Transcriptome profiling of human oocytes experiencing recurrent total fertilization failure
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT There exist some patients who face recurrent total fertilization failure during assisted reproduction treatment, but the pathological mechanism underlying is elusive. Here, by using
sc-RNA-seq method, the transcriptome profiles of ten abnormally fertilized zygotes were assessed, including five zygotes from one patient with recurrent Poly-PN zygotes, and five zygotes
from a patient with pronuclear fusion failure. Four zygotes with three pronuclear (Tri-PN) were collected from four different patients as controls. After that, we identified 951 and 1697
significantly differentially expressed genes (SDEGs) in Poly-PN and PN arrest zygotes, respectively as compared with the control group. KEGG analyses indicated down regulated genes in the
Poly-PN group included oocyte meiosis related genes, such as _PPP2R1B, YWHAZ, MAD2L1, SPDYC, SKP1_ and _CDC27_, together with genes associated with RNA processing, such as _SF3B1, LOC645691,
MAGOHB, PHF5A, PRPF18, DDX5, THOC1_ and _BAT1_. In contrast, down regulated genes in the PN arrest group, included cell cycle genes, such as _E2F4, DBF4, YWHAB, SKP2, CDC23, SMC3, CDC25A,
CCND3, BUB1B, MDM2, CCNA2_ and _CDC7_, together with homologous recombination related genes, such as _NBN, XRCC3, SHFM1, RAD54B_ and _RAD51_. Thus, our work provides a better understanding
of transcriptome profiles underlying RTFF, although it based on a limited number of patients. SIMILAR CONTENT BEING VIEWED BY OTHERS _ARHGAP26_ DEFICIENCY DRIVES THE OOCYTE ANEUPLOIDY AND
EARLY EMBRYONIC DEVELOPMENT FAILURE Article 23 September 2024 SINGLE CELL RNA-SEQ REVEALS GENES VITAL TO IN VITRO FERTILIZED EMBRYOS AND PARTHENOTES IN PIGS Article Open access 13 July 2021
AN INTEGRATED ANALYSIS OF MULTIPLE DATASETS REVEALS NOVEL GENE SIGNATURES IN HUMAN GRANULOSA CELLS Article Open access 06 September 2024 INTRODUCTION Despite nearly forty years of scientific
and clinical advance in the field of assisted reproduction, there still exist some rarely patients, even though rarely occur, who have to face recurrent total fertilization failure (RTFF)
without any visual precautionary indicator1,2,3, even some of them could be rescued by assisted oocyte activation4. Therefore, elucidating the internal mechanism of fertilization failure is
of great importance for these patients. However, until now, the relevant etiological analysis was often restricted to morphology during IVF, such as immature oocytes5, improper meiosis6,
zygotes with abnormal pronuclei7, and di-pronuclear zygote failing mitotic cleavage8. Due to small amount of material available, deciphering mechanisms underlying these defects remain
technical challenging. Recent technical advances in single-cell sequencing open a new era for exploring the biological state of a single cell at both the DNA and RNA levels for studying
variations in genome9,10, transcriptome, and epigenome11 separately or in parallel12. Originally adopted by Surani’s research team13, this approach has been applied successfully in
discriminating cell types14,15,16,17,18,19, elucidating regulatory circuits20, and investigating tumor heterogeneity21,22. In reproductive biology fields, this technique has been used for
screening transcriptome of tissues23 and germline cells at different stages24,25,26,27,28,29. The single cell sequencing technique has great potential in clinical implication30,31,32,
especially in the diagnosis for clarifying the molecular mechanisms of fertilization failure at a single cell resolution. So the aim of this work was to characterize the pathological changes
of human zygotes with RTFF at the transcriptional level. RESULTS CLINICAL TREATMENT HISTORY OF THE RTFF PATIENTS As clinical treatment history shown (Table 1), one patient experienced two
stimulated cycles under different procedures with 4 and 5 Poly-PN fertilized eggs after ICSI treatment, respectively. Another patient had all zygotes with PN arrest, with 18, 7 and 9 matured
oocytes retrieved separately in three cycles although three different ovarian stimulation procedures were employed each time. Moreover, There were no significant differences in serum levels
of FSH, LH, E2 and progesterone at baseline and trigger day in patients with Poly-PN, PN arrest, and the control groups (Supplementary Fig. 1), indicating that the observed defects in the
zygotes were more likely associated with oocyte original molecular defects rather than ovarian stimulation protocol. TRANSCRIPTOME PROFILES IN POLY-PN AND PN-ARREST ZYGOTES It is crucial for
oocyte to accumulate indispensable mRNAs to ensure its later use for fertilization and subsequent cell division before the zygotic gene activation33. As the scarce of the oocytes for RTFF
patients, it was difficult to collect enough donated oocytes for our study. Therefore, we investigate the transcriptome profile using the unfertilized oocyte after clinical treatment. The
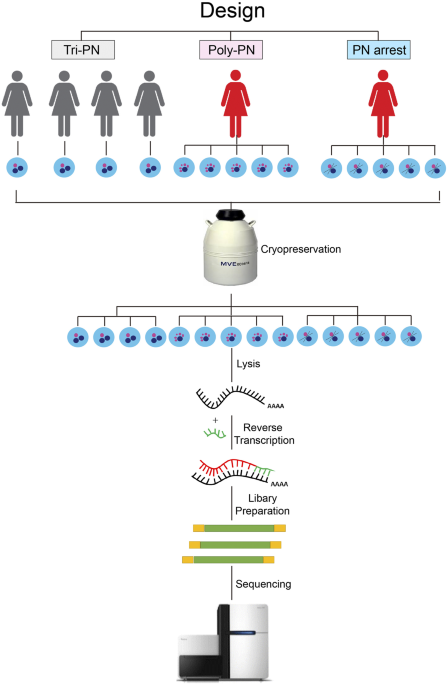
procedure of our work was shown in Fig. 1. After sequencing using Illumina HiSeq 2,500 sequencer, we obtain about 142 million clean reads, of which 116 million clean reads mapped to human
genome reference. On average, 15,058, 14,995 and 17,713 genes (FPKM ≥ 0.1), 10,471, 10,451 and 10,289 genes (FPKM ≥ 1) or 4,539, 4,410 and 3,630 genes (FPKM ≥ 10) were acquired in Tri-PN,
Poly-PN and PN arrest groups, respectively (Fig. 2a). These results were consistent with the data from a previous study24, implying that our technology has the similar sensitivity and
coverage. To compare the global transcriptome profiles of unfertilized eggs or zygotes among different groups, we analyzed data by hierarchical clustering, and the results indicated that 14
zygotes from 3 groups were clustered into corresponding groups and separated from each other (Fig. 2b). Four Tri-PN zygotes from different patients have the similar transcriptome profile in
spite of the heterogeneity in patient source. Interestingly, we also found that five PN arrest zygotes clustered closer with four Tri-PN zygotes, but away from the five Poly-PN zygotes. This
finding indicated that the underlying mechanisms was quite different between the Poly-PN and PN arrest group. To clarify underlying mechanisms, we analyzed major differences of expression
profile among different groups. To rule out technical errors causing artifacts of gene expression, all reference genes with average FPKM > 0.5 in any of three groups were used for
subsequent analysis. According to the criteria of fold change > 2 or < 0.5 and _P_ < 0.001, 951 (227 up regulated and 724 down regulated) and 1,697 genes (205 up regulated and 1,492
down regulated) were found to be significantly differentially expressed genes (SDEGs) in Poly-PN and PN arrest zygotes, respectively, as compared with the control group (Fig. 2c,d and
Supplementary Tables 1 and 2). THE SDEGS ARE INVOLVED IN DIFFERENT BIOLOGICAL PROCESSES BETWEEN POLY-PN AND PN ARREST ZYGOTES In order to clarify the biological function of these
differential expressed genes, the KEGG analyses were applied to SDEGs of Poly-PN and PN arrest zygotes, separately. As the results shown (Table 2), for the up regulated genes, there was a
enrichment of genes whose products are related to RNA processing and translation, such as RNA splicing (_P_-value = 1.90 × 10−2 for Poly-PN) or Ribosome biogenesis (_P_-value = 5.80 × 10−9
for Poly-PN and 1.10 × 10−84 for PN arrest), together with energy consuming related items, such as Huntington’s disease (_P_-value = 6.40 × 10−3 for Poly-PN), Parkinson’s disease (_P_-value
= 1.20 × 10−3 for PN arrest) and Oxidative phosphorylation (_P_-value = 5.20 × 10−5 for PN arrest). Genes involved in Wnt signaling pathway (_P_-value = 1.00 × 10−2 for Poly-PN), Notch
signaling pathway (_P_-value = 2.00 × 10−2 for Poly-PN) and some other signaling pathways in cancer were also enriched (Table 2 and Supplementary Tables 3 and 4). For down regulated genes,
the Poly-PN specific down regulated genes were mainly enriched in Oocyte meiosis (_P_-value = 3.60 × 10−2), Spliceosome (_P_-value = 4.00 × 10−3), Pyrimidine metabolism (_P_-value = 1.60 ×
10−2), Citrate cycle (_P-_value = 3.80 × 10−3) (Table 3 and Supplementary Table 5). Whereas, the PN arrest specific down regulated genes mainly belonged to Cell cycle (_P_-value = 7.60 ×
10−3), Homologous recombination (_P_-value = 2.40 × 10−2) and Amino sugar or nucleotide sugar metabolism (_P_-value = 4.60 × 10−2) (Table 3 and Supplementary Table 6). Furthermore, The SDEGs
down regulated overlapped in both of this two groups were mainly related to Basal transcription factors (_P_-value = 3.10 × 10−2 for Poly-PN and 4.20 × 10−3 for PN arrest), Ubiquitin
mediated proteolysis (_P_-value = 4.70 × 10−2 for Poly-PN and 7.70 × 10−3 for PN arrest) and Glycan biosynthesis (_P_-value = 2.00 × 10−2 for Poly-PN and 3.10 × 10−2 for PN arrest). Among
the total 1,956 down regulated genes in different annotations, there were about 464 (23.7%) genes specifically down regulated in Poly PN, 1,233 (63.0%) genes specifically down regulated in
PN arrest and only 259 (13.3%) genes down regulated overlap in both of this two groups (Fig. 3a). The Poly-PN specific down regulated genes included oocyte meiosis related genes such as
Protein Phosphatase (_PPP2R1B_), _YWHAZ, MAD2L1, SPDYC, SKP1_ and _CDC27_ (Fig. 3b). Certain genes associated with RNA processing, such as those encoding splicing factor genes _SF3B1,
LOC645691, MAGOHB, PHF5A, PRPF18, DDX5, THOC1_ and _BAT1_ were also down regulated (Fig. 3c), perhaps contributing to the fertilization failure in Poly-PN group. In contrast, the PN arrest
specific down regulated genes, such as _E2F4_, _DBF4, YWHAB, SKP2, CDC23, SMC3, CDC25A, CCND3, BUB1B, MDM2, CCNA2, CDC7_ were involved in Cell cycle (Fig. 3d) and _NBN, XRCC3, SHFM1, RAD54B,
RAD51_ were Homologous recombination related genes (Fig. 3e). These results implied that the Poly-PN might have defects during oocyte meiosis, whereas defects of PN arrest zygotes were
involved in Cell cycle and Homologous recombination. FUNCTIONAL VALIDATION OF THE SELECTED GENES BY GENE KNOCK DOWN We randomly chose two of these meiosis related genes (PPP2CA and SKP1) and
validated their function in mice oocyte, the results indicated that both PPP2CA and SKP1 knock down did not show any difference with the corresponding control group in either oocyte
maturation or fertilization (Fig. 4a,b). In order to clarify the mechanism underlying, we analyzed available published single cell RNA-Seq data sets corresponding to fertilization process of
human and mice, focusing on the 43 selected genes, which enriched in Meiosis, Spliceosome, Cell cycle and Homologous recombination items separately. As the results shown (Fig. 4c), human
and mice have very different expression patterns of the selected genes during their fertilization process. Thus, more clinical cases, but not the mice, might be an ideal model for validation
of the function of these selected genes in future. DISCUSSION Some patients have oocytes incapable of completing the whole process of fertilization, including defective sperm entry, oocyte
activation, pronuclear formation or fusion34, as well as some failure in mitotic division35. In this work, we profiled the transcriptome of the Poly-PN and PN arrest zygotes from two
patients with RTFF, and found Poly-PN zygotes showed defects in Meiosis and RNA processing and PN arrest zygotes had defects in Cell cycle and DNA homologous recombination. For meiosis,
oocytes need to undergo meiotic DNA replication and homologous chromosomes segregation, and then arrest in metaphase of meiosis II awaiting fertilization33. After sperm penetration, oocyte
resumes meiosis and segregates sister chromatids and completes the meiosis II. So the differentially expressed genes in Poly-PN might play critical roles in this biological process. For
example, the subunit of the SCF E3 ubiquitin ligase (SKP1) has been reported to be important in the progression of recombination during oocyte meiosis36. The APC core subunit (CDC27) and the
checkpoint protein (MAD2) play critical roles in segregating sister chromatids during oocyte meiosis37. Similarly, some other genes down regulated in Poly-PN zygotes, such as Protein
Phosphatase (_PPP2R1B_), _YWHAZ_ and _SPDYC_ were also in associated with meiosis38,39. Furthermore, during oocyte maturation, it also needs to accumulate sufficient maternal RNA to ensure
oocyte maturation, fertilization and subsequently embryo development until the embryonic genome is activated40. So certain RNA processing genes identified in Poly-PN zygotes, such as those
encoding splicing factor genes _THOC1_41, _SF3B1_42, _LOC645691_, exon junction complex core component related gene (_MAGOHB_)43, PHD finger-like domain-containing protein 5 A (_PHF5A_)44,
some pre-mRNA processing factor 18 related gene (_PRPF18_)45 and RNA helicases related genes (_DDX5_ and _BAT1_)46,47, are involved in regulating RNA secondary structure and pre-mRNA
splicing, which might be responsible for RNA maturation during oocyte meiosis. Upon fertilization, the zygotes undergoes chromatin remodeling, genomes reprogramming or DNA repairing, and the
cell cycle machinery must be switched from meiotic to mitotic chromosome segregation48. Our results indicated that the PN arrest specific down regulated genes mainly related to these
biological process. For example, the cyclin associated kinase (_CCNA2_ and _CCND3_) were required for sister chromatid segregation49, and structural maintenance and segregation of chromosome
proteins (_SMC3_ and _BUB1B_) have been reported to be in associated with developmental potential of human pre-implantation zygotes50. Cell cycle related genes (_CDC7_, _CDC23_ and
_CDC25A_) and some other genes including _DBF4, YWHAB, SKP2 and MDM2_ were also found to be significantly down regulated in PN arrest group. Moreover, some other genes specifically
down-regulated in PN arrest zygotes, including check point proteins codon genes (_RAD54B_ and _RAD51_)51,52, DNA repair related genes (_XRCC3_)53, chromosome integrity maintenance genes
(_NBN_)54,55 and _SHFM1_, all of which were involved in key proteins for homologous recombination. In addition, we also found some genes down regulated overlap for both Poly-PN and PN arrest
groups and these genes in different annotations were classified according to the expression specification and illustrated in a model (Fig. 5). Taken together, our work found Poly-PN have
some problems in oocyte meiosis and RNA processing, whereas PN arrest showed defects during mitosis cell cycle or homologous recombination during meiosis and this could provide new targets
for therapeutic intervention by modulating these corresponding signaling pathways in the future. Remarkably, as the scarce of the RTFF patients, we could not collect enough oocyte samples
for single cell RNA sequencing. So more clinical cases need to be collected and further verification need to be performed in the future. METHODS ETHICS STATEMENT All procedures were approved
by the Research Ethics Committee of Shanghai Jiao tong University School of Medicine and informed consent was obtained from participants at IVF center of the Ninth people’s hospital. We
confirmed that all patients have written informed consent for the use of their zygotes for this research. Animals were maintained at 23 °C in a 12-h (7:00–19:00) light and 12-h (19:00–7:00)
dark schedule, and all experimental procedures were performed in accordance with Institutional Animal Care and Use Committee guidelines of Shanghai Jiao Tong University School of Medicine.
PATIENTS, OVARIAN STIMULATION, OOCYTE RETRIEVAL, AND THE IVF/ICSI PROCEDURE For all patients in our study, five types of ovarian stimulation protocols were used: (1) Human Menotrophins
Gonadotrophin (hmG, Lizhu Pharmaceutical Trading Co.) co-treated with Medroxyprogesterone acetate (MPA, Shanghai Sine Pharmaceutical Ltd.) (hMG + MPA); (2) Human Menotrophins Gonadotrophin
co-treated with Clomifene Citrate (CC, Medochemie Ltd.) (hMG + CC); (3) Human Menotrophins Gonadotrophin co-treated with Medroxyprogesterone acetate and Clomifene Citrate (hMG + MPA + CC);
(4) Human Menotrophins Gonadotrophin co-treated with Medroxyprogesterone acetate and ethinyl estradiol (EE, Shanghai Sine Pharmaceutical Ltd.) (hMG + MPA + EE) and (5) Short protocol, in
which patients were administered with GnRHa daily beginning on menstrual cycle day 2 and with hMG daily beginning on menstrual cycle day 3. Follicle growth was monitored by ultrasound
examination. Serum FSH, LH, E2, and progesterone concentrations were measured serially using the chemiluminescence (Abbott Biologicals B.V.) method on the same days as the ultrasound exams.
Human Chorionic Gonadotrophin (hCG, Lizhu Pharmaceutical Trading Co.) at a 1000–5000 IU dose was administered when the dominant follicles reached 18 mm in diameter. Cumulus oocyte complexes
were recovered transvaginally with ultrasound guidance 34–36 hours post hCG. After retrieval, oocytes were maintained in human tubal fluid (HTF; Irvine Scientific) medium plus 10% synthetic
serum substitute (SSS; Irvine Scientific) for about 2 hours before _In vitro_ fertilization (IVF)/Intracytoplasmic sperm injection (ICSI). For ICSI treatment, the cumulus oophorus were
removed mechanically from oocytes with denuding pipettes in solution with 80IU hyaluronidase (Sigma) followed by injection. For IVF treatment, cumulus oocyte complexes were inseminated with
about 0.3–0.5 × 106/ml motile spermatozoa in HTF medium and the cumulus oophorus were removed 18 hours later. Fertilized eggs from both IVF and ICSI groups were cultured in 20 μl continuous
single culture medium (CSC: Irvine Scientic: USA) individually under oil and incubated at 37 °C humidified atmosphere under 5% CO2, 5% O2, and 90% N2 for pre-implantation culture. As a
policy of our center, fertilization was assessed by the presence of two pronuclei 16–18 hours post insemination, followed by confirming the embryonic development 66–68 hours post
insemination. The zygotes with more than three tiny pronuclei following the ICSI procedure were recognized as Poly-PN zygotes. The zygotes with normal pronuclei but failed to fuse until
66–68 hours post fertilization were name as PN-arrest zygotes. Tri-PN zygotes from four different IVF patients were used as controls. All samples above were collected and vitrified using
Cryotip method and then stored in liquid nitrogen until subsequent experimental treatment. PREPARATION AND QUALITY CONTROL OF SINGLE-CELL CDNAS The method for RNA extraction was carried out
as described previously56. Briefly, after thawing, each zygote was washed twice and transferred into lysate buffer. Then the reverse transcription reaction was performed directly on whole
cell lysate using SuperScript II reverse transcriptase (Life Technologies). We performed 15 cycles of PCR to amplify cDNA and the PCR product was purified by using AMPure XP beads (Beckman
Coulter). Agilent high-sensitivity DNA chip kit on a BioAnalyzer (Agilent Technologies) was used for checking the quality of cDNAs according the size distribution to ensure cDNAs contained
few short fragments (<500 bp) and showed peak sizes between 1.5 kb–2 kb. RNA-SEQ LIBRARY CONSTRUCTION AND SEQUENCING According to the manual of TruePrep DNA Library Prep Kit V2 for
Illumina (Vazyme Biotech), the quality of RNA-Seq sequencing library was checked by using Agilent high-sensitivity DNA chip. The libraries showing the peak around 300 bp was chosen for
high-throughput sequencing on the Illumina HiSeq 2500 platform using the dual index sequencing strategy with single-end reads length of 50 bp. BIOINFORMATICS PROCESS FOR SEQUENCING DATA
Individual sample from different zygotes has its own unique barcode sequence and could be separated from clean data. We used Tophat v2.0.957 to assemble the reads into NCBI build 37 hg19
genome and used Cufflinks v2.1.158 to calculate gene expression level. Clustering was used to process hierarchical clustering using Euclidean distance metric in the R packages59. Gene
expression levels were measured by using fragment per kilobase of exon per million mapped reads (FPKM). To rule out technical errors and increase the power to detect biological function, all
reference genes with average FPKM > 0.5 in any of three groups and the criterion of _P_ < 0.001 or _P_ < 0.01 together with FC (fold change) >2 or <0.5 were used to identify
differentially expressed genes for subsequent biological analysis using ArrayTrackTM software (FDA’s own bioinformatics and genomics tool,
http://www.fda.gov/ScienceResearch/BioinformaticsTools/Arraytrack/default.htm). KEGG PATHWAY ANALYSIS Database for Annotation Visualization and Integrated Discovery (DAVID V6.7;
https://david.ncifcrf.gov/) was used to perform KEGG pathway analysis60,61. SHRNA DESIGN AND _IN VITRO_ TRANSCRIPTION For short hairpin RNA (shRNA) design, we selected an siRNA-target
sequence on the NCBI RNAi database for each targeted genes, and the forward and reverse primers for each gene (SKP1 F: ATAGGGGGCT GCAAACTACT TAGACATTTC AAGAGA ATGT CTAAGTAGTT TGCAGCCTTT
TTTG; SKP1 R: GATCCAAAAA AGGCTGCAAA CTACTTAGAC ATTCTCTTGA AATGTCTAAG TAGTTTGCAG CCCC; PPP2CA F: ATAGGGTGGA ACTTGACGAC ACTCTTATTC AAGAGATAAG AGTGTCGTCA AGTTCCATTT TTTGPPP2CA R: GATCCAAAAA
ATGGAACTTG ACGACACTCT TATCTCTTGA ATAAGAGTGT CGTCAAGTTC CACC) were annealed and cloned into a T7 promoter containing vector pcDNA3.1(+) using Bsa1 and BamH1 restriction enzyme site, shRNA was
transcribed _in vitro_ from linearized pcDNA3.1-shRNA plasmid using MEGA short script T7 kit (Life Technology) and purified using MEGA clear kit (Life Technology) and mixed in RNase-free
water at the concentration of 50 ng/μl for subsequent use. OOCYTE MICROINJECTION, PARTHENOGENETIC ACTIVATION AND DEVELOPMENT ASSESSMENT Female mice aged 6–8 weeks were induced to
superovulate by i.p. injection of 10 IU of pregnant mare’s gonadotrophin (PMSG) (Ningbo Hormone Products Co.). Cumulus oocyte complexes (COCs) were collected at 46 h post PMSG. For COCs
retrieval, the ovaries were removed immediately and put into 4 ml HTF medium plus 10% SSS (Irvine Scientific) and 0.2 mM IBMX (Sigma). The COCs were released into this medium by puncturing
ovaries with a 27 g needle. The cumulus cells were released mechanically using mouth pipette and only those with normal morphologies were used for RNA injection. All injected oocytes were
cultured for maturation in a CO2 incubator for 16 hours for maturation before activation. The activation medium used was KSOM (Millipore) supplemented with 10 mM SrCl2. After being washed
twice in activation medium, oocytes were incubated first in activation medium for 2.5 hours and then in activation medium without SrCl2 for 3.5 hours at 37 °C in a humidified atmosphere with
5% CO2, 5% O2, and 90% N2. Both the activation medium and KSOM for subsequent short culture of oocytes were supplemented with 5 μg/mL cytochalasin B. Six hours after the onset of
activation, the fertilization rate was assessed by count pronuclear formation. STATISTICAL ANALYSIS Serum hormone data were analyzed by GraphPad Prism software using two-way repeated
measures ANOVA. Bonferroni post tests were used for pairwise comparisons. **_P_ < 0.01; ***_P_ < 0.001. Data for relative expression levels in Poly-PN and PN arrest zygotes were
separately compared with control were analyzed using a two-tailed, unpaired Student’s t test. _P_ < 0.001 indicated as significantly different. The maturation and fertilization rate of
the oocytes between gene knock down and control group were analyzed by using Chi-Squared Test. REFERENCES * Flaherty, S. P., Payne, D. & Matthews, C. D. Fertilization failures and
abnormal fertilization after intracytoplasmic sperm injection. _Hum Reprod_ 13(Suppl 1), 155–164 (1998). Article Google Scholar * Mahutte, N. G. & Arici, A. Failed fertilization: is it
predictable? _Current opinion in obstetrics & gynecology_ 15, 211–218 (2003). Article Google Scholar * Combelles, C. M. _et al_. Diagnosing cellular defects in an unexplained case of
total fertilization failure. _Hum Reprod_ 25, 1666–1671 (2010). Article Google Scholar * Kuentz, P. _et al_. Assisted oocyte activation overcomes fertilization failure in globozoospermic
patients regardless of the DPY19L2 status. _Hum Reprod_ 28, 1054–1061 (2013). Article CAS Google Scholar * Thornton, M. H., Francis, M. M. & Paulson, R. J. Immature oocyte retrieval:
lessons from unstimulated IVF cycles. _Fertility and sterility_ 70, 647–650 (1998). Article CAS Google Scholar * Filges, I. _et al_. Recurrent triploidy due to a failure to complete
maternal meiosis II: whole-exome sequencing reveals candidate variants. _Molecular human reproduction_ 21, 339–346 (2015). Article CAS Google Scholar * Rosenbusch, B. E. & Schneider,
M. Recurrent failure of pronucleus formation after intracytoplasmic sperm injection. _Arch Gynecol Obstet_ 262, 185–188 (1999). Article CAS Google Scholar * Rawe, V. Y., Olmedo, S. B.,
Nodar, F. N., Ponzio, R. & Sutovsky, P. Abnormal assembly of annulate lamellae and nuclear pore complexes coincides with fertilization arrest at the pronuclear stage of human zygotic
development. _Hum Reprod_ 18, 576–582 (2003). Article CAS Google Scholar * Lu, S. _et al_. Probing meiotic recombination and aneuploidy of single sperm cells by whole-genome sequencing.
_Science_ 338, 1627–1630 (2012). Article ADS CAS Google Scholar * Yan, L. _et al_. Live births after simultaneous avoidance of monogenic diseases and chromosome abnormality by
next-generation sequencing with linkage analyses. _Proceedings of the National Academy of Sciences of the United States of America_ 112, 15964–15969 (2015). Article ADS CAS Google Scholar
* Angermueller, C. _et al_. Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity. _Nature methods_ 13, 229–232 (2016). Article CAS Google Scholar *
Macaulay, I. C. _et al_. Separation and parallel sequencing of the genomes and transcriptomes of single cells using G&T-seq. _Nature protocols_ 11, 2081–2103 (2016). Article CAS Google
Scholar * Tang, F. _et al_. mRNA-Seq whole-transcriptome analysis of a single cell. _Nature methods_ 6, 377–382 (2009). Article CAS Google Scholar * Poulin, J. F., Tasic, B.,
Hjerling-Leffler, J., Trimarchi, J. M. & Awatramani, R. Disentangling neural cell diversity using single-cell transcriptomics. _Nature neuroscience_ 19, 1131–1141 (2016). Article Google
Scholar * Zeisel, A. _et al_. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. _Science_ 347, 1138–1142 (2015). Article ADS CAS Google
Scholar * Li, J. _et al_. Single-cell transcriptomes reveal characteristic features of human pancreatic islet cell types. _EMBO reports_ 17, 178–187 (2016). Article CAS Google Scholar *
Treutlein, B. _et al_. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. _Nature_ 509, 371–375 (2014). Article ADS CAS Google Scholar * Lawlor,
N. _et al_. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. _Genome research_ 27, 208–222 (2017). Article
CAS Google Scholar * Han, X. _et al_. Mapping the Mouse Cell Atlas by Microwell-Seq. _Cell_ 172, 1091–1107 e1017 (2018). Article CAS Google Scholar * Jaitin, D. A. _et al_. Dissecting
Immune Circuits by Linking CRISPR-Pooled Screens with Single-Cell RNA-Seq. _Cell_ 167, 1883–1896 e1815 (2016). Article CAS Google Scholar * Patel, A. P. _et al_. Single-cell RNA-seq
highlights intratumoral heterogeneity in primary glioblastoma. _Science_ 344, 1396–1401 (2014). Article ADS CAS Google Scholar * Zheng, C. _et al_. Landscape of Infiltrating T Cells in
Liver Cancer Revealed by Single-Cell Sequencing. _Cell_ 169, 1342–1356 e1316 (2017). Article CAS Google Scholar * Krjutskov, K. _et al_. Single-cell transcriptome analysis of endometrial
tissue. _Hum Reprod_ 31, 844–853 (2016). Article CAS Google Scholar * Xue, Z. _et al_. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. _Nature_
500, 593–597 (2013). Article ADS CAS Google Scholar * Yan, L. _et al_. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. _Nature structural &
molecular biology_ 20, 1131–1139 (2013). Article CAS Google Scholar * Petropoulos, S. _et al_. Single-Cell RNA-Seq Reveals Lineage and X Chromosome Dynamics in Human Preimplantation
Embryos. _Cell_ 167, 285 (2016). Article CAS Google Scholar * Blakeley, P. _et al_. Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. _Development_ 142,
3151–3165 (2015). Article CAS Google Scholar * Shi, J. _et al_. Dynamic transcriptional symmetry-breaking in pre-implantation mammalian embryo development revealed by single-cell RNA-seq.
_Development_ 142, 3468–3477 (2015). Article CAS Google Scholar * Liu, W. _et al_. Identification of key factors conquering developmental arrest of somatic cell cloned embryos by
combining embryo biopsy and single-cell sequencing. _Cell discovery_ 2, 16010 (2016). Article CAS Google Scholar * Liu, Q. _et al_. Single-cell analysis of differences in transcriptomic
profiles of oocytes and cumulus cells at GV, MI, MII stages from PCOS patients. _Scientific reports_ 6, 39638 (2016). Article ADS CAS Google Scholar * Zhang, R. _et al_. RNA-Seq-Based
Transcriptome Analysis of Changes in Gene Expression Linked to Human Pregnancy Outcome After _In Vitro_ Fertilization-Embryo Transfer. _Reprod Sci_ 23, 134–145 (2016). Article CAS Google
Scholar * Fragouli, E., Lalioti, M. D. & Wells, D. The transcriptome of follicular cells: biological insights and clinical implications for the treatment of infertility. _Human
reproduction update_ 20, 1–11 (2014). Article CAS Google Scholar * Clift, D. & Schuh, M. Restarting life: fertilization and the transition from meiosis to mitosis. _Nature reviews.
Molecular cell biology_ 14, 549–562 (2013). Article CAS Google Scholar * Kort, D. H. _et al_. Human embryos commonly form abnormal nuclei during development: a mechanism of DNA damage,
embryonic aneuploidy, and developmental arrest. _Hum Reprod_ 31, 312–323 (2016). CAS PubMed Google Scholar * Azzarello, A., Hoest, T. & Mikkelsen, A. L. The impact of pronuclei
morphology and dynamicity on live birth outcome after time-lapse culture. _Hum Reprod_ 27, 2649–2657 (2012). Article CAS Google Scholar * McLoud, J. D. & Yang, M. The conserved
function of skp1 in meiosis. _Frontiers in genetics_ 3, 179 (2012). Article Google Scholar * Peter, M. _et al_. The APC is dispensable for first meiotic anaphase in Xenopus oocytes.
_Nature cell biology_ 3, 83–87 (2001). Article ADS CAS Google Scholar * Hu, M. W. _et al_. Scaffold subunit Aalpha of PP2A is essential for female meiosis and fertility in mice. _Biology
of reproduction_ 91, 19 (2014). Article Google Scholar * Meng, J. _et al_. The role of 14-3-3epsilon interaction with phosphorylated Cdc25B at its Ser321 in the release of the mouse
oocyte from prophase I arrest. _PloS one_ 8, e53633 (2013). Article ADS CAS Google Scholar * Li, L., Lu, X. & Dean, J. The maternal to zygotic transition in mammals. _Molecular
aspects of medicine_ 34, 919–938 (2013). Article Google Scholar * Wang, X. _et al_. Thoc1 deficiency compromises gene expression necessary for normal testis development in the mouse.
_Molecular and cellular biology_ 29, 2794–2803 (2009). Article CAS Google Scholar * Golas, M. M., Sander, B., Will, C. L., Luhrmann, R. & Stark, H. Molecular architecture of the
multiprotein splicing factor SF3b. _Science_ 300, 980–984 (2003). Article ADS CAS Google Scholar * Singh, K. K., Wachsmuth, L., Kulozik, A. E. & Gehring, N. H. Two mammalian MAGOH
genes contribute to exon junction complex composition and nonsense-mediated decay. _RNA biology_ 10, 1291–1298 (2013). Article CAS Google Scholar * Will, C. L. _et al_. Characterization
of novel SF3b and 17S U2 snRNP proteins, including a human Prp5p homologue and an SF3b DEAD-box protein. _The EMBO journal_ 21, 4978–4988 (2002). Article CAS Google Scholar * Minakuchi,
M. _et al_. Pre-mRNA Processing Factor Prp18 Is a Stimulatory Factor of Influenza Virus RNA Synthesis and Possesses Nucleoprotein Chaperone Activity. _Journal of virology_ 91 (2017). *
Gonzalez-Duarte, R. J. _et al_. The expression of RNA helicase DDX5 is transcriptionally upregulated by calcitriol through a vitamin D response element in the proximal promoter in SiHa
cervical cells. _Molecular and cellular biochemistry_ 410, 65–73 (2015). Article CAS Google Scholar * Peelman, L. J. _et al_. The BAT1 gene in the MHC encodes an evolutionarily conserved
putative nuclear RNA helicase of the DEAD family. _Genomics_ 26, 210–218 (1995). Article CAS Google Scholar * Ladstatter, S. & Tachibana-Konwalski, K. A Surveillance Mechanism Ensures
Repair of DNA Lesions during Zygotic Reprogramming. _Cell_ 167, 1774–1787 e1713 (2016). Article Google Scholar * Touati, S. A. _et al_. Cyclin A2 is required for sister chromatid
segregation, but not separase control, in mouse oocyte meiosis. _Cell reports_ 2, 1077–1087 (2012). Article CAS Google Scholar * Yanez, L. Z., Han, J., Behr, B. B., Reijo Pera, R. A.
& Camarillo, D. B. Human oocyte developmental potential is predicted by mechanical properties within hours after fertilization. _Nature communications_ 7, 10809 (2016). Article ADS CAS
Google Scholar * Wang, A. T. _et al_. A Dominant Mutation in Human RAD51 Reveals Its Function in DNA Interstrand Crosslink Repair Independent of Homologous Recombination. _Molecular cell_
59, 478–490 (2015). Article CAS Google Scholar * Tanaka, K., Kagawa, W., Kinebuchi, T., Kurumizaka, H. & Miyagawa, K. Human Rad54B is a double-stranded DNA-dependent ATPase and has
biochemical properties different from its structural homolog in yeast, Tid1/Rdh54. _Nucleic acids research_ 30, 1346–1353 (2002). Article CAS Google Scholar * Kurumizaka, H. _et al_.
Homologous-pairing activity of the human DNA-repair proteins Xrcc3.Rad51C. _Proceedings of the National Academy of Sciences of the United States of America_ 98, 5538–5543 (2001). Article
ADS CAS Google Scholar * Carney, J. P. _et al_. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage
response. _Cell_ 93, 477–486 (1998). Article CAS Google Scholar * Varon, R. _et al_. Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome.
_Cell_ 93, 467–476 (1998). Article CAS Google Scholar * Picelli, S. _et al_. Full-length RNA-seq from single cells using Smart-seq2. _Nature protocols_ 9, 171–181 (2014). Article CAS
Google Scholar * Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: discovering splice junctions with RNA-Seq. _Bioinformatics_ 25, 1105–1111 (2009). Article CAS Google Scholar *
Trapnell, C. _et al_. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. _Nature protocols_ 7, 562–578 (2012). Article CAS Google
Scholar * Deng, W., Wang, Y., Liu, Z., Cheng, H. & Xue, Y. HemI: a toolkit for illustrating heatmaps. _PloS one_ 9, e111988 (2014). Article ADS Google Scholar * Huang da, W.,
Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. _Nucleic acids research_ 37, 1–13 (2009).
Article Google Scholar * Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. _Nature protocols_
4, 44–57 (2009). Article Google Scholar Download references ACKNOWLEDGEMENTS We thank the voluntary research participants and all the doctors and embryologists in our center. We also
thank Qiang Wu (SJTU) for technique assistance for RNA sequencing and Aaron J. Hsueh (Stanford) for critical reading of manuscript. This work was supported by grants from the National
Natural Science Foundation of China (Grant No. 31200825, 81571397 and 81571486), the Fundamental Research Funds for the Central Universities (17JCYB12), Shanghai Committee of Science and
Technology, China (Grant No. 16411963800) and Shanghai Three-year Plan on Promoting TCM Development, China (Grant No. ZY3-LCPT-2-2006). AUTHOR INFORMATION Author notes * Lun Suo and Yu xiao
Zhou contributed equally. AUTHORS AND AFFILIATIONS * Department of Assisted Reproduction, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Lun Suo, Hai bo Wu, Qi feng Lyu, Li hua Sun & Yan ping Kuang * Center for Comparative Biomedicine, MOE Key Laboratory of Systems Biomedicine, Institute of Systems Biomedicine, SCSB,
Shanghai Jiao Tong University (SJTU), Shanghai, 200240, China Yu xiao Zhou, Li ling Jia & Jin Zheng * Department of Genetics, School of Medicine, Stanford University, Stanford, CA,
94305, USA Han Sun Authors * Lun Suo View author publications You can also search for this author inPubMed Google Scholar * Yu xiao Zhou View author publications You can also search for this
author inPubMed Google Scholar * Li ling Jia View author publications You can also search for this author inPubMed Google Scholar * Hai bo Wu View author publications You can also search
for this author inPubMed Google Scholar * Jin Zheng View author publications You can also search for this author inPubMed Google Scholar * Qi feng Lyu View author publications You can also
search for this author inPubMed Google Scholar * Li hua Sun View author publications You can also search for this author inPubMed Google Scholar * Han Sun View author publications You can
also search for this author inPubMed Google Scholar * Yan ping Kuang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.S., Y.X.Z. and
Y.P.K. designed the study; L.S., Q.F.L. and L.H.S. collected the samples; Y.X.Z., L.L.J. and J.Z. constructed the library; L.S. and H.B.W. performed the RNAi experiment; L.S., Y.X.Z. and
H.S. analyzed the data; L.S. and Y.P.K. supervised the study; L.S. wrote the manuscripts. CORRESPONDING AUTHORS Correspondence to Lun Suo or Yan ping Kuang. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION DATASET 1 DATASET 2 DATASET 3 DATASET 4 DATASET 5 DATASET 6 RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as
you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party
material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s
Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Suo, L., Zhou, Y.x., Jia, L.l. _et al._
Transcriptome profiling of human oocytes experiencing recurrent total fertilization failure. _Sci Rep_ 8, 17890 (2018). https://doi.org/10.1038/s41598-018-36275-6 Download citation *
Received: 11 July 2018 * Accepted: 16 November 2018 * Published: 17 December 2018 * DOI: https://doi.org/10.1038/s41598-018-36275-6 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative