Microplastic in riverine fish is connected to species traits
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Microplastic is a contaminant of concern worldwide. Rivers are implicated as major pathways of microplastic transport to marine and lake ecosystems, and microplastic ingestion by
freshwater biota is a risk associated with microplastic contamination, but there is little research on microplastic ecology within freshwater ecosystems. Microplastic uptake by fish is
likely affected by environmental microplastic abundance and aspects of fish ecology, but these relationships have rarely been addressed. We measured the abundance and composition of
microplastic in fish and surface waters from 3 major tributaries of Lake Michigan, USA. Microplastic was detected in fish and surface waters from all 3 sites, but there was no correlation
between microplastic concentrations in fish and surface waters. Rather, there was a significant effect of functional feeding group on microplastic concentration in fish. _Neogobius
melanostomus_ (round goby, a zoobenthivore) had the highest concentration of gut microplastic (19 particles fish−1) compared to 10 other fish taxa measured, and had a positive linear
relationship between body size and number of microplastic particles. Surface water microplastic concentrations were lowest in the most northern, forested watershed, and highest in the most
southern, agriculturally dominated watershed. Results suggest microplastic pollution is common in river food webs and is connected to species feeding characteristics. Future research should
focus on understanding the movement of microplastic from point-source and diffuse sources and into aquatic ecosystems, which will support pollution management efforts on inland waters.
SIMILAR CONTENT BEING VIEWED BY OTHERS MICROPLASTIC BURDEN IN MARINE BENTHIC INVERTEBRATES DEPENDS ON SPECIES TRAITS AND FEEDING ECOLOGY WITHIN BIOGEOGRAPHICAL PROVINCES Article Open access
04 December 2023 CURRENT LEVELS OF MICROPLASTIC POLLUTION IMPACT WILD SEABIRD GUT MICROBIOMES Article Open access 27 March 2023 FIELD EVIDENCE FOR MICROPLASTIC INTERACTIONS IN MARINE BENTHIC
INVERTEBRATES Article Open access 22 October 2021 INTRODUCTION In the mid-1900s, plastic became an integral component of human cultures and commerce globally1. Plastic contributes to ~10%
of all municipal waste2 and 50–80% of waste on beaches and in the oceans3. Plastic litter is an emerging concern in ecosystems worldwide. Approximately 20 million tons of plastic enters the
marine environment each year4, and plastic litter is predicted to outweigh fish in the ocean by the year 20505. Plastic is abundant in the most remote, un-inhabited parts of the world such
as the Barents Sea (Artic Ocean)6, Henderson Island (South Pacific)7, and the deep ocean8,9. Sources of plastic litter to aquatic ecosystems include wastewater treatment plant effluent10,11,
industrial production12, synthetic textiles13, and the breakdown of larger anthropogenic litter (AL; trash) into smaller pieces14,15,16. While a growing body of research shows plastic is
ubiquitous globally, its biological and ecological effects are less well known. Microplastic (particles <5 mm) is a focus of research on interactions between plastic and biota, including
microbes, invertebrates, fish, birds, and aquatic mammals17,18,19,20. Microplastic can adsorb hydrophobic compounds such as persistent organic pollutants and contaminants of emerging concern
(_e.g_. Triclosan and polyaromatic hydrocarbons (PAHs))21,22,23. Once ingested, compounds can be desorbed in the anaerobic environment of the gut and absorbed by animal tissues21,23. This
may accelerate bioaccumulation of microplastic and adsorbed compounds as they move through food webs via trophic transfer24. For example, up to 60 ng g−1 dry weight of pyrene (a PAH) was
measured in the gill tissue of a filter-feeding mussel (_Mytilus galloprovincialis_ Lamarck) after consuming microplastic exposed to pyrene22. Ingestion of microplastic could also reduce
nutrient assimilation via digestive tract blockages and irritation of epithelial lining25,26. Finally, microplastic supports unique microbial communities compared to natural habitats and
substrates20,27 and could facilitate pathogenic ‘hitch-hikers’ such as the bacteria Campylobacteraceae and _Vibrio_10,19,20. Although microplastic ecology is a rapidly growing field of
research, most studies have focused on marine organisms and habitats, with fewer studies conducted in rivers. Understanding the abundance, movement, and biological interactions of
microplastic in freshwaters is critical to documenting its global impacts28. In addition, because freshwater ecosystems are closely connected to the terrestrial landscape and have much
smaller volumes of water than the oceans, freshwaters represent important sites for prevention and management of microplastic. Landscape features in a watershed (_e.g_., land-use, riparian
vegetation, and geomorphology) influence particle transport and concentration in rivers29,30,31,32,33, but few studies have examined how landscape features influence the abundance and
biological interactions of microplastic in freshwaters. Microplastic originates from point and non-point sources. Earlier studies have focused on point sources of microplastic pollution such
as WWTP effluent10,34,35, while non-point sources of pollution are less well understood. Recent evidence suggests non-point sources include application of biosolids to agricultural
fields36, atmospheric deposition37, and stormwater runoff38. Landscape features that serve as sinks for other types of fine particles most likely promote deposition of microplastic, such as
dams, lakes, and low-velocity zones39. Understanding the composite effect of landscape sources and sinks on microplastic abundances in freshwater food webs requires comparisons across
watersheds of different land-use types40. The rapidly growing field of microplastic ecology has allowed for developments in methodology and thereby facilitated new approaches to data
collection and processing41,42,43. Many studies of microplastic use neuston or plankton nets to collect samples44. However, Barrows _et al_.42 compared ‘grab’ samples of 1 L of seawater to
the conventional neuston net approach and found grab samples collected 3 orders of magnitude (5.9 ± 4.4 SD L−1) more microplastic than the net approach (0.005 ± 0.004 SD L−1). The authors
concluded most microplastic escaped the neuston net (0.363 mm mesh) and that grab samples collected a wider size range of microplastic, which better reflect microplastic abundance in the
environment42. In addition to changes in data collection procedures, contamination of samples with microplastic is common. Sources include airborne particles in the lab and field, as well as
microplastic in reagent chemicals and filtered lab water45,46. Previous research accounts for contamination in each step10, but no previous studies have quantified the size, shape, and
color of contamination particles compared to environmental samples. This comparison will help isolate the sources of contamination and more accurately account for contaminants in field
samples. In this study, we measured microplastic abundance in fish and water from three major tributaries of Lake Michigan, which spanned a land-use gradient. We expected _(H_1) that
microplastic abundance in fish would increase with body size and trophic position, and _(H_2) that a positive relationship would be observed between microplastic concentrations in fish
digestive tissue and the water column. In addition, we hypothesized _(H_3) that microplastic would predominantly be small or medium sized fibers across river sites and fish taxa, with a
similar composition as a recent study using the same grab sample approach in the Gulf of Maine43. Finally, _(H_4) we predicted that analytical controls (to account for contamination) would
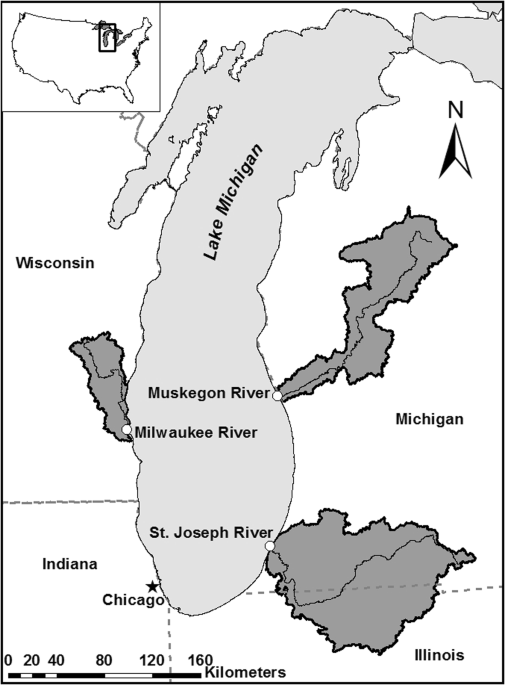
show substantially fewer microplastic fibers and would be distinct in size, color, and relative composition compared to environmental samples. METHODS STUDY SITES The Muskegon River (MG),
Milwaukee River (MK), and St. Joseph River (SJ) are major tributaries of Lake Michigan, USA (Fig. 1). We selected these sites to span a land-use gradient dominated by forest (MG),
urban-agriculture (MK), and agriculture (SJ; Table 1). Dominant land-use categories were determined by calculating relative abundance of land-use in forest, urban, and agriculture categories
within each watershed using the 2011 National Land Cover Dataset in ArcMap Geographic Information Systems (GIS)47. All field work was conducted upriver of each river mouth to ensure surface
water current was unidirectional toward Lake Michigan. The Muskegon River was sampled within Muskegon State Park, 1.1 km upstream from the river mouth in Muskegon, MI (43°13′56.2″N,
86°19′40.1″W). Milwaukee River field work was conducted 1.5 km upstream from the river mouth, just downstream of the confluence of the MK and Menomonee Rivers in Milwaukee, WI (43°01′49.8′N,
87°54′27.9″W). The St. Joseph River field site was 0.93 km upstream from the river mouth in Benton Harbor and St. Joseph, MI (42°06′44.4″N, 86°28′41.7″W). All field work was conducted with
approval of state and local officials. FISH AND WATER COLLECTION Fish were opportunistically collected with wading seine nets adjacent to MG and SJ water collection sites and 1.5 km
downstream from the MK water collection site. Fish were preliminarily identified in the field and up to 15 fish per taxa were euthanized with MS-222 (Tricaine-S; 0.25 g L−1) while the
remaining fish were released. Fish were preserved in 70% ethanol in the field and transported to the laboratory where they were identified to species or genus48 and processed for
microplastic gut content. All methods were carried out in accordance with ethical guidelines and regulations and approved by Loyola University Chicago’s Institutional Animal Care and Use
Committee. Microplastic was collected from surface water habitats via grab samples with 2 L glass bottles (_n_ = 4 bottles per site) from the MG, MK, and SJ Rivers during summer 2016.
Bottles were rinsed three times with DI water filtered through a 0.363 µm mesh in the lab pre-sampling. Collection bottles were filled with water directly below the river surface along the
left and right side of river channels (_n_ = 2 bottles per in-stream river location), capped immediately to prevent atmospheric contamination of microplastic, and transported to the
laboratory for microplastic processing42. MICROPLASTIC QUANTIFICATION AND CHARACTERIZATION In the laboratory, the body length of each fish was measured, the digestive tracts were dissected,
and the tissue was placed in individual clean beakers. Digestive tissue was dried at 75 °C for at least 24 h and underwent wet peroxide oxidation (0.05 M Fe(II) and 30% H2O2) at 75 °C to
dissolve organic material49. Microplastic is resistant to wet peroxide oxidation49. Samples were filtered onto gridded 0.45 µm filters (WhatmanTM, Pittsburgh, Pennsylvania, USA). Filters
were examined at 25–50 × magnification under dissecting microscopes and checked two separate times to confirm microplastic counts were consistent and conservative. Microplastic was counted
and categorized as either fiber, fragment, bead, foam, or film and classified into a color category20,50. Due to the high abundance of fibers, length was measured on a randomly selected
sub-sample of fibers on each filter. Fiber length was measured along the longest dimension with an ocular micrometer or estimated using the filter grid width (3.2 mm)42. We measured size and
color of 554 fibers from fish (_n_ = 819 counted). Fish were classified into functional feeding groups (FFG) and assigned a trophic fraction based on collective data available on FishBase51
coupled with visual identification of macroinvertebrates in gut content post wet peroxide oxidation (Table 2). A total of 17 fish were classified as detritivores, 30 as omnivores, and 27 as
zoobenthivores (Table 2). Microplastic concentration in fish taxa and FFG were calculated as the No. microplastic fish−1. River surface water samples were vacuum filtered onto gridded 0.45
µm filters (~300 mL filter−1) and oven dried at 75 °C for 24 h42. Microplastic was quantified as explained above. Microplastic concentration was calculated by dividing the total number of
microplastic in each sample by the sample’s total volume (L). Microplastic polymer type was identified using Fourier Transform Infrared Spectroscopy (FTIR) on randomly selected microplastic
from environmental samples. This technique produces infrared absorption bands that are unique to each polymer type. The small size of most of the microplastic and impurities (_e.g_., organic
material and minerals) that can adhere to the microplastic is challenging for FTIR14,52,53. Of 160 number of fibers analyzed by FTIR, 5.6% were successfully identified. QUANTIFICATION OF
LABORATORY MICROPLASTIC CONTAMINATION We carried out filter controls to account for microplastic contamination associated with surface water samples. We placed gridded 0.45 µm filters onto
the vacuum filtration apparatus and rinsed the collection cup with 0.363 µm filtered DI water (_n_ = 10 filter controls). In addition, a second set of controls were completed to account for
microplastic contamination from the digestion and filtering processes (_i.e_., ‘digestion control’) that fish samples were exposed to. Twenty mL of 30% H2O2 and 20 mL of 0.05 M Fe(II)
solution were added to a clean beaker, heated at 75 °C, and vacuum filtered onto a gridded 0.45 µm filter (_n_ = 10). Microplastic was quantified and characterized as explained for surface
water and fish samples. Microplastic contamination (mean No. filter−1) consisted of 2.3 (±0.63 SE) and 4 (±0.39 SE) fibers for filter and digestion controls, respectively. Mean fiber
contamination from each control was accounted for in each sample type (_i.e_., digestion control was used for fish and filter control was used for grab samples). We corrected fiber color and
size category by removing fibers from fish (4) and surface water samples (2) following the color and size class frequencies recorded on control filters. Finally, we compared the abundance,
category, size, and color of contamination fibers to those found in environmental samples. STATISTICAL ANALYSES We compared microplastic concentration in fish among sites and taxa using both
one-way ANOVA and Kruskal-Wallis analyses with _anova(lm())_ and _kruskal.test()_ using the R statistical program54. We used the same test to compare surface water microplastic
concentrations among sites. Presentation of both non-parametric and parametric analyses were included to balance the interpretation of the lower power non-parametric test with the robust
parametric analyses since data sets were a mix of both normal and non-normal Gaussian distributions, which were similar to analyses conducted with our previous work55. To identify which
sites were significantly different from one another, pairwise post-tests were conducted between sites with both _pairwise.t.test()_ with a Bonferroni correction (parametric) and
_post.hoc.kruskal.nemenyi.test()_ (non-parametric) in ‘PMCMR’ R56. Microplastic concentration categorized by fish FFG (_i.e_., detritivore, omnivore, zoobenthivore) was non-normal and
analyzed with the Kruskal-Wallis test54 followed by _post.hoc.kruskal.nemenyi.test()_ to determine which FFG was significantly different from one another. We conducted linear regression
analyses between fish microplastic concentration and surface water microplastic concentration using _lm()_. We also conducted linear regression analyses between fish traits (_i.e_., body
size and trophic fraction) and microplastic concentrations in fish with linear regression models to determine if microplastic abundance in fish increased with increasing fish body size and
trophic fraction. To determine if larger fish ingested larger fibers, fiber length and fish body length were also analyzed with linear regression models. All fish trait regression models
were conducted with pooled and individual species data, with the exception of fish trophic fraction (_i.e_., each taxa was assigned one trophic fraction). Microplastic category, size class,
and color patterns were analyzed with Chi-square test of independence after data were converted to ratios for each sampling site with _chisq.test()_. This analysis also included data
collected from the Gulf of Maine using the same grab sampling and laboratory processing methods as used in our study42. These analyses allowed for a test of independence to determine if
microplastic category, size class, or color patterns were independent of sampling sites57. To identify if microplastic patterns in the environment were similar to patterns found in
laboratory controls, Chi-square test of independence analyses were conducted with microplastic category, size class, and color data (surface water and fish) and their respective controls
(_i.e_., filter and digestion). Microplastic concentration comparisons between environmental data and controls were analyzed with student’s t-test (_t.test()_) and Wilcoxon rank-sum test,
(_wilcox.test()_), after data were found to have mixed normality post Shapiro-Wilk normality tests54. All analyses were conducted using the ‘base’ package in R version 3.3.0 unless stated
otherwise. DATA AVAILABILITY Data will be made available when requested from corresponding author. ETHICAL STATEMENT All methods were carried out in accordance with ethical guidelines and
regulations and approved by Loyola University Chicago’s Institutional Animal Care and Use Committee. RESULTS MICROPLASTIC ABUNDANCE A total of 74 fish spanning eleven taxa were collected
throughout the study, with 10 taxa (85% of individuals) containing microplastic in their digestive tracts (Table 3). Microplastic abundance in fish was not significantly different among the
three study sites (_P_ > 0.05) and ranged from 10 (±2.3) to 13 (±1.6) microplastic particles fish−1 (Table 3; Fig. 2a). However, there was a significant difference in microplastic
concentration across fish species (Table 3; Fig. 3). Round goby (_Neogobius melanostomus_) microplastic concentration was significantly greater than fathead minnow (_Pimephales promelas_)
and white sucker (_catostomus commersonii_) (all _P_ < 0.05), with no microplastic in gizzard shad (_Dorosoma cepedianum_) (Fig. 3). Round goby was the only fish present across all three
sites, and gobies from SJ River had approx. 50% less microplastic concentration compared to those collected from the MG and MK Rivers (Suppl. Table 1). Microplastic concentrations in surface
waters were significantly different among the 3 sites (Fig. 2b, Table 3), and abundance in fish was unrelated to patterns in the water (_r_2 = −0.011, _P_ > 0.05). There was also a
significant effect of fish FFG on microplastic concentration in fish regardless of river site (Table 3), with zoobenthivores greater in microplastic concentration than detritivores (_P_ <
0.001). Zoobenthivore fish had significantly greater microplastic compared to the omnivore FFG in the MK River (_X_2 = 10.92, df = 2, _P_ < 0.01), whereas there was no difference in
microplastic abundance among FFG present in other river sites (all _P_ > 0.05; Suppl. Fig. 1). Omnivore fish concentration was significantly influenced by site (F = 4.92, df = 2, _P_ =
0.015), with omnivore fish from the MG River significantly greater in microplastic concentration compared to omnivore fish from the MK River (_P_ = 0.018) but was similar in concentration to
fish from the SJ River (_P_ > 0.05). Zoobenthivore fish in the MK River had the greatest microplastic concentration compared to zoobenthivore fish from MG and SJ Rivers; however, this
comparison was not statistically significant (F = 2.169, df = 2, _P_ > 0.05). Fiber polymer composition from fish digestive tissues consisted of polyethylene, polyacrylonitrile,
polyacetal, and polyvinyl acetate (Suppl. Table 2). There were some links between fish body size and trophic fraction and the abundance of microplastic in digestive tissue. For example, gut
microplastic in round goby increased with body length (_r_2 = 0.393; _P_ = 0.010; Suppl. Table 3; Fig. 4b), but fish body size was not related to microplastic abundance in other taxa or when
taxa were pooled (Suppl. Table 3; Fig. 4a). Microplastic abundance in fish was also positively related to an increase in fish trophic fraction, although the model explained a small portion
of the variation in the data (_r_2 = 0.050; _P_ = 0.032; Fig. 4c). Microplastic abundance in zoobenthivores was positively related to increasing fish body length (_r_2 = 0.188; _P_ = 0.014;
Suppl. Table 3), which was most likely driven by the round goby (classified as zoobenthivore; Table 2). There were no such patterns observed for the other FFG (Suppl. Table 3). Fiber length
within fish digestive tracts had no relationship with fish body length, indicating fibers of all size classes were found equally in small and large fish (Suppl. Table 4). CHARACTERIZATION OF
MICROPLASTIC Microplastic category, size class, and color patterns were significantly different in surface water river sites and the Gulf of Maine42 (Fig. 5, Suppl. Table 5, Suppl. Figs 2
and 3). Fibers comprised approximately 97–100% of all microplastic found in the water and fish (Suppl. Fig. 2). Fragments comprised 1.5–3% of microplastic collected from water in the MK and
SJ Rivers respectively. Foam was only found in the MK River and accounted for 0.4% of the water column microplastic at that site. Fragments were also rare in fish and accounted for
approximately 2.5–3% of the microplastic (Suppl. Fig. 2). The relative abundance of fiber colors significantly depended on river site (_X_2 = 66.06, df = 6, _P_ < 0.001), with clear
fibers dominant at the SJ River (approx. 80%) and blue fibers (approx. 44%) most common at the MG River (Suppl. Fig. 3a). In contrast, fiber color patterns were similar in fish across sites,
with clear and blue fibers predominant (Suppl. Fig. 3b). A total of 526 (out of 980) fibers were classified as small (<1.5 mm), medium (1.6–3.2 mm), or large (>3.3 mm). Small fibers
were the most common size found across water samples and fish (Fig. 5). However, medium sized fibers were common from water samples at the SJ River but not at the other sites (Fig. 5a).
Surface water microplastic category, color, and fiber size class patterns in the Gulf of Maine were similar to data from the MG, MK, and SJ Rivers (Fig. 5a; Suppl. Figs 2a and 3a).
CHARACTERIZING MICROPLASTIC IN LABORATORY CONTROLS Analyses of microplastic in control procedures could help reveal potential sources of microplastic contamination. Microplastic
concentrations were significantly lower in laboratory controls compared to environmental samples (Fig. 5a; tRiver = −3.544, df = 72.5, _P_ < 0.001; tFish = 9.563, df = 37.4, _P_ <
0.001). Microplastic concentrations on surface water (approx. 16 ± 4 pieces filter−1) and fish filters (approx. 13 ± 0.7 pieces filter−1) were 8× and 3× greater than filter controls (2 ± 0.4
pieces filter−1) and digestion controls (4 ± 0.63 pieces filter−1; Fig. 6a) respectively. Microplastic composition in filter and digestion controls was different in size class and color
patterns compared to water column and fish results (Suppl. Table 6; Fig. 6b,c). Fibers were the dominant microplastic type found in controls, which was similar to surface water and fish
(Fig. 6b). Small fibers were the dominant size class found across environmental samples and controls. However, large fibers were more common in filter controls than surface water samples
(Fig. 6c). Clear was the dominant color in both surface water and filter control samples (Fig. 6d), but purple and red/clear bi-colored fibers were more common in filter controls compared to
surface water samples. Similarly, red and gray fibers were common in the water samples, but not in filter controls (Fig. 6d). Clear and blue fibers were the most common fibers in both fish
and digestion controls, but digestion controls were characterized by more blue/clear fibers compared to fibers from fish, which had more gray fibers (Fig. 6d). DISCUSSION Microplastic
pollution is pervasive worldwide, but research on its abundance, movement, and biological interactions in freshwater ecosystems is newly emerging. In this study, we present evidence that
microplastic is present in major tributaries of Lake Michigan, including fish digestive tissue and surface waters. Understanding the factors which drive microplastic patterns in freshwater
food webs will be critical for management policies. MICROPLASTIC IN FISH DIGESTIVE TRACTS WAS DIFFERENT AMONG SPECIES AND FEEDING GROUPS Plastic and other anthropogenic litter is commonly
found in marine fish58, but its abundance, introduction pathway, and physiological effects on freshwater fish are largely unknown. In this study, 85% of fish had microplastic in their
digestive tissues with an average of approximately 13 particles fish−1 with fibers the dominant microplastic. Pazos _et al_.59 found microplastic abundance was approximately 8–55 particles
fish−1, and fibers were also the dominant microplastic category across all fish taxa and from fish collected from the Rio de la Plata estuary, Argentina59, which is comparable to gut
microplastic in fish in our study. In contrast, Lusher _et al_.60 found 11% (84 of 761) of mesopelagic fish (_e.g_., glacial lantern fish (_Benthoseoma glacial_)), spotted barracudina
(_Arctozenus risso_), and lancet fish (_Notoscopelus kroyeri_) from the Northeast Atlantic Ocean had microplastic, with an average of 1.2 particles fish−1. Anthropogenic litter has been
found in 25–28% of fish collected from markets prepared for human consumption (seafood) in the USA and Indonesia ranging from 0.3–5.9 particles fish−1 58. These studies suggest microplastic
abundance in fish could vary across a gradient of aquatic habitats. Microplastic in riverine fish might be higher than marine fish due to lower water volume:surface water area ratio and
proximity to terrestrial microplastic point-sources; however, a systematic comparison has not yet been completed. Fish ecological and morphological traits were linked to gut microplastic
abundance from fish in Lake Michigan major tributaries. As we expected, microplastic abundance was positively related to fish trophic fraction. Zoobenthivores had greater microplastic
abundance compared to omnivores and detritivores, suggesting predator oriented fish may obtain microplastic via trophic transfer from prey items. Farrell _et al_.24 demonstrated the
potential for microplastic trophic transfer from mussels (_Mytilus edulis_) to crabs (_Carcinus maenas_). The authors reported microplastic concentrations were up to 163,111 particles mL−1
of crab haemolymph, which represented 0.24% of the microplastic retained by mussels. Two fish species of particular interest in our study were the round goby and the gizzard shad. The round
goby is invasive in the Great Lakes and is a voracious, opportunistic feeder61,62. This could explain why this species had the highest microplastic concentration in its digestive tissues
(approx. 20 particles fish−1). Microplastic abundance in round gobies increased with fish body length and suggested microplastic in these fish could accumulate with age. The gizzard shad was
the only taxa with no microplastic in its gastrointestinal tract, which could be attributed to its common diet of plants and detritus instead of aquatic fauna63. In contrast, Pazos _et
al_.59 found no relationship between microplastic abundance in fish and fish trophic group and body length in the Rio de la Plata estuary, Argentina. Ferreira _et al_.64 reported 64% of
_Cynoscion acoupa_ (Acoupa weakfish, Lacepéde) collected from the Goiana Estuary (South America) had microplastic in their stomachs, with adult fish containing more microplastic than
juvenile and sub-adult fish, suggesting fish ontogeny may play a role in microplastic abundance in fish. Collective findings from this study suggest fish species traits could help explain
microplastic abundance, but can be species dependent. Future research should include identifying functional traits linked with microplastic abundance in aquatic biota to help identify
wildlife taxa susceptible to microplastic. These results do not indicate the source(s) of microplastic in fish digestive tracts. Microplastic abundance in fish were similar across the 3
study sites, indicating microplastic concentration in the water column was not a reliable predictor of microplastic abundance in fish and may not be the primary source of ingested
microplastic for these taxa. In contrast, Pazos _et al_.59 found fish collected closer to WWTPs had significantly greater microplastic concentrations in their digestive tissues compared to
fish from further locations in the freshwater zone of the Rio de la Plata estuary, Argentina. Most studies have focused on quantifying microplastic abundance in fish and invertebrates65,66,
but little research has been conducted to identify the movement of microplastic between the environment and organisms24. Aquatic biota may ingest microplastic either directly from their
habitat (_i.e_., water column or benthos) or indirectly via trophic transfer. It is unknown the proportion of microplastic each pathway contributes to microplastic abundance in aquatic
biota, or what aspects of gut tissue anatomy may affect internal microplastic retention. Retention of microplastic in the gut could lead to irritation of the epithelial lining and blockage
in the digestive tract due to the shape and filamentous structure of microplastic25, which could impact aquatic biota ingestion and egestion rates. Future research is needed to explore the
pathways in which microplastic is incorporated into aquatic biota and food webs. SOURCES OF MICROPLASTIC TO RIVERS Watersheds with urban and agricultural land-use can have increased point-
and non-point sources of microplastic pollution. We noted higher microplastic concentration was in human-dominated watersheds relative to the forested watershed. Some microplastic sources in
urban and agricultural environments are well documented, while others will require additional research to measure. For example, McCormick _et al_. (2016) reported microplastic flux
downstream of WWTPs in the Chicago region, a densely populated area in the USA, was on average 1.3 million particles d−1 and was higher downstream of WWTP outfalls compared to upstream
locations. Litter can accumulate on freshwater beaches and in riparian zones of rivers27,67 and is similar in composition in benthic habitats28, suggesting urban terrestrial zones are
sources of AL, and possibly microplastic, to rivers. Plasticulture, the use of plastic film/mulch to cover and protect seedbeds, is a worldwide agricultural practice that could also
contribute microplastic to the landscape across large spatial scales. For example, plasticulture is present in 156,900 ha in the Shandong province, China68, creating the potential for
microplastic to be introduced throughout this region as large plastic degrades into smaller particles. Microplastic concentrations in biosolids (_i.e_., WWTP sludge converted to a
fertilizer) from seven WWTPs in Ireland ranged from 4,196–15,385 particles kg−1 dry weight36. Therefore, biosolid application on agricultural fields may be a non-point source of microplastic
pollution to nearby rivers and lakes; however, this has not yet been measured. COMPARING RESULTS TO LITERATURE AND CONSIDERATIONS FOR SCALING Comparing the values for water column
microplastic from this study to published results requires consideration of methodology and location. Previous work using neuston nets in freshwater ecosystems found lower values than those
documented with grab samples. In a study measuring microplastic abundance in 29 Laurention Great Lakes tributaries using neuston nets, microplastic concentrations ranged from 0.05–32
particles m−3 and was positively related to an increase in urban land-use40. The Seine River at Paris had microplastic concentrations of 3–106 particles m−3 11 with the same method. Finally,
McCormick _et al_. (2016) also used neuston nets to report microplastic concentrations downstream from WWTP effluent (0.80–11.22 particles m−3) were greater than concentrations upstream of
WWTPs (0.48–5.92 particles m−3). In contrast, a grab sample approach in the Gulf of Maine estimated microplastic concentrations at 3,400–10,000 particles m−3 42, approximately one order of
magnitude less than our results. Barrows _et al_.42 also reported similar composition of microplastic as our results, where particles were primarily small fibers (<1.5 mm) and most
commonly clear or blue42 (supporting _H_4). These collective results show microplastic pollution is abundant in freshwater habitats and that rivers are sources of microplastic pollution to
downstream ecosystems. Scaling up microplastic results to larger volumes of water and time periods will require careful attention to replication and consideration of distinct river habitats.
We did not extrapolate our water column samples to estimate flux (No. particles day−1) or export (No. particles km−2 d−1) due to the volume and lack of temporal replication in our surface
water collection. Estimates of flux and export are needed to inform global budgets of plastic movement in rivers. To do so, we suggest that more samples should be collected across the width
of a river, with depth-integrated collection, and at multiple times of year. In addition, we propose that investigators simultaneously measure microplastic at the benthic surface, in the
water column, and floating at the water surface. Initial assessments of these habitats in rivers suggest high variation among sampling locations and times, including deposition and
resuspension of microplastic, as is common for naturally occurring particles39. Rigorous sampling regimes will allow for initial budgets of microplastic to be constructed for rivers.
COMPOSITION OF MICROPLASTIC IN CONTROLS Analyses of microplastic in control procedures could help reveal potential sources of microplastic contamination in laboratory settings. Laboratory
microplastic contamination was minimal in this study (2–4 particles filter−1) and similar to other studies20,59 (_e.g_., McCormick _et al_.20, Pazos _et al_.59). As we predicted,
microplastic in controls were typically large fibers and unique in colors (_e.g_., purple) compared to environmental samples. Sources of microplastic contamination could have come from
laboratory technician clothing, atmospheric deposition, and the water supply. In this study, DI water with a 363 µm mesh covering the faucet was used for all solutions and rinsing of
glassware. De-ionized water was used due to greater microplastic contamination in MilliQ and tap water in the laboratory (_personal observation_, McNeish). Microplastic contamination has
been documented in a diversity of commercial salt brands ranging from 1–10 particles kg−1 of salt46, which is important since density separation of microplastic using salts is a common
protocol in the microplastic field49. Possible contamination may have also come from pre-ordered 30% hydrogen peroxide. Although we did not isolate the sources of microplastic contamination,
this study is the first to report microplastic contamination is unique in size and color compared to environmental samples. Polymer identification in small plastic fibers is a major
challenge for this field of research. It is possible some fibers in this study were mis-identified as plastic instead of other anthropogenic sources of fibers (_e.g_., cotton and viscose
fibers). Lenz _et al_.69 reported a 25% error in mis-identification of fibers as plastic when comparing visual versus FTIR identification of fibers as plastic. This suggests the possibility
that 25% of the fibers in this study could not be plastic. However, the fibers that were identified by FTIR in our study were all plastic. Moreover, the patterns observed across sites and
fish taxa would still be the same assuming this error was consistent throughout sample processing. As technology for polymer identification on small fibers develops, this outstanding
question of misidentification will inform research on microplastic pollution across ecosystem types. SUMMARY Microplastic is abundant in rivers and fish connected to Lake Michigan, which
serve as conduits of contamination via river currents and fish movement. Species functional traits may help predict microplastic abundance in fish and could be applied to other fauna.
Understanding traits that make fauna susceptible to microplastic pollution could enhance our understanding of how these organisms interact with microplastic and could enable us to target
species for conservation efforts. Microplastic concentrations in Lake Michigan tributaries were also higher than what has been reported for marine coastal habitats42 and the open ocean70;
although, greater harmony in methodological approaches would be needed for more robust comparisons of microplastic concentrations across large spatial scales. In particular, more research is
needed to pinpoint the landscape features which serve as point and non-point sources of microplastic pollution to freshwaters, and its accumulation in food webs. These collective findings
highlight the need for future research to focus on the movement of microplastic across the terrestrial-aquatic boundary and the importance of focusing pollution management efforts on inland
waters. REFERENCES * Thompson, R. C., Swan, S. H., Moore, C. J. & vom Saal, F. S. Our plastic age. _Philos. Trans. R. Soc. B Biol. Sci._ 364, 1973–1976 (2009). Article Google Scholar *
Barnes, D. K. A., Galgani, F., Thompson, R. C. & Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. _Philos. Trans. R. Soc. Lond. B. Biol. Sci._ 364,
1985–1998 (2009). Article PubMed PubMed Central CAS Google Scholar * Derraik, J. G. B. The pollution of the marine environment by plastic debris: a review. _Mar. Pollut. Bull._ 44,
842–852 (2002). Article PubMed CAS Google Scholar * Vannela, R. Are we ‘Digging our own grave’ under the oceans? _Environ. Sci. Technol._ 46, 7932–7933 (2012). Article ADS PubMed CAS
Google Scholar * World Economic Forum, The Ellen MacArthur Foundation & Company, M. _The new plastics economy: Rethinking the future of plastics_ (2016). * Lusher, A. L., Tirelli, V.,
Connor, I. O. & Officer, R. Microplastics in Arctic polar waters: the first reported values of particles in surface and sub-surface samples. _Nature_ 1–9,
https://doi.org/10.1038/srep14947 (2015). * Lavers, J. L. & Bond, A. L. Exceptional and rapid accumulation of anthropogenic debris on one of the world’s most remote and pristine islands.
_Proc. Natl. Acad. Sci_., https://doi.org/10.1073/pnas.1619818114 (2017). * Woodall, L. C. _et al_. The deep sea is a major sink for microplastic debris. _R. Socient Open Sci._ 1, 140317
(2014). Article Google Scholar * Van Cauwenberghe, L., Vanreusel, A., Mees, J. & Janssen, C. R. Microplastic pollution in deep-sea sediments. _Environ. Pollut._ 182, 495–499 (2013).
Article PubMed CAS Google Scholar * McCormick, A. R. _et al_. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. _Ecosphere_ 7
(2016). * Dris, R. _et al_. Microplastic contamination in an urban area: A case study in Greater Paris. _Environ. Chem_., https://doi.org/10.1071/EN14167 (2015). * Cole, M., Lindeque, P.,
Halsband, C. & Galloway, T. S. Microplastics as contaminants in the marine environment: A review. _Mar. Pollut. Bull._ 62, 2588–2597 (2011). Article PubMed CAS Google Scholar *
Browne, M. A. _et al_. Accumulation of microplastic on shorelines woldwide: sources and sinks. _Environ. Sci. Technol._ 45, 9175–9179 (2011). Article ADS PubMed CAS Google Scholar *
Andrady, A. L. The plastic in microplastics: A review. _Mar. Pollut. Bull._ 119, 12–22 (2017). Article PubMed CAS Google Scholar * O’Brine, T. & Thompson, R. C. Degradation of
plastic carrier bags in the marine environment. _Mar. Pollut. Bull._ 60, 2279–2283 (2010). Article PubMed CAS Google Scholar * Sheavly, S. B. & Register, K. M. Marine debris &
plastics: Environmental concerns, sources, impacts and solutions. _J. Polym. Environ._ 15, 301–305 (2007). Article CAS Google Scholar * Blight, L. K. & Burger, A. E. Occurrence of
plastic particles in sea- birds from the Eastern North Pacific. _Mar. Pollut. Bull._ 34, 323–325 (1997). Article CAS Google Scholar * Cole, M. _et al_. Microplastic ingestion by
zooplankton. _Environ. Sci. Technol._ 47, 6646–6655 (2013). Article ADS PubMed CAS Google Scholar * Zettler, E. R., Mincer, T. J. & Amaral-Zettler, L. A. Life in the ‘plastisphere’:
Microbial communities on plastic marine debris. _Environ. Sci. Technol._ 47, 7137–7146 (2013). Article ADS PubMed CAS Google Scholar * McCormick, A., Hoellein, T. J., Mason, S. A.,
Schluep, J. & Kelly, J. J. Microplastic is an Abundant and distinct microbial habitat in an urban river. _Environ. Sci. Technol._ 48, 11863–11871 (2014). Article ADS PubMed CAS
Google Scholar * Browne, M. A., Niven, S. J., Galloway, T. S., Rowland, S. J. & Thompson, R. C. Microplastic moves pollutants and additives to worms, reducing functions linked to health
and biodiversity. _Curr. Biol._ 23, 2388–2392 (2013). Article PubMed CAS Google Scholar * Avio, C. G. _et al_. Pollutants bioavailability and toxicological risk from microplastics to
marine mussels. _Environ. Pollut._ 198, 211–222 (2015). Article PubMed CAS Google Scholar * Rochman, C. M., Hoh, E., Kurobe, T. & Teh, S. J. Ingested plastic transfers hazardous
chemicals to fish and induces hepatic stress. _Sci. Rep._ 3, 1–7 (2013). Article Google Scholar * Farrell, P., Farrell, P. & Nelson, K. Trophic level transfer of microplastic: Mytilus
edulis (L.) to Carcinus maenas (L.). _Environ. Pollut._ 177, 1–3 (2013). Article PubMed CAS Google Scholar * Wright, S., Thompson, R. C. & Galloway, T. S. The physical impacts of
microplastics on marine organisms: a review. _Environ. Pollut._ 178, 483–492 (2013). Article PubMed CAS Google Scholar * Gregory, M. R. Environmental implications of plastic debris in
marine settings-entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. _Philos. Trans. R. Soc. B Biol. Sci._ 364, 2013–2025 (2009). Article Google Scholar *
Hoellein, T., Rojas, M., Pink, A., Gasior, J. & Kelly, J. Anthropogenic Litter in Urban Freshwater Ecosystems: Distribution and Microbial Interactions. _PLoS One_ 9 (2014). * McCormick,
A. R. & Hoellein, T. J. Anthropogenic litter is abundant, diverse, and mobile in urban rivers: Insights from cross-ecosystem analyses using ecosystem and community ecology tools.
_Limnol. Oceanogr._ 61, 1718–1734 (2016). Article ADS CAS Google Scholar * Chazdon, R. L. Beyond deforestation: Restoring forests and ecosystem services on degraded lands. _Science._
320, 1458–1460 (2008). Article ADS PubMed CAS Google Scholar * Vogt, R. J., Frost, P. C., Nienhuis, S., Woolnough, D. A. & Xenopoulos, M. A. The dual synchronizing influences of
precipitation and land use on stream properties in a rapidly urbanizing watershed. 7, 1–15 (2016). * Foster, D. _et al_. The importance of land-use legacies to ecology and conservation.
_Bioscience_ 53, 77–88 (2003). Article Google Scholar * Hladyz, S. _et al_. Stream ecosystem functioning in an agricultural landscape: the importance of terrestrial-aquatic linkages. _Adv.
Ecol. Res._ 44, 211–276 (2011). Article Google Scholar * Horton, A. A., Walton, A., Spurgeon, D. J., Lahive, E. & Svendsen, C. Microplastics in freshwater and terrestrial
environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. _Science_ 586, 127–141 (2017). CAS Google Scholar * Habib, D., Locke, D.
C. & Cannone, L. J. Synthetic fibers as indicators of municipal sewage sludge, sludge products, and sewage treatment plant effluents. _Water, Air, Soil Pollut._ 103, 1–8 (1998). Article
ADS CAS Google Scholar * Murphy, F., Ewins, C., Carbonnier, F. & Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. _Environ. Sci.
Technol._ 50, 5800–5808 (2016). Article ADS PubMed CAS Google Scholar * Mahon, A. M. _et al_. Microplastics in sewage sludge: Effects of treatment. _Environ. Sci. Technol._ 51, 810–818
(2017). Article ADS PubMed CAS Google Scholar * Dris, R., Gasperi, J., Saad, M., Mirande, C. & Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the
environment? _Mar. Pollut. Bull._ 104, 290–293 (2016). Article PubMed CAS Google Scholar * Driedger, A. G. J., Durr, H. H., Mitchell, K. & Van Cappellen, P. Plastic debris in the
Laurentian Great Lakes: A review. _J. Great Lakes Res._ 41, 9–19 (2015). Article CAS Google Scholar * Hoellein, T. J. _et al_. Longitudinal patterns of microplastic concentration and
bacterial assemblages in surface and benthic habitats of an urban river. _Freshw. Sci._ 36, 491–507 (2017). Article Google Scholar * Baldwin, A. K., Corsi, S. R. & Mason, S. A. Plastic
debris in 29 Great Lakes tributaries: Relations to watershed attributes and hydrology. _Environ. Sci. Technol_, https://doi.org/10.1021/acs.est.6b02917 (2016). * Claessens, M., Van
Cauwenberghe, L., Vandegehuchte, M. B. & Janssen, C. R. New techniques for the detection of microplastics in sediments and field collected organisms. _Mar. Pollut. Bull._ 70, 227–233
(2013). Article PubMed CAS Google Scholar * Barrows, A. P. W., Neumann, C. A., Berger, L. & Shaw, S. D. Grab vs. neuston tow net: a microplastic sampling performance comparison and
possible advances in the field. _Anal. Methods_ 1–8, https://doi.org/10.1039/C6AY02387H (2016). * Rocha-Santos, T. & Duarte, A. C. A critical overview of the analytical approaches to the
occurrence, the fate and the behavior of microplastics in the environment. _Trends Anal. Chem._ 65, 47–53 (2015). Article CAS Google Scholar * Hidalgo-Ruz, V., Gutow, L., Thompson, R. C.
& Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. _Environ. Sci. Technol._ 46, 3060–3075 (2012). Article ADS
PubMed CAS Google Scholar * Davison, P. & Asch, R. G. Plastic ingestion by mesopelagic fishes in the North Pacific Subtropical Gyre. _Mar. Ecol. Prog. Ser._ 432, 173–180 (2011).
Article ADS Google Scholar * Karami, A. _et al_. The presence of microplastics in commercial salts from different countries. _Sci. Rep._ 7, 46173 (2017). Article ADS PubMed PubMed
Central CAS Google Scholar * Homer, C. _et al_. [downloaded file] Completion of the 2011 National Land Cover Database for the conterminous United States - Representing a decade of land
cover change information. _Photogramm. Eng. Remote Sensing_ 81, 345–354 (2015). Google Scholar * Becker, G. C. _Fishes of Wisconsin_. (University of Wisconsin Press, 1983). * Masura, J.,
Baker, J., Foster, G. & Arthur, C. Laboratory methods for the analysis of microplastics in the marine environment: Recommendations for quantifying synthetic particles in waters and
sediments. _NOAA Tech. Memo. NOS-OR&R-48_ (2015). * Eriksen, M. _et al_. Microplastic pollution in the surface waters of the Laurentian Great Lakes. _Mar. Pollut. Bull_. 177–182,
https://doi.org/10.1016/j.marpolbul.2013.10.007 (2013). * Froese, R. & Pauly, D. FishBase. _World Wide Web electronic publicatio_n (2017). Available at: www.fishbase.org. (Accessed: 1st
January 2017). * Qiu, Q. _et al_. Occurrence of microplastics in the coastal marine environment: First observation on sediment of China. _Mar. Pollut. Bull._ 98, 274–280 (2015). Article
PubMed CAS Google Scholar * Imhof, H. K. _et al_. Pigments and plastic in limnetic ecosystems: A qualitative and quantitative study on microparticles of different size classes. _Water
Res._ 98, 64–74 (2016). Article PubMed CAS Google Scholar * Zar, J. _Biostatistical Analysis_. (Prentice-Hall, 1999). * McNeish, R. E., Benbow, M. E. & McEwan, R. W. Riparian forest
invasion by a terrestrial shrub (Lonicera maackii) impacts aquatic biota and organic matter processing in headwater streams. _Biol. Invasions_ 14, 1881–1893 (2012). Article Google Scholar
* Pohlert, A. PMCMR: Calculate multiple comparisons of mean rank sums. _4.0_ (2015). * Sokal, R. & Rohlf, F. _Biometery: the Principles and Practice of Statistics in Biological
Research_. (Freeman, W. H. and Company, 1981). * Rochman, C. M. _et al_. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human
consumption. _Nature_ 1–10, https://doi.org/10.1038/srep14340 (2015). * Pazos, R. S., Maiztegui, T., Colautti, D. C., Paracampo, A. H. & Gomez, N. Microplastics in gut contents of
coastal freshwater fish from Rio de la Plata estuary. _Mar. Pollut. Bull_., https://doi.org/10.1016/j.marpolbul.2017.06.007 (2017). * Lusher, A. L., O’Donnell, C., Officer, R. &
O’Connor, I. Microplastic interations with North Atlantic mesopelagic fish. _ICES J. Mar. Sci._ 73, 1214–1225 (2016). Article Google Scholar * Polačik, M. _et al_. Invasive gobies in the
Danube: Invasion success facilitated by availability and selection of superior food resources. _Ecol. Freshw. Fish_ 18, 640–649 (2009). Article Google Scholar * Brush, J. M., Fisk, A. T.,
Hussey, N. E. & Johnson, T. B. Spatial and seasonal variability in the diet of round goby (Neogobius melanostomus): stable isotopes indicate that stomach contents overestimate the
importance of dreissenids. _Can. J. Fish. Aquat. Sci._ 69, 573–586 (2012). Article CAS Google Scholar * Whitehead, P. _Culpeoid fishes of the world (suborder Culpeioidei). An annotated
and illustrated catalague of the herrings, sardines, pilchards, sprats, shads, anchovies and world-herrings_. _FAO Fish. Synop_ 7, (FAO, 1985). * Ferreira, G. V. B. _et al_. Plastic debris
contamination in the life cycle of Acoupa weakfish (Cynoscion acoupa) in a tropical estuary. _ICES J. Mar. Sci. J. du Cons._ 73, 2695–2707 (2016). Article Google Scholar * Lusher, A. L.,
Mchugh, M. & Thompson, R. C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. _Mar. Pollut. Bull._ 67, 94–99 (2012).
Article PubMed CAS Google Scholar * Van Cauwenberghe, L. & Janssen, C. R. Microplastics in bivalves cultured for human consumption. _Environ. Pollut._ 193, 65–70 (2014). Article
PubMed CAS Google Scholar * Williams, A. T., Randerson, P., Allen, C. & Cooper, J. A. G. Beach litter sourcing: A trawl along the Northern Ireland coastline. _Mar. Pollut. Bull_.,
https://doi.org/10.1016/j.marpolbul.2017.05.066 (2017). * Yang, D., Sun, S., Chen, J. & Liu, X. Analysis for the spatial and temporal patterns of plasticulture In Shandong Province,
China with remotely sensed data. in _Interantional Conference on Agro-geoinformatics_, https://doi.org/10.1109/Agro-Geoinformatics.2016.7577663 (2015). * Lenz, R., Enders, K., Stedmon, C.
A., MacKenzie, D. M. A. & Nielsen, T. G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. _Mar. Pollut. Bull._
100, 82–91 (2015). Article PubMed CAS Google Scholar * Lusher, A. L., Tirelli, V., O’Connor, I. & Officer, R. Microplastics in Arctic polar waters: the first reported values of
particles in surface and sub-surface samples. _Sci. Rep_. 5 (2015). Download references ACKNOWLEDGEMENTS We thank officials from Benton Harbor and St. Joseph, MI, the Milwaukee Park
District, WI, and Muskegon State Park, MI for use of the field sites. Special appreciation to Marty Berg for use of laboratory equipment and to Brenainn Turner, Paul Risteca, Veronica
Lourich, Anthony Overhiser, Anna Vincent and the nine other graduate and undergraduate students at Loyola University Chicago and the University Notre Dame who contributed to field and
laboratory work. This work was supported by a grant from the Illinois-Indiana Sea Grant of the National Oceanic Atmospheric Administration (074483-15907) to JJK, TJH and SAM and by a grant
from the National Science Foundation (CAREER 1553835) to TJH. Any opinions, findings, and conclusions or recommendations expressed are those of the authors and do not necessarily reflect the
views of Sea Grant, the National Oceanic Atmospheric Administration, or the National Science Foundation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Loyola University Chicago – Biology,
1032 West Sheridan Road, Chicago, IL, 60660, USA R. E. McNeish, L. H. Kim, J. J. Kelly & T. J. Hoellein * Department of Geology and Environmental Sciences, The State University of New
York at Fredonia, 280 Central Ave., Science Complex 340, Fredonia, NY, 14063, USA H. A. Barrett & S. A. Mason Authors * R. E. McNeish View author publications You can also search for
this author inPubMed Google Scholar * L. H. Kim View author publications You can also search for this author inPubMed Google Scholar * H. A. Barrett View author publications You can also
search for this author inPubMed Google Scholar * S. A. Mason View author publications You can also search for this author inPubMed Google Scholar * J. J. Kelly View author publications You
can also search for this author inPubMed Google Scholar * T. J. Hoellein View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.J.K., T.J.H.,
and S.A.M. designed the research; R.E.M. conducted the research and statistical analyses; L.H.K. conducted field work and training on sample processing; S.A.M. and H.A.B. conducted FTIR
analysis; All authors contributed to writing and editing of the manuscript. CORRESPONDING AUTHOR Correspondence to R. E. McNeish. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare
no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTAL MATERIALS RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which
permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE McNeish, R.E., Kim, L.H., Barrett, H.A. _et al._ Microplastic in riverine fish is connected to species traits. _Sci Rep_ 8, 11639 (2018).
https://doi.org/10.1038/s41598-018-29980-9 Download citation * Received: 11 September 2017 * Accepted: 23 July 2018 * Published: 03 August 2018 * DOI:
https://doi.org/10.1038/s41598-018-29980-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative