Multifunctional paper-based analytical device for in situ cultivation and screening of escherichia coli infections
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Point-of-care testing (POCT) for uropathogen detection and chemical screening has great benefits for the diagnosis of urinary tract infections (UTIs). The goal of this study was to
develop a portable and inexpensive paper-based analytical device (PAD) for cultivating bacteria _in situ_ and rapidly testing for nitrite on the same device. The PAD was fabricated using a
wax printing technique to create a pattern on Whatman No. 1 filter paper, which was then combined with a cotton sheet to support bacterial growth. Nitrite detection was based on the
principle of the Griess reaction, and a linear detection range of 0–1.6 mg/dL (R2 = 0.989) was obtained. Scanning electron microscopy (SEM) analysis demonstrated that the bacteria were able
to grow and formed a cluster on the cellulose fibres within 2 hours. The enzyme β-glucuronidase, which is specifically produced by _Escherichia coli_, was able to convert the pre-immobilized
5-bromo-4-chloro-3-indolyl-β-D-glucuronide sodium salt (X-GlcA), a colourless substrate, generating a blue colour. Under optimum conditions, the proposed device allowed bacterial
concentrations in the range of 104–107 colony forming units (CFU)/mL to be quantified within 6 hours. Moreover, the use of this device enables the identification of _E_. _coli_ pathogens
with selectivity in real urine samples. In conclusion, the PAD developed in this study for UTI screening provides a rapid, cost-effective diagnostic method for use in remote areas. SIMILAR
CONTENT BEING VIEWED BY OTHERS VALIDATION OF QUANTITATIVE LOOP-MEDIATED ISOTHERMAL AMPLIFICATION ASSAY USING A FLUORESCENT DISTANCE-BASED PAPER DEVICE FOR DETECTION OF _ESCHERICHIA COLI_ IN
URINE Article Open access 31 October 2023 RAPID ANTIMICROBIAL SUSCEPTIBILITY TESTING USING CARBON SCREEN PRINTED ELECTRODES IN A MICROFLUIDIC DEVICE Article Open access 11 February 2025
RAPID UROPATHOGEN IDENTIFICATION USING SURFACE ENHANCED RAMAN SPECTROSCOPY ACTIVE FILTERS Article Open access 22 April 2021 INTRODUCTION In the developing world, infectious diseases are the
most common cause of illness, resulting in more than 1.2 million deaths each year in those countries1,2. The development of simple, inexpensive, robust and portable point-of-care diagnostic
devices for the early detection of infectious diseases remains an urgent need for use in most developing countries1,2,3,4. To cover the guidelines recommended by the World Health
Organization (WHO)5, the ideal diagnostic test should follow the ASSURED criteria, including being affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free and
deliverable5. Among all the types of point of care diagnostic devices, paper-based sensors have become attractive and promising to meet the ASSURED criteria, because as paper is cost
effective, flexible and biocompatible1,5,6,7. Moreover, microfluidic paper-based analytical devices (µPADs) have received particularly interest for detecting various types of analytes,
including biological fluid biomarkers8,9,10, pathogens and contamination11,12,13,14,15,16, and metal compound monitoring17. For pathogen monitoring, contamination caused by foodborne and/or
pathogens are also a significant public health issue18,19, and bacterial infections, such as blood, urinary tract, and respiratory tract infections are regarded as a major cause of morbidity
and mortality20. Thus, the development of a rapid test for the early detection of bacterial infections would be valuable for the diagnosis of such infectious diseases. Urinary tract
infections (UTIs) are one of the most frequent hospital-acquired infections and are caused by a wide range of pathogens, including bacteria, fungi, viruses and parasites21. For the global
burden of disease study 2016, the interstitial nephritis and urinary tract infections affected health loss more than 4 million people in 195 countries and territories22. Members of the
family Enterobacteriaceae are gram-negative bacilli that are the most common cause of UTIs, with _E_. _coli_ being the most common pathogen causing both uncomplicated and complicated UTIs21.
To date, the gold standard for the diagnosis of UTIs requires both a physical examination and a microbiological assay in urine culture23,24,25. The presence of bacterial cells above 105
CFU/mL, together with the detection of inflammatory cells in sterile urine, is clinically significant for UTIs24. In addition to a microbiological test, nitrite and leukocyte esterase
testing have been used to confirm _E_. _coli_ infection25,26. Although the conventional methods used to diagnose UTIs are widely used in most clinical laboratories, the development of an
alternative method that is faster and easier would be a significant advancement27. The long incubation time, at least 1–2 days, is a major shortcoming of the conventional culture method.
This long incubation time contributes to the delay of treatment and the spread of infectious disease, leading to the misuse of antibiotics and the development of antibiotic resistance28.
Novel approaches enabling faster bacterial analysis must be able to accurately identify pathogens, which would contribute to the effectively antimicrobial therapy25,28. Currently, rapid
bacterial detection methods, e.g., the FLEXICULT™ SSI-Urinary Kit, are available in the market29. However, this kit is rather expensive and still requires one day for bacterial culturing. To
date, molecular biology techniques have been used to detect microorganisms and in epidemiological studies30,31,32, such as multiplex PCR methods used to detect _E_. _coli_ serogroups31.
Nevertheless, molecular techniques are limited to the laboratory and should be performed in a closed system to prevent contamination30,32. The use of paper7 and/or other types of
biocompatible substrates, such as cotton threads33,34, cloth35, cotton36 and lignocellulose37 have become attractive in biosensors research because of their flexibility and cost
effectiveness. Several studies have reported on the use of paper-based analytical devices for the quantitative analysis of nitrite and nitrate based on colourimetric assays8,38,39,40. These
assays can be used to quantify the target analytes in variety type of samples, such as saliva8,38 and drinking water39. However, previous reports have not focused on the development of
sensors for monitoring UTIs8,38,39,40. A paper-based device has been reported for the culturing and identification of bacteria based on the T4 bacteriophage infection of _E_. _coli_ cells
and the detection of released β-galactosidase, and this device has been used for environment monitoring41. A major drawback of this method is the utilization of T4 bacteriophage, which is
known to infect only 60% of _E_. _coli_ strains41, raising the possibility of false negative results being obtained. To pre-concentrate the bacteria from complex sample matrices,
immunomagnetic separation (IMS) has been utilized in which samples are mixed with antibody-attached beads to capture the cells of interest11,42. Combining IMS with paper-based devices has
been reported for the detection of _E_. _coli_42 and _S_. _typhimurium_11 in complicated sample matrices. To detect UTIs and gonorrhoea on µPADs, the principle used is based on the
immunoagglutination of antibody-conjugated particles and the specific targeting and detection of nitrite using a commercial strip. The detection limit determined for both _E_. _coli_ and
gonorrhoea using this method was 10 CFU/mL43. In addition to their use as PADs, lateral flow test strips have been successfully developed for multiplex analysis of whole bacterial cells,
which were applied in point-of-care diagnostics tests because these tests allow real-time monitoring, simultaneous detection and short analysis times44,45. A significant benefit of
immunoassays is the high specificity and high sensitivity for detecting low concentrations of bacteria. However, the limitation of antibody-based bacterial detection is its inability to
distinguish between living and dead cells41. The differentiation of live or dead cells is regarded as an important requirement for antibiotic treatment. Recently, the use of PADs to detect
β-lactamase producing bacteria based on the reaction between β-lactamase and nitrocefin was demonstrated and was used to detect β-lactam resistance in wastewater and sewage12. The properties
of PADs make them useful as simple and low-cost platforms for bacterial growth46, identification14,16 and susceptibility testing15. However, only a few studies have described the
development of PADs for both the cultivation and identification of bacterial species and for their use in screening for urinary tract infections. The goal of this study was to develop a new,
simpler PAD for the simultaneous detection of nitrite and bacterial cultivation and identification from urine samples on the same device. This is the first report on the use of Whatman No.
1 filter paper with cotton pads for supporting bacterial growth on a PAD. The moisture absorption and biocompatibility36 qualities of cotton make it an excellent choice of materials for
supporting bacterial growth. To allow for rapid screening of gram-negative bacteria, nitrite detection based on the Griess reaction47 was utilized. In concurrence with nitrite detection, a
biochemical test for the production of the β-glucuronidase was also included in the PAD. Because approximately 95% of _E_. _coli_ strains release β-glucuronidase48, our proposed PAD should
be specific for _E_. _coli_ detection in clinical samples. Importantly, the colour change on the culture area can be visually detected. Because the bacterial isolation step is omitted using
this device, our proposed PAD has promise for use in the rapid screening of UTIs, especially in remote areas. RESULTS FABRICATION AND CHARACTERIZATION OF PAPER-BASED ANALYTICAL DEVICE (PAD)
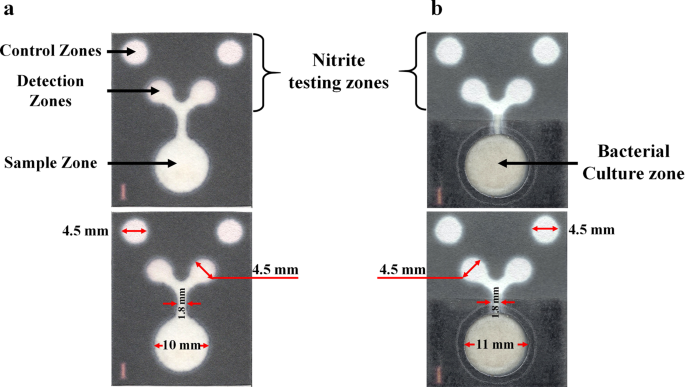
During the PAD fabrication process, a hydrophobic area was created using a wax printing technique, and the prototype PAD for nitrite determination is shown in Fig. 1a. To allow for the
multiple functions of the PAD, the printed papers were successfully combined with the cotton pad, in which the latter was intended for use as the supporting area for bacterial cultivation
(Fig. 1b). The PAD fabrication process is illustrated in Supplementary Fig. S1. The surface properties of the paper before and after sterilization were characterized using Field Emission
Scanning Electron Microscopy (FESEM). To avoid contamination, the selection of a sterilization process is very important and is inevitably performed on every PAD before used. In this study,
an autoclave was used to sterilize the PADs. The high temperature reached during sterilization affected the wax melting, leading to a narrowing of the hydrophilic flow channel that resulted
in a non-uniform pattern formation49. To solve this problem, clear packing tape was attached to one side of paper and then was placed in a heat-resistant plastic bag before being autoclaved.
Interestingly, the attachment of the packing tape underneath the paper resulted in a uniformly patterned PAD as shown in Supplementary Fig. S2. To confirm the surface morphology of the
cellulose-based paper after exposure to high temperature, three types of surface paper treatment were performed and assayed by FESEM, including (1) a plain Whatman No. 1, (2) Whatman No. 1
heated at 150 °C for 2 minutes and (3) Whatman No. 1 heated at 150 °C for 2 minutes and autoclaved at 121 °C for 20 minutes. Figure 2 displays the microstructures of the three different
papers as determined by the FESEM analysis. The visual assessment suggests that heating the paper did not affect the structure of the paper device. STANDARD CURVE OF NITRITE AND REAL SAMPLE
ANALYSIS The first pattern of papers (Fig. 1a) was used to generate a standard curve for nitrite and to quantify the nitrite level in the urine control. The nitrite test was based on the
Griess reaction, wherein nitrite reacts with sulfanilamide under acidic conditions to form a diazonium salt and then couples to N-(1-naphthyl)-ethylenediamine dihydrochloride (NED) to
produce a red-pink colour47. NED is photosensitive and changes to pink when exposed to ambient light. To overcome this limitation, control areas were created on the proposed paper, and the
mean colour intensity of each concentration was calculated by subtracting the intensity of the testing areas from the intensity of the control areas8. Figures 3a,b display a colour change on
the paper-based device that is proportional to the nitrite concentration. The curve shows increasing colour intensity with increasing nitrite concentration. The linear function of this
curve is y = 24.90x + 12.03, R2 = 0.970 for Whatman No. 1 filter paper and y = 22.56x + 11.32, R2 = 0.989 for sterilized Whatman No. 1 filter paper, as shown in Fig. 3c,d. The data from the
FESEM analysis and the slope of linear function of the standard curve indicate that the sterilization process did not affect the surface morphology of the cellulose paper or the nitrite
measurement. For real sample analysis, urine controls (level 1) spiked with various concentrations of nitrite were used to test the performance of the sensor, and the measured nitrite values
are shown in Table 1. Surprisingly, when using volumes less than 20 µL, the nitrite concentrations in the control urine did not correlate well with spiked concentrations. These inconsistent
results may have occurred because of the difference in viscosity between water and the control urine, particularly when an insufficient sample volume was used. The nitrite standard
dissolved in distilled water has a specific gravity of 1, whereas the MAS® UA control (level 1) has a specific gravity of approximately 1.005–1.020 (Manufacturer leaflet of MAS® UA control,
Thermo Scientific, MA, USA). Therefore, the time required for the standard nitrite solution and the spiked control urine to reach the detection zones was different. This difference led to
the reaction time of the control urine being shorter than those of standard nitrite solutions, leading to lower concentrations being calculated. This phenomenon has also been observed with
respect to tear fluid viscosity50 and saliva viscosity8. A good correlation was obtained for the control urine at sample volumes of 20 µL and higher. However, when using volumes 22 or 25 µL,
the PADs became soaked and required assay times longer than 10 min until the PADs were ready for imaging. Because the sample zone became overloaded when 30 µL of sample was used, this
volume was not suitable for this size of PAD (Supplementary information, Fig. S3). Therefore, a sample volume of 20 µL was used for the subsequent experiments. The reproducibility of the
sensor for measuring nitrite was evaluated at nitrite concentrations of 0.5 and 1.0 mg/dL, and coefficients of variation (CVs) of 9.07% (0.58 ± 0.05) and 7.90% (1.02 ± 0.08) (n = 20) were
observed, respectively. These results demonstrate that the proposed PAD has the capability of quantifying nitrite in urine samples. OPTIMUM CONCENTRATION OF
5-BROMO-4-CHLORO-3-INDOLYL-Β-D-GLUCURONIDE SODIUM SALT (X-GLCA) SUBSTRATE The hydrolytic properties of the β-glucuronidase enzyme from _E_. _coli_ with the X-GlcA substrate were studied on
LB agar. The colour change of bacterial colonies on agar from colourless to blue can be observed with the naked eye at substrate concentrations ranging from 4 to 12 mg/mL. Figure 4 shows the
generation of blue colonies in the presence of various concentrations of the X-GlcA substrate. The substrate concentrations that produced deep blue colonies, i.e., 6, 8, 10 and 12 mg/mL,
were selected for further testing with the proposed PAD. Subsequently, the optimum X-GlcA substrate concentration and reaction time on the proposed paper were studied using the proposed
device. For the sensor, colour changes could be observed at 4 hours for all substrate concentrations. An X-GlcA concentration of 10 mg/mL and a reaction time of 6 hours were selected for the
generation of analytical curves for bacterial detection. The colour intensities of the paper devices using varying substrate concentrations and incubation times are shown in Supplementary
Fig. S4. FIELD EMISSION SCANNING ELECTRON MICROSCOPY (FESEM) ANALYSIS OF BACTERIAL CULTURE ON THE PAD FESEM analysis was used to ensure the growth of the bacterial cells on the PAD. As shown
in Fig. 5d, a visual assessment of the PAD demonstrated that the bacteria grew and formed a cluster on the cellulose fibres of Whatman No. 1 filter paper within 2 hours. The fibrous and
porous properties of filter paper promote bacterial cell attachment, as shown in Fig. 5d–e. Moreover, the bacterial growth on the proposed devices was supported by the addition of a cotton
pad, which has absorptive properties and provides a stable platform36. Therefore, it can be concluded that cellulose fibres combined with cotton and agar can be used for bacterial
cultivation. ANALYTICAL RANGE FOR _E_. _COLI_ DETECTION ON THE PROPOSED PAD Based on the intensity of the blue pigment, colour changes on the proposed device can be observed within 4 hours,
which corresponds to the exponential phase of bacterial growth. The proposed device could quantify _E_. _coli_ with a detection range of 104–107 CFU/mL within 6 hours. Moreover, below 104
CFU/mL, the concentration of _E_. _coli_ could be monitored by the naked eye when incubated on the device for 10 hours, as shown in Supplementary Fig. S5. The observed colour changes for
different quantities of _E_. _coli_ cells on the PADs are presented in Fig. 6a. Figure 6b shows the correlation between colour intensity and the logarithm of the concentration of _E_. _coli_
cells, R2 = 0.982. As shown in Fig. 6a, the minimum concentration of _E_. _coli_ at which a change in the X-GlcA substrate from colourless to a blue colour could be visually observed by the
naked eye at 6 hours was 104 CFU/mL. To confirm the limit of detection (LOD) of this proposed method, a single colony of _E_._coli_ was inoculated in LB broth followed by incubation for 4
hours at 37 °C with continuous shaking. Subsequently, the 10-fold serial dilutions of bacterial solution were prepared and each bacterial suspension was portioned to culture on the proposed
PADs. The actual concentration of _E_. _coli_ in each suspension was determined by a standard plate counting method. The minimum concentration of _E_. _coli_ able to convert the colourless
X-GlcA substrate to the blue pigment as visually observed on the PAD at 6 hours was considered as the LOD of this method. The result demonstrated that the concentration of _E_. _coli_ in LB
broth was 1.57 ± 0.46 × 107 CFU/mL, as determined in triplicate experiments by plate counting method. After 10-fold serial dilutions, the lowest concentration of _E_. _coli_ able to produce
blue pigment as visually observed by the naked eye at 6 hours was 1.57 × 104 CFU/mL (n = 4), as shown in the Supplementary data Fig. S6a. Interestingly, the concentrations of _E_. _coli_ up
to 103 CFU/mL could be visually detected after 7 hours incubation, and the image is shown in Supplementary Fig. S6b. However, based on 6 hours incubation, the detection limit of 104 CFU/mL
is quite high compared to that observed in other studies41,43. Nevertheless, the range for _E_. _coli_ detection by our proposed paper sensor for screening bacteria in urinary tract
infection (UTIs) covers that needed to diagnose UTIs, which requires the presence of bacterial cells above 105 CFU/mL24. However, the proposed device could be used to monitor _E_. _coli_ in
single step without adding the inducer or indicator dye and omitting the bacterial isolation step. Moreover, the PAD could be combined with additional chemical screening functionalities,
including for nitrite, protein and leukocyte esterase testing to confirm an infection25,26. REAL SAMPLE ANALYSIS AND SPECIFICITY STUDY After a prototype device for nitrite determination and
bacterial cultivation was successfully developed, urine samples were tested on the proposed device. As shown in Fig. 7, the nitrite detection results at the nitrite detection zones show a
good correlation with the nitrite strip test assay. However, the colour change in the nitrite detection area of the PAD are paler than that observed using a strip test and the nitrite
standard on PADs, which may result from the effect of sample loss in µPADs51. Moreover, the immersion of the paper surface in the culture area with medium resulted in a decrease in the
absorption properties of the cellulose fibre. For the bacterial analysis, two urine samples from healthy subjects (samples 1 and 2) were assayed that contained approximately <500
bacterial cells as determined by a standard plate count. Samples 3, 4, 5 and 6 contained _E_. _coli_, _P_. _mirabilis_, _S_. _saprophyticus_ and _K_. _pneumoniae_, respectively, and all of
the urine samples contained >105 CFU/mL of the assayed bacteria. As shown in Fig. 7, the blue colour in the culture area of sample 3 could be observed via the naked eye within 6 hours,
whereas the samples containing other bacteria (_P_. _mirabilis_, _S_. _saprophyticus_ or _K_. _pneumoniae_) could not produce the blue pigment in the proposed PAD. Therefore, it can be
concluded that the proposed PAD holds considerable promise for the simultaneous determination of nitrite and _E_. _coli_ in urine samples with high specificity. DISCUSSION In the present
study, we describe a simple and low-cost PAD for the simultaneous determination of nitrite and for bacterial cultivation and identification in a single step. The various viscosities of
biological samples have a significant impact on the fluid flow in paper channels8. Although, the described PAD is promising for nitrite detection, the inconsistent nitrite measurement
results may result from the viscosity of the urine solution. The nitrite standard dissolved in distilled water has a specific gravity of 1, whereas the MAS® UA control (level 1) has a
specific gravity of approximately 1.005–1.020 (Leaflet of MAS® UA control, Thermo Scientific). This phenomenon is similar to that observed for tear fluid viscosity50 and saliva viscosity8,
which is particularly important when making PAD-based measurements. Previous results have revealed that the wicking distance in the paper channel depends on the time of sample flow and on
the fluid viscosity50. This behaviour can be described by a modified Navier- Stokes equation, as shown in (1)50. According to this equation, the fluid viscosity can be $$t=\eta
{L}^{{\bf{2}}}/k{\rm{\Delta }}P$$ (1) where _t_ is the time, _η_ is the viscosity, _L_ is the wicking distance, _k_ is the permeability of the fluid, and Δ_P_ is the applied pressure
difference50. It is generally known that Griess reagent is photosensitive and unstable when exposed to ambient light. Therefore, several studies have reported a strategy to prevent the
degradation of Griess reagent, i.e., separating the components of the Griess reagent38 and by adding Nafion to the nitrite cocktail to avoid the leaching of the cationic azo-dye formed by
the reaction40. However, in this study, the problem of high background colour was overcome by providing a control area on the PAD for subtracting the colour background intensity.
Furthermore, the combined use of a paper-based device and a cotton pad was designed especially for supporting bacterial growth. Generally, the growth of bacteria in liquid medium is divided
into 4 phases, including the lag, exponential, stationary and death phases. In the optimal culture media, _E_. _coli_ adapts and divides at a constant rate for approximately 100 minutes52.
As shown in the FESEM image, the observed rate of _E_. _coli_ growth on the PAD was similar to the growth of the bacterium in liquid medium. Because both paper and cotton are biocompatible
materials, they have been used as substrates for a variety of biomedical applications53. Although the bacteria were able to grow and formed a cluster on the PADs within 2 hours, the number
of bacteria was not sufficient for monitoring by colourimetric assay. However, a detection time of 6 hours is significantly shorter than that of a conventional bacterial culture, which
requires at least 24 hours for a UTI diagnosis26. Using our proposed devices, 104–107 CFU/mL of _E_. _coli_ could be quantified within 6 hours. However, the limit of detection is rather high
when compared to those reported in previous studies. Using a method based on β-galactosidase gene-carrying T4 bacteriophage to infect bacterial cells, less than 10 CFU/mL of _E_. _coli_ can
be detected within 8 hours by a colourimetric assay and within 5.5 hours by a bioluminescence assay41. Unfortunately, T4 bacteriophage only infects 60% of _E_. _coli_ strains41. The purpose
of this study was to develop a screening method for UTIs, and the sensitivity of the described device was sufficient to detect bacteria in range required for the diagnosis of UTIs, as
bacteria were detected above 105 CFU/mL24. The PAD design concept used in this study was similar to previous reports describing the use of a paper substrate, packing tape and PDMS membrane
to fabricate culture devices46. However, we used low-cost material and instrumentation that is available in developing countries to create our culture device. For specificity testing,
indoxyl-β-D-glucuronide chromogenic β-glucuronidase has a sensitivity of 88 to 90% and a specificity of 100% for the rapid detection of _E_. _coli_ on MacConkey agar54. Our results showed
that only _E_. _coli_, which causes more than 70% of UTIs, could change the colour of the X-GlcA substrate. The chromatic change can be observed with the naked eye, whereas fluorogenic
substrates such as 4-methylumbelliferyl-β-D-glucuronide (MUG) require specific wavelengths of light for visualization55. CONCLUSIONS In this study, a portable and cost-effective paper-based
analytical device (PAD) for screening UTIs was successfully developed. The method offers an advantage of _in situ_ bacterial cultivation and identification, in which the method was faster
than the standard culture method26. The specificity of the proposed method was achieved using a β-glucuronidase-specific substrate, as approximately 95% of _E_. _coli_ strains can release
this enzyme48. Quantification of the number of bacterial cells in urine samples greatly benefits the diagnosis and prognosis of UTIs. Moreover, this platform can be used to quantify nitrite
over the entire range of normal and clinically significant levels. Therefore, UTIs caused by _E_. _coli_ can be confirmed using this novel PAD. The system is not limited to nitrite and
bacterial detection, as it can be extended by being combined with other biochemical tests, e.g., drug susceptibility testing. Moreover, this paper-based platform can be incorporated with
other detection techniques, such as fluorescence-based assays and electrochemical techniques, for rapid monitoring of bacterial cells. MATERIALS AND METHODS MATERIALS AND REAGENTS The
materials used to fabricate the PAD described in this study included Whatman No. 1 filter paper (Cat No. 1001–185), obtained from GE Healthcare UK Limited, UK, and cotton pads (Shiseido
Cleansing Cotton, Shiseido, Japan), purchased from a cosmetic shop in Thailand. Adhesive tape (CROCO, clear, 72-mm) and aluminium foil were obtained from a local grocery store. The chemicals
used to prepare the detection areas and to generate the calibration curve for nitrite included sulfanilamide (≥99%, CAS No. 63741), citric acid (≥99.5%, CAS No. 77929), and N-(1-naphthyl)
ethylenediamine dihydrochloride (≥98%, CAS No. 1465254), as well as a nitrite standard for IC 1,000 mg/L ± 4 mg/L (Pcode No. 101693502) were purchased from Sigma-Aldrich, USA. For bacterial
culture and identification, 5-bromo-4-chloro-3-indolyl-β-D-glucuronide sodium salt (≥98%, Pcode 1001943317) was purchased from Sigma-Aldrich, USA. Quality control bacterial strains,
including _Escherichia coli_ ATCC® 25922TM, _Proteus mirabilis_ ATCC®12453TM, _Klebsiella pneumoniae_ subsp. _pneumoniae_ ATCC® 13883TM and _Staphylococcus saprophyticus_ ATCC® 15305TM were
purchased from Microbiologics, Inc., USA. Agar A, yeast extract and tryptone powder were obtained from Bio Basic Canada, Inc., Canada. Sodium chloride (CAS No. 7647145) was purchased from
Merck, Germany. Tryptic soy broth (CAS No. 63741) was obtained from Sigma-Aldrich, USA. Tryptone soy agar and nutrient agar plates were obtained from Oxoid Limited, UK. Two levels of MAS® UA
control (liquid assayed urinalysis control) were purchased from Thermo Scientific, USA. PAPER-BASED DEVICE FABRICATION PROCESS The prototype PAD consists of two regions, one for a nitrite
assay and one for bacterial culturing. The PAD pattern was created by using a wax printing technique to construct hydrophobic barriers on the paper. The first prototype consisted of one
sample zone (12-mm diameter) for sample application, two detection zones (6.5-mm diameter) and two control zones (6.5-mm diameter) for nitrite determination. The pattern was printed on
Whatman No. 1 filter paper using a wax printer, after which the printed paper was placed on a hot plate at 150 °C for 2 minutes. Subsequently, clear packing tape was adhered to one side of
the PAD prior to sterilization of the PAD by autoclaving. The dimensions of the proposed device are shown in Fig. 1a. For the nitrite assay, Griess reagent was immobilized onto the detection
and control zones and was subsequently allowed to air-dry in darkness at room temperature. In this study, the second PAD prototype consisted of an area for nitrite detection combined with
an area for bacterial cultivation (Fig. 1b). An 11-mm diameter hole was cut out of the sample zone of the wax printed paper using a circle cutter, and then the back side of the entire PAD
was coated with a clear packing tape. Next, a 10-mm diameter cotton pad was inserted into the hole of the PAD, where the cotton pad was placed over the packing tape. For sterilization, the
PADs were placed in a heat-resistant plastic bag to protect it from penetration by water stream and then were autoclaved and allow to dry in a hot-air oven at 65 °C overnight. To prepare the
bacterial culture area, 2% Luria-Bertani (LB) agar was pipetted over the cotton pad and the 5-bromo-4-chloro-3-indolyl-β-D-glucuronide sodium salt (X-GlcA) substrate was added to the
Whatman No. 1 filter paper. The paper was then folded, and the device was exposed to ultraviolet (UV) light for 15 minutes before use. The PAD fabrication process is illustrated in
Supplementary Fig. S1 and the pattern of the PAD is shown in Fig. 1b. NITRITE DETERMINATION ON THE PAD A stock solution of a 1,000 mg/L ± 4 mg/L (100 mg/dL) nitrite standard was diluted with
milliQ water to create standard solutions at concentrations of 0.05, 0.1, 0.2, 0.4, 0.8, 1.6 and 3.2 mg/dL. Nitrite determination on the PAD was performed based on the Griess reaction. The
reagent solution for the nitrite assay contains 50 mM sulfanilamide, 330 mM citric acid and 10 mM N-(1-naphthyl) ethylenediamine dihydrochloride. All the chemicals were dissolved in milliQ
water and were stored in the dark. To quantify nitrite alone, 0.7 μL of the nitrite solution was immobilized in the testing and control areas and was left to dry in the dark for 15 minutes.
Subsequently, 13 μL of nitrite standard was aliquoted into the sample area. After 10 minutes of colour development, the PAD was scanned using a flatbed scanner (Epson Perfection V39 colour
scanner, Epson Thailand Co Ltd. Bangkok, Thailand), and the colour intensity of the image was analysed using Adobe Photoshop CC 2015. The colour intensity was calculated by measuring the
magenta channel in Photoshop® (CMYK mode). BACTERIAL PREPARATION AND CULTIVATION To optimize the concentration of the 5-bromo-4-chloro-3-indolyl-β-D-glucuronide sodium salt (X-GlcA)
substrate for bacterial detection, of 2, 4, 6, 8 and 10 mg/dL solutions of the substrate were evaluated by spreading 40 μL of the solutions onto LB agar. Subsequently, _E_. _coli_ ATCC®
25922TM was streaked onto the agar medium and incubated at 37 °C for 24 hours. The concentrations that produced blue pigmented colonies were then selected for testing on the proposed PAD.
For bacterial detection on the PAD, the different concentrations of the X-GlcA substrate were aliquoted into the culture area of each PAD, and _E_. _coli_ ATCC 25922 at 104–105 CFU/mL,
determined based on the colony count method, was subsequently immobilized in this zone. The PAD was covered with a sterilized plastic sheet to prevent contamination and evaporation and was
incubated at 37 °C. The colour change on the PAD was recorded at 2, 4, 6, and 8 hours. FIELD EMISSION SCANNING ELECTRON MICROSCOPY (FESEM) ANALYSIS To confirm the surface properties of the
paper after sterilization, the surfaces of three types of paper, including Whatman No. 1 filter paper, Whatman No. 1 filter paper heated at 150 °C for 2 minutes and Whatman No. 1 filter
paper heated at 150 °C for 2 minutes, affixed with packing tape and autoclaved at 121 °C for 20 minutes, were analysed via Field Emission Scanning Electron Microscopy (FESEM; JEOL, model
JSM7610F, JAPAN). The papers were coated with platinum using a sputter coater (Quorum model Q150RS) and were observed by FESEM. To ensure bacterial growth on the paper, an _E_. _coli_
culture on the PAD was prepared to obtain a concentration of approximately 104–105 CFU/mL according to the colony count method, and the solution was then applied to the culture area.
Bacteria were grown on the filter paper for 0, 1, 2 and 3 hours, fixed overnight in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) and then were rinsed twice with phosphate buffer
and once with distilled water. The samples were dehydrated by a gradient series of ethanol (30, 50, 70, and 95% for 5 minutes each and 100% three times for 5 minutes each). The dehydrated
papers were dried to a critical point, coated with platinum and observed by FESEM. Filter paper with LB broth was used as a control. GENERATION OF A BACTERIAL STANDARD CURVE A standard curve
of _E_. _coli_ was obtained by inoculating one colony of _E_. _coli_ in LB broth followed by incubation at 37 °C with shaking overnight. The bacteria were enumerated by plating 10-fold
dilutions (10−1, 10−2, 10−3, 10−4, 10−5 and 10−6), and the number of bacteria was confirmed by the plate counting method. Subsequently, each bacterial culture was applied onto the PAD and
incubated at 37 °C for 6 hours. The colour change on the surface of the paper was recorded using a flat scanner, and the colour intensity was measured using the cyan channel in Photoshop®
(CMYK mode). REAL SAMPLE ANALYSIS AND SPECIFICITY TEST All research work was performed in accordance with relevant guidelines of a protocol approved by the Ethics Review Committee for
Research Involving Human Research Subjects, Health Science Group, Chulalongkorn University, Bangkok, Thailand. (COA No. 061.1/59). Midstream urine from healthy volunteers was collected in
sterile containers. and informed consent was obtained from all subjects involved in this study. The agar plates with four types of bacteria, including _E_. _coli_, _P_. _mirabilis_, _S_.
_saprophyticus_ and _K_. _pneumoniae_ were incubated at least 18 hours at 37 °C. A single colony of each bacterial species was picked and inoculated into sterile urine and incubated for 7–8
hours at 37 °C. Next, the nitrite standard was spiked into healthy urine to obtain a positive control as confirmed using a commercial rapid test. To perform the assay with the PAD, 20 µL of
urine was added onto the culture area and then was allowed to stand for 10 minutes to observe the colour change for nitrite detection. After incubating for 6 hours at 37 °C, the PAD images
were captured and analysed. The colour changes on the surface of the paper were recorded using a flat scanner. REFERENCES * Mabey, D., Peeling, R. W., Ustianowski, A. & Perkins, M. D.
Diagnostics for the developing world. _Nat. Rev. Microbiol._ 2, 231–240 (2004). Article CAS PubMed Google Scholar * Drain, P. K. _et al_. Evaluating Diagnostic Point-of-Care Tests in
Resource-Limited Settings. _Lancet Infect Dis._ 14, 239–249 (2014). Article PubMed Google Scholar * Lin, S.-C. _et al_. Paper-based CRP monitoring devices. _Sci. Rep._ 6, 38171 (2016).
Article ADS CAS PubMed PubMed Central Google Scholar * Sher, M., Zhuang, R., Demirci, U. & Asghar, W. Paper-based analytical devices for clinical diagnosis: recent advances in the
fabrication techniques and sensing mechanisms. _Expert Rev. Mol. Diagn._ 17, 351–366 (2017). Article CAS PubMed PubMed Central Google Scholar * Kosack, C. S., Page, A.-L. & Klatser,
P. R. A guide to aid the selection of diagnostic tests. _Bull World Health Organ._ 95, 639–645 (2017). Article PubMed PubMed Central Google Scholar * Mahato, K., Srivastava, A. &
Chandra, P. Paper based diagnostics for personalized health care: Emerging technologies and commercial aspects. _Biosens Bioelectron_ 96, 246–259 (2017). Article CAS PubMed Google Scholar
* Martinez, A. W., Phillips, S. T., Butte, M. J. & Whitesides, G. M. Patterned paper as a platform for inexpensive, low‐volume, portable bioassays. _Angew. Chem. Int. Ed. Engl._ 46,
1318–1320 (2007). Article CAS PubMed PubMed Central Google Scholar * Noiphung, J. _et al_. Development of paper-based analytical devices for minimizing the viscosity effect in human
saliva. _Theranostics_ 8, 3797–3807 (2018). Article CAS PubMed PubMed Central Google Scholar * Berry, S. B., Fernandes, S. C., Rajaratnam, A., DeChiara, N. S. & Mace, C. R.
Measurement of the hematocrit using paper-based microfluidic devices. _Lab Chip_ 16, 3689–3694 (2016). Article CAS PubMed Google Scholar * Songjaroen, T., Dungchai, W., Chailapakul, O.,
Henry, C. S. & Laiwattanapaisal, W. Blood separation on microfluidic paper-based analytical devices. _Lab Chip_ 12, 3392–3398 (2012). Article CAS PubMed Google Scholar * Srisa-Art,
M., Boehle, K. E., Geiss, B. J. & Henry, C. S. Highly Sensitive Detection of Salmonella typhimurium Using a Colorimetric Paper-Based Analytical Device Coupled with Immunomagnetic
Separation. _Anal. Chem._ 90, 1035–1043 (2017). Article PubMed Google Scholar * Boehle, K. E. _et al_. Utilizing Paper‐Based Devices for Antimicrobial‐Resistant Bacteria Detection.
_Angew. Chem. Int. Ed. Engl._ 56, 6886–6890 (2017). Article CAS PubMed PubMed Central Google Scholar * Suaifan, G. A., Alhogail, S. & Zourob, M. Paper-based magnetic
nanoparticle-peptide probe for rapid and quantitative colorimetric detection of _Escherichia coli_ O157: H7. _Biosens Bioelectron_ 92, 702–708 (2017). Article CAS PubMed Google Scholar *
Shih, C.-M. _et al_. Paper-based ELISA to rapidly detect _Escherichia coli_. _Talanta._ 145, 2–5 (2015). Article CAS PubMed Google Scholar * Deiss, F., Funes-Huacca, M. E., Bal, J.,
Tjhung, K. F. & Derda, R. Antimicrobial susceptibility assays in paper-based portable culture devices. _Lab Chip_ 14, 167–171 (2014). Article CAS PubMed Google Scholar * Jokerst, J.
C. _et al_. Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. _Anal. Chem._ 84, 2900–2907 (2012). Article CAS PubMed Google Scholar
* Hofstetter, J. C. _et al_. Quantitative colorimetric paper analytical devices based on radial distance measurements for aqueous metal determination. _Analyst_ 143, 3085–3090 (2018).
Article ADS CAS PubMed Google Scholar * Cabral, J. P. Water microbiology. _Bacterial pathogens and water. Int J Environ Res Public Health_ 7, 3657–3703 (2010). Article PubMed Google
Scholar * Fu, L., Valentino, H. & Wang, Y. In _Antimicrobial Food Packaging_ 35–43 (Elsevier 2016). * Simon, L., Gauvin, F., Amre, D. K., Saint-Louis, P. & Lacroix, J. Serum
procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. _Clin. Infect. Dis._ 39, 206–217 (2004). Article CAS PubMed Google
Scholar * Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. _Nat. Rev.
Microbiol._ 13, 269–284 (2015). Article CAS PubMed PubMed Central Google Scholar * Hay, S. I. _et al_. Global, regional, and national disability-adjusted life-years (DALYs) for 333
diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. _Lancet._ 390,
1260–1344 (2017). Article Google Scholar * Schmiemann, G., Kniehl, E., Gebhardt, K., Matejczyk, M. M. & Hummers-Pradier, E. The diagnosis of urinary tract infection: a systematic
review. _Dtsch Arztebl Int_ 107, 361–367 (2010). PubMed PubMed Central Google Scholar * Lee, J. B. & Neild, G. H. Urinary tract infection. _Medicine_ 35, 423–428 (2007). Article
Google Scholar * Sheerin, N. S. Urinary tract infection. _Medicine_ 39, 384–389 (2011). Article Google Scholar * Wilson, M. L. & Gaido, L. Laboratory diagnosis of urinary tract
infections in adult patients. _Clin. Infect. Dis._ 38, 1150–1158 (2004). Article PubMed Google Scholar * Paniel, N., Baudart, J., Hayat, A. & Barthelmebs, L. Aptasensor and genosensor
methods for detection of microbes in real world samples. _Methods_ 64, 229–240 (2013). Article CAS PubMed Google Scholar * Kelley, S. O. New technologies for rapid bacterial
identification and antibiotic resistance profiling. _SLAS TECHNOLOGY: Translating Life Sciences Innovation_ 22, 113–121 (2017). Google Scholar * Blom, M., Sørensen, T. L., Espersen, F.
& Frimodt-Møller, N. Validation of FLEXICULT™ SSI-Urinary Kit for Use in the Primary Health Care Setting. _Scand. J. Infect. Dis._ 34, 430–435 (2002). Article PubMed Google Scholar *
Liao, J. C. _et al_. Use of electrochemical DNA biosensors for rapid molecular identification of uropathogens in clinical urine specimens. _J Clin Microbiol_ 44, 561–570 (2006). Article CAS
PubMed PubMed Central Google Scholar * Li, D. _et al_. A multiplex PCR method to detect 14 _Escherichia coli_ serogroups associated with urinary tract infections. _J. Microbiol.
Methods_ 82, 71–77 (2010). Article CAS PubMed Google Scholar * Mach, K. E. _et al_. A biosensor platform for rapid antimicrobial susceptibility testing directly from clinical samples.
_J. Urol._ 185, 148–153 (2011). Article CAS PubMed Google Scholar * Nilghaz, A., Ballerini, D. R., Fang, X.-Y. & Shen, W. Semiquantitative analysis on microfluidic thread-based
analytical devices by ruler. _Sens Actuators B Chem_ 191, 586–594 (2014). Article CAS Google Scholar * Agustini, D., Bergamini, M. F. & Marcolino-Junior, L. H. Low cost microfluidic
device based on cotton threads for electroanalytical application. _Lab Chip_ 16, 345–352 (2016). Article CAS PubMed Google Scholar * Nilghaz, A. _et al_. Flexible microfluidic
cloth-based analytical devices using a low-cost wax patterning technique. _Lab Chip_ 12, 209–218 (2012). Article CAS PubMed Google Scholar * Lin, S.-C. _et al_. Cotton-based diagnostic
devices. _Sci_. _Rep_. 4 (2014). * Kuan, C.-M., York, R. L. & Cheng, C.-M. Lignocellulose-based analytical devices: bamboo as an analytical platform for chemical detection. _Sci. Rep._
5, 18570 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Bhakta, S. A., Borba, R., Taba, M., Garcia, C. D. & Carrilho, E. Determination of nitrite in saliva using
microfluidic paper-based analytical devices. _Anal. Chim. Acta_ 809, 117–122 (2014). Article CAS PubMed Google Scholar * Jayawardane, B. M., Wei, S., McKelvie, I. D. & Kolev, S. D.
Microfluidic paper-based analytical device for the determination of nitrite and nitrate. _Anal. Chem._ 86, 7274–7279 (2014). Article CAS PubMed Google Scholar * Lopez-Ruiz, N. _et al_.
Smartphone-based simultaneous pH and nitrite colorimetric determination for paper microfluidic devices. _Anal. Chem._ 86, 9554–9562 (2014). Article CAS PubMed Google Scholar * Burnham,
S. _et al_. Towards rapid on-site phage-mediated detection of generic _Escherichia coli_ in water using luminescent and visual readout. _Anal. Bioanal. Chem._ 406, 5685–5693 (2014). Article
CAS PubMed Google Scholar * Hossain, S. Z. _et al_. Multiplexed paper test strip for quantitative bacterial detection. _Anal. Bioanal. Chem._ 403, 1567–1576 (2012). Article CAS PubMed
Google Scholar * Cho, S., San Park, T., Nahapetian, T. G. & Yoon, J.-Y. Smartphone-based, sensitive µPAD detection of urinary tract infection and gonorrhea. _Biosens Bioelectron_ 74,
601–611 (2015). Article CAS PubMed Google Scholar * Li, C.-z. _et al_. Paper based point-of-care testing disc for multiplex whole cell bacteria analysis. _Biosens Bioelectron_ 26,
4342–4348 (2011). Article CAS PubMed Google Scholar * Zhao, Y. _et al_. Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel
lateral flow assay. _Sci_. _Rep_. 6 (2016). * Funes-Huacca, M. _et al_. Portable self-contained cultures for phage and bacteria made of paper and tape. _Lab Chip_ 12, 4269–4278 (2012).
Article CAS PubMed Google Scholar * Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the
L-arginine/nitric oxide area of research. _J Chromatogr B_ 851, 51–70 (2007). Article CAS Google Scholar * Adams, M., Grubb, S. & Hamer, A. & Clifford, M. Colorimetric enumeration
of _Escherichia coli_ based on beta-glucuronidase activity. _Appl. Environ. Microbiol._ 56, 2021–2024 (1990). CAS PubMed PubMed Central Google Scholar * Derda, R. _et al_. Multizone
paper platform for 3D cell cultures. _PLoS ONE_ 6, e18940 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Yetisen, A. K. _et al_. Paper-based microfluidic system for
tear electrolyte analysis. _Lab Chip_ 17, 1137–1148 (2017). Article CAS PubMed PubMed Central Google Scholar * Nguyen, M. P., Meredith, N. A., Kelly, S. P. & Henry, C. S. Design
considerations for reducing sample loss in microfluidic paper-based analytical devices. _Anal. Chim. Acta_ 1017, 20–25 (2018). Article CAS PubMed Google Scholar * Hall, B. G., Acar, H.,
Nandipati, A. & Barlow, M. Growth rates made easy. _Mol. Biol. Evol._ 31, 232–238 (2014). Article CAS PubMed Google Scholar * Ng, K. _et al_. Paper-based cell culture platform and
its emerging biomedical applications. _Mater. Today_ 20, 32–44 (2017). Article CAS Google Scholar * Delisle, G. & Ley, A. Rapid detection of _Escherichia coli_ in urine samples by a
new chromogenic beta-glucuronidase assay. _J Clin Microbiol_ 27, 778–779 (1989). CAS PubMed PubMed Central Google Scholar * Moberg, L. J. Fluorogenic assay for rapid detection of
_Escherichia coli_ in food. _Appl. Environ. Microbiol._ 50, 1383–1387 (1985). CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS J.N. gratefully acknowledges
the Thailand Research Fund through the Royal Golden Jubilee PhD Program (Grant No. PHD/0017/2556). This research was financially supported by the 90th Anniversary of Chulalongkorn University
Fund (Ratchadaphiseksomphot Endowment Fund) and The Faculty of Allied Health Sciences, Chulalongkorn University, Research grant. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Graduate
Program in Clinical Biochemistry and Molecular Medicine, Faculty of Allied Health Sciences, Chulalongkorn University, Patumwan, Bangkok, 10330, Thailand Julaluk Noiphung * Department of
Clinical Chemistry, Faculty of Allied Health Sciences, Chulalongkorn University, Patumwan, Bangkok, 10330, Thailand Wanida Laiwattanapaisal * Electrochemistry and Optical Spectroscopy Center
of Excellence (EOSCE), Chulalongkorn University, Bangkok, 10330, Thailand Wanida Laiwattanapaisal Authors * Julaluk Noiphung View author publications You can also search for this author
inPubMed Google Scholar * Wanida Laiwattanapaisal View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.N. and W.L. designed the experiments.
J.N. performed the experiments. Both authors analysed the data, discussed the results, and wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Wanida Laiwattanapaisal. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Noiphung, J., Laiwattanapaisal, W. Multifunctional Paper-Based Analytical Device
for _In Situ_ Cultivation and Screening of _Escherichia coli_ Infections. _Sci Rep_ 9, 1555 (2019). https://doi.org/10.1038/s41598-018-38159-1 Download citation * Received: 10 September 2018
* Accepted: 20 December 2018 * Published: 07 February 2019 * DOI: https://doi.org/10.1038/s41598-018-38159-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative