymsfield, S. B. & Ross, R. Waist circumference and abdominal adipose tissue distribution: influence of
age and sex. _Am J Clin Nutr_ 81, 1330–1334 (2005). Article CAS PubMed Google Scholar * Hopper, J. Jr, Trew, P. A. & Biava, C. G. Membranous nephropathy: its relative benignity in
women. _Nephron_ 29, 18–24 (1981). Article PubMed Google Scholar * Gretz, N., Zeier, M., Geberth, S., Strauch, M. & Ritz, E. Is gender a determinant for evolution of renal failure? A
study in autosomal dominant polycystic kidney disease. _Am J Kidney Dis_ 14, 178–183 (1989). Article CAS PubMed Google Scholar * Tozawa, M. _et al_. Influence of smoking and obesity on
the development of proteinuria. _Kidney Int_ 62, 956–962, https://doi.org/10.1046/j.1523-1755.2002.00506.x (2002). Article PubMed Google Scholar * Neugarten, J., Acharya, A. &
Silbiger, S. R. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. _J Am Soc Nephrol_ 11, 319–329 (2000). CAS PubMed Google Scholar * Henegar, J. R.,
Bigler, S. A., Henegar, L. K., Tyagi, S. C. & Hall, J. E. Functional and structural changes in the kidney in the early stages of obesity. _J Am Soc Nephrol_ 12, 1211–1217 (2001). CAS
PubMed Google Scholar * Haslam, D. W. & James, W. P. Obesity. _Lancet_ 366, 1197–1209, https://doi.org/10.1016/S0140-6736(05)67483-1 (2005). Article PubMed Google Scholar * Stengel,
B. Chronic kidney disease and cancer: a troubling connection. _J Nephrol_ 23, 253–262 (2010). PubMed PubMed Central Google Scholar * Porta, C., Cosmai, L., Gallieni, M., Pedrazzoli, P.
& Malberti, F. Renal effects of targeted anticancer therapies. _Nat Rev Nephrol_ 11, 354–370, https://doi.org/10.1038/nrneph.2015.15 (2015). Article CAS PubMed Google Scholar * Shin,
H. Y., Linton, J. A., Shim, J. Y. & Kang, H. T. Cancer survivors aged 40 years or elder are associated with high risk of chronic kidney disease: the 2010–2012 Korean National Health and
Nutrition Examination Survey. _Asian Pac J Cancer Prev_ 16, 1355–1360 (2015). Article PubMed Google Scholar * Wong, G. _et al_. Association of CKD and cancer risk in older people. _J Am
Soc Nephrol_ 20, 1341–1350, https://doi.org/10.1681/ASN.2008090998 (2009). Article CAS PubMed PubMed Central Google Scholar * Jorgensen, L., Heuch, I., Jenssen, T. & Jacobsen, B. K.
Association of albuminuria and cancer incidence. _J Am Soc Nephrol_ 19, 992–998, https://doi.org/10.1681/ASN.2007060712 (2008). Article PubMed PubMed Central Google Scholar * Morley, J.
E., Thomas, D. R. & Wilson, M. M. Cachexia: pathophysiology and clinical relevance. _Am J Clin Nutr_ 83, 735–743 (2006). Article CAS PubMed Google Scholar * Lecker, S. H., Goldberg,
A. L. & Mitch, W. E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. _J Am Soc Nephrol_ 17, 1807–1819, https://doi.org/10.1681/ASN.2006010083
(2006). Article CAS PubMed Google Scholar * Kalantar-Zadeh, K. _et al_. Risk factor paradox in wasting diseases. _Curr Opin Clin Nutr Metab Care_ 10, 433–442,
https://doi.org/10.1097/MCO.0b013e3281a30594 (2007). Article PubMed Google Scholar * Park, J. _et al_. Obesity paradox in end-stage kidney disease patients. _Prog Cardiovasc Dis_ 56,
415–425, https://doi.org/10.1016/j.pcad.2013.10.005 (2014). Article PubMed Google Scholar * Conus, F., Rabasa-Lhoret, R. & Peronnet, F. Characteristics of metabolically obese
normal-weight (MONW) subjects. _Appl Physiol Nutr Metab_ 32, 4–12, https://doi.org/10.1139/H07-926 (2007). Article PubMed Google Scholar * Vivante, A. _et al_. Body mass index in 1.2
million adolescents and risk for end-stage renal disease. _Arch Intern Med_ 172, 1644–1650, https://doi.org/10.1001/2013.jamainternmed.85 (2012). Article PubMed PubMed Central Google
Scholar * Li, W. C. _et al_. Association between waist-to-height ratio and chronic kidney disease in the Taiwanese population. _Intern Med J_ 44, 645–652, https://doi.org/10.1111/imj.12459
(2014). Article CAS PubMed Google Scholar * Kramer, H. _et al_. Association of waist circumference and body mass index with all-cause mortality in CKD: The REGARDS (Reasons for
Geographic and Racial Differences in Stroke) Study. _Am J Kidney Dis_ 58, 177–185, https://doi.org/10.1053/j.ajkd.2011.02.390 (2011). Article PubMed PubMed Central Google Scholar *
Postorino, M., Marino, C., Tripepi, G., Zoccali, C. & Group, C. W. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. _J Am Coll Cardiol_ 53,
1265–1272, https://doi.org/10.1016/j.jacc.2008.12.040 (2009). Article PubMed Google Scholar * Brown, R. N. _et al_. Body mass index has no effect on rate of progression of chronic kidney
disease in non-diabetic subjects. _Nephrol Dial Transplant_ 27, 2776–2780, https://doi.org/10.1093/ndt/gfr757 (2012). Article PubMed Google Scholar * Lee, M. J., Wu, Y. & Fried, S. K.
Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. _Mol Aspects Med_ 34, 1–11, https://doi.org/10.1016/j.mam.2012.10.001 (2013).
Article CAS PubMed Google Scholar * Mooney, S. J., Baecker, A. & Rundle, A. G. Comparison of anthropometric and body composition measures as predictors of components of the metabolic
syndrome in a clinical setting. _Obes Res Clin Pract_ 7, e55–66, https://doi.org/10.1016/j.orcp.2012.10.004 (2013). Article PubMed Google Scholar * Chang, S. H., Beason, T. S., Hunleth,
J. M. & Colditz, G. A. A systematic review of body fat distribution and mortality in older people. _Maturitas_ 72, 175–191, https://doi.org/10.1016/j.maturitas.2012.04.004 (2012).
Article PubMed PubMed Central Google Scholar * National Kidney, F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. _Am J
Kidney Dis_ 39, S1–266 (2002). Google Scholar * Levey, A. S. _et al_. A new equation to estimate glomerular filtration rate. _Ann Intern Med_ 150, 604–612 (2009). Article PubMed PubMed
Central Google Scholar * Levey, A. S. & Coresh, J. Chronic kidney disease. _Lancet_ 379, 165–180, https://doi.org/10.1016/S0140-6736(11)60178-5 (2012). Article PubMed Google Scholar
* Stevens, P. E. & Levin, A. & Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group, M. Evaluation and management of chronic kidney
disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. _Ann Intern Med_ 158, 825–830, https://doi.org/10.7326/0003-4819-158-11-201306040-00007
(2013). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by grants from the Health Promotion Administration, Ministry of Health and Welfare,
Institute for Biotechnology and Medicine Industry, Taiwan, ROC (MOHW104-HPA- H-114-134101). AUTHOR INFORMATION Author notes * Senyeong Kao and Yuh-Feng Lin contributed equally to this work.
AUTHORS AND AFFILIATIONS * Graduate Institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan Tian-Jong Chang, Jing-Quan Zheng, Senyeong Kao & Yuh-Feng Lin *
Performance Appraisal Section, Secretary Office, Shuang Ho Hospital, Taipei Medical University, Taipei, Taiwan Tian-Jong Chang * Division of Nephrology, Department of Internal Medicine,
School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan Cai-Mei Zheng, Mei-Yi Wu, Yung-Ho Hsu & Yuh-Feng Lin * Division of Nephrology, Department of Internal
Medicine, Shuang Ho Hospital, Taipei Medical University, Taipei, Taiwan Cai-Mei Zheng, Mei-Yi Wu, Yung-Ho Hsu & Yuh-Feng Lin * Graduate Institute of Clinical Medicine, College of
Medicine, Taipei Medical University, Taipei, Taiwan Cai-Mei Zheng & Yuh-Feng Lin * Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan
University, Taipei, Taiwan Tzu-Ting Chen & Yun-Chun Wu * School of Nursing, College of Nursing, Taipei Medical University, Taipei, Taiwan Yi-Lien Wu * Kidney Disease Prevention
Foundation, Taipei, Taiwan Yi-Lien Wu & Yuh-Feng Lin * Department of Ophthalmology, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan Hsin-Ting Lin *
Department of Critical Care Medicine, Shuang Ho Hospital, Taipei Medical University, Taipei, Taiwan Jing-Quan Zheng * School of Public Health, National Defense Medical Center, Taipei, Taiwan
Nain-Feng Chu, Sui-Lung Su & Senyeong Kao * Department of Medical Education and Research, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan Nain-Feng Chu * School of Public Health,
College of Public Health and Nutrition, Taipei Medical University, Taipei, Taiwan Yu-Me Lin & Hung-Yi Chiou * Division of Nephrology, Department of Medicine, Fu-Jen Catholic University
Hospital, School of Medicine, Fu-Jen Catholic University, Taipei, Taiwan Kuo-Cheng Lu * Division of Nephrology, Department of Medicine, Tri-Service General Hospital, National Defense Medical
Center, Taipei, Taiwan Jin-Shuen Chen & Yuh-Feng Lin * School of Public Health, Graduate Institute of Clinical Medical Science, China Medical University, Taichung, Taiwan Fung-Chang
Sung * Division of Nephrology, Kaohsiung Chang Gung Memorial Hospital, Chang Gung Medical University, Kaohsiung, Taiwan Chien-Te Lee * The Division of Nephrology, Changhua Christian
Hospital, Changhua, Taiwan Yu Yang * Division of Nephrology, Department of Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan Shang-Jyh Hwang * Division of Nephrology,
Department of Internal Medicine, Cheng Kung University Medical Center, Tainan, Taiwan Ming-Cheng Wang Authors * Tian-Jong Chang View author publications You can also search for this author
inPubMed Google Scholar * Cai-Mei Zheng View author publications You can also search for this author inPubMed Google Scholar * Mei-Yi Wu View author publications You can also search for this
author inPubMed Google Scholar * Tzu-Ting Chen View author publications You can also search for this author inPubMed Google Scholar * Yun-Chun Wu View author publications You can also
search for this author inPubMed Google Scholar * Yi-Lien Wu View author publications You can also search for this author inPubMed Google Scholar * Hsin-Ting Lin View author publications You
can also search for this author inPubMed Google Scholar * Jing-Quan Zheng View author publications You can also search for this author inPubMed Google Scholar * Nain-Feng Chu View author
publications You can also search for this author inPubMed Google Scholar * Yu-Me Lin View author publications You can also search for this author inPubMed Google Scholar * Sui-Lung Su View
author publications You can also search for this author inPubMed Google Scholar * Kuo-Cheng Lu View author publications You can also search for this author inPubMed Google Scholar *
Jin-Shuen Chen View author publications You can also search for this author inPubMed Google Scholar * Fung-Chang Sung View author publications You can also search for this author inPubMed
Google Scholar * Chien-Te Lee View author publications You can also search for this author inPubMed Google Scholar * Yu Yang View author publications You can also search for this author
inPubMed Google Scholar * Shang-Jyh Hwang View author publications You can also search for this author inPubMed Google Scholar * Ming-Cheng Wang View author publications You can also search
for this author inPubMed Google Scholar * Yung-Ho Hsu View author publications You can also search for this author inPubMed Google Scholar * Hung-Yi Chiou View author publications You can
also search for this author inPubMed Google Scholar * Senyeong Kao View author publications You can also search for this author inPubMed Google Scholar * Yuh-Feng Lin View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS For research articles with several authors, a short paragraph specifying their individual contributions
must be provided. The following statements should be used “Senyeong Kao and Cai-Mei Zheng conceived and designed the experiments; Cai-Mei Zheng, Hsin-Ting Lin, Jing-Quan Zheng, Nain-Feng
Chu, Yu-Me Lin, Sui-Lung Su, Kuo-Cheng Lu, Jin-Shuen Chen, Fung-Chang Sung, Chien-Te Lee, Yu Yang, Shang-Jyh Hwang, Ming-Cheng Wang, Yung-Ho Hsu and Hung-Yi Chiou performed the experiments;
Tian-Jong Chang, Mei-Yi Wu, Tzu-Ting Chen, Yun-Chun Wu and Yi-Lien Wu analyzed the data; Yuh-Feng Lin contributed reagents/materials/analysis tools and coordinate experiment performance;
Tian-Jong Chang wrote the paper”. Authorship must be limited to those who have contributed substantially to the work reported. CORRESPONDING AUTHORS Correspondence to Senyeong Kao or
Yuh-Feng Lin. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chang, TJ., Zheng, CM., Wu, MY. _et al._ Relationship between body mass index and
renal function deterioration among the Taiwanese chronic kidney disease population. _Sci Rep_ 8, 6908 (2018). https://doi.org/10.1038/s41598-018-24757-6 Download citation * Received: 12
October 2017 * Accepted: 22 March 2018 * Published: 02 May 2018 * DOI: https://doi.org/10.1038/s41598-018-24757-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative