Three-dimensional stereoscopic visualization shortens operative time in laparoscopic gastrectomy for gastric cancer
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Laparoscopic gastrectomy for gastric cancer is now widely accepted and has become a standard surgery. This study investigated the advantages of three-dimensional (3D) stereoscopic
visualization for laparoscopic gastrectomy over a conventional two-dimensional (2D) planar screen. The primary outcome of this study was operative time. Ninety-four consecutive cases of
gastric cancer patients who underwent laparoscopic total gastrectomy (LTG) (25 cases) or laparoscopic distal gastrectomy (LDG) (69 cases) were enrolled in this study before and after the
introduction of the 3D system. Operative time was significantly shorter in the 3D groups for both LTG (351 vs. 406 min, _P_ = 0.026) and LDG (269 vs. 344 min, _P_ < 0.01). During
intracorporeal procedures, dissection time was significantly shorter in the 3D groups for both LTG (183 vs. 232 min, _P_ = 0.011) and LDG (161 vs. 213 min, _P_ < 0.01), although the time
needed for anastomosis was similar between the groups. However, operators preferred intracorporeal knot-tying as a ligature for anastomosis under 3D (LTG, _P_ = 0.012; LDG, _P_ < 0.01).
These data suggest that 3D stereoscopic visualization shortens the operative time of laparoscopic gastrectomy by reducing the intracorporeal dissection time. SIMILAR CONTENT BEING VIEWED BY
OTHERS INTEGRATING MINIMALLY INVASIVE BARIATRIC SURGERY WITH LESSONS FROM GASTRIC CANCER SURGERY Article Open access 24 March 2025 SAFETY AND FEASIBILITY OF REDUCED-PORT ROBOTIC DISTAL
GASTRECTOMY FOR GASTRIC CANCER: A PHASE I/II CLINICAL TRIAL USING THE DA VINCI SINGLE PORT(SP) ROBOTIC SYSTEM Article Open access 30 October 2023 ADVANTAGES OF THE SPLIT-LEG SUPINE POSITION
SINGLE-PORT PLUS ONE LAPAROSCOPIC SURGERY APPROACH Article Open access 09 November 2024 INTRODUCTION Laparoscopic gastrectomy has been widely accepted as a minimally invasive surgery and has
become one of the standard surgeries for early gastric cancer. Although there is no evidence for the oncological superiority of laparoscopic surgery over open surgery for early gastric
cancers, recent meta-analyses have demonstrated its clinical benefits such as fewer postoperative complications and shorter postoperative hospital stays1,2. Moreover, its application has
been extended to advanced gastric cancers that require D2 lymphadenectomy and/or combined resections3,4. However, it can still be technically challenging, especially when advanced
lymphadenectomy or oesophagojejunostomy (E-J) is required. For these advanced procedures, the magnifying effect of the laparoscope is an advantage. Conversely, the lack of depth perception
with a conventional two-dimensional (2D) laparoscopic planar screen may be a disadvantage that frustrates surgeons, resulting in increased operative time. Recent advances in surgical devices
are outstanding with new, innovative designs available almost every year. Three-dimensional (3D) stereoscopic laparoscopy is one such recent innovation. To date, there are some reports
showing the benefit of 3D stereoscopic visualization in dry box training for tasks that require high levels of precise spatial perception, such as suturing and knot-tying5,6. In addition to
the benefit of dry box training, there are some reports showing the clinical benefit of 3D displays during laparoscopic/thoracoscopic surgeries in urology, gynaecology, thoracic and rectal
surgery7,8,9,10,11. As for laparoscopic gastric surgery, there were no previous studies showing the direct benefit of 3D stereoscopic visualization to reduce total operative time, although
some have shown only a limited 3D benefit12,13. Therefore, a detailed study was necessary to draw conclusions about the possible benefit of 3D stereoscopic visualization for laparoscopic
gastrectomy for gastric cancer. Here, we report that 3D stereoscopic visualization shortened total operative time for both laparoscopic total gastrectomies (LTG) and laparoscopic distal
gastrectomies (LDG) for gastric cancer. We set operative time as the primary outcome and compared 94 consecutive cases of gastric cancer patients who underwent LTG or LDG before and after
introduction of the 3D system at our hospital. Multivariate analyses revealed that operative time performed under 3D was significantly shorter than under 2D. We measured time needed for
intracorporeal dissection and anastomosis and found that dissection time under 3D was significantly shorter, although anastomosis times were similar. Our data demonstrates the benefit of 3D
stereoscopic visualization during laparoscopic gastrectomy for gastric cancer by confirming shorter operative times. METHODS PATIENTS We started using the Olympus 3D laparoscopic system
(LTF-190-10-3D ENDOEYE FLEX 3D) in May 2017, at Kyoto University hospital. A total of 94 consecutive patients (25 LTG and 69 LDG) who underwent LTG or LDG for primary gastric cancer 12
months before and after introduction of the 3D system were evaluated in this study. Patients who underwent open surgery, robot-assisted surgery, laparoscopic proximal gastrectomy, remnant
total gastrectomy or additional splenectomy were excluded. Of these 94 patients, 13 LTG and 40 LDG patients underwent surgery using the Olympus 2D flexible scope (LTF-S190-10 ENDOEYE) with
planar monitors, whereas 12 LTG and 29 LDG patients underwent surgery using the Olympus 3D flexible scope with stereoscopic monitors through polarized glasses. Clinical, surgical, and
pathological outcomes of the patients were retrospectively analysed. The study protocol was approved by the institutional review board of Kyoto University (approval number R1537) and was
performed in accordance with its guidelines and regulations. All patients provided written informed consent for the use of their clinical data. SURGICAL PROCEDURES All surgeries were
performed under the supervision of one of four qualified endoscopic surgeons (KO, ST, HH, or SH) who are board certified by the Japan Society of Endoscopic Surgery14. Over the last 10 years
at our institute, we have performed 69–107 annual gastric cancer operations (Supplementary Figure S1). The level of lymphadenectomy, either D1 + for early gastric cancer or D2 for advanced
cancers, was determined by a pre-operative clinical stage evaluation, based on an upper gastrointestinal endoscopy, an upper gastrointestinal series, and thoracic-abdominal computed
tomography in accordance with Japanese gastric cancer treatment guidelines15,16. Precise procedures of D2 lymphadenectomy for LTG and LDG are reported elsewhere17,18. Roux-Y reconstruction
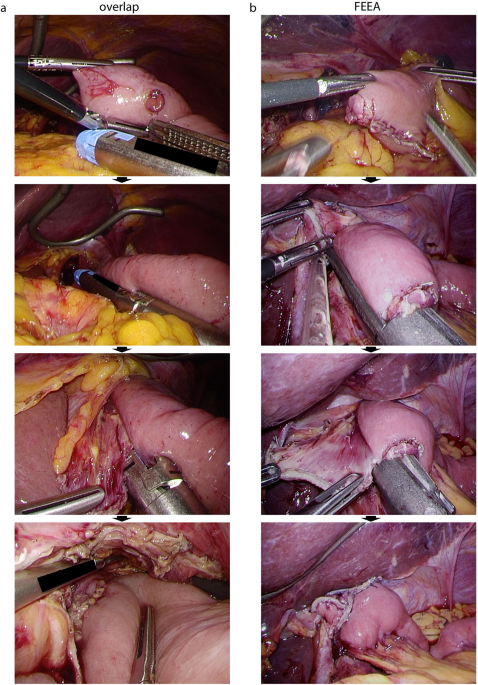
was employed for LTG with intracorporeal E-J and extracorporeal jejunojejunostomy (J-J). For E-J, linear stapled anastomosis was performed by either the overlap method or the
functional-end-to-end anastomosis (FEEA), based on the cancer location19. For tumours located close to the oesophagogastric junction (EGJ), the abdominal oesophagus must be resected to
maintain a proximal surgical safety margin. In these cases, the overlap method was performed20. When the tumour was further away from the EGJ, FEEA was performed. The E-J anastomosis
procedure is described in Fig. 1 and elsewhere19. FEEA was also employed for J-J anastomoses. Petersen’s defect was sutured continuously and intracorporeally, whereas the J-J anastomosis gap
was sutured extracorporeally. For LDG, a Roux-Y, Billroth-I, or Billroth-II anastomosis was performed based on previous reports21,22. Intracorporeal anastomosis requires two linear stapler
cartridges in addition to gut transections, as previously reported21,23: one linear stapler is used to create an anastomosis opening with the oral and anal sides of the gut, and the other is
used to close the first entry hole. Just before using the second linear stapler, the entry hole of the first stapler has to be closed temporarily either with minimal suturing or
laparoscopic hernia staplers (LHS) (Covidien, Dublin, Ireland) (Fig. 2). The procedure used to close the opening [intracorporeal knot-tying (IKT), extracorporeal knot-tying (EKT), LHS or
running suture] was determined based on the operator’s preference. Operative time was defined as the period from skin incision to skin closure. Dissection time was from the first cut of the
omentum to the end of the final cut of the stomach for resection. Anastomosis time began at the opening of the first hole and ended with the final staple or suture for anastomosis. For
dissection and anastomosis times, we excluded the times when the scope was outside of the abdomen, e.g., during cleaning of the scope lens. POSTOPERATIVE COMPLICATIONS Postoperative
complications were defined as greater than Grade 2 of the Clavien-Dindo classification24. STATISTICAL ANALYSIS All continuous variables are expressed as medians (interquartile range) and
were analysed using the Mann-Whitney _U_ test. Categorical independence was analysed using Fisher’s exact test. Multivariate analyses were performed with factors that showed associations
with _P_ values < 0.2 in the univariate analyses. Statistical analysis was performed using JMP software, ver.8 (SAS Institute, Japan). RESULTS PATIENT CHARACTERISTICS, OPERATIVE FACTORS
AND PATHOLOGICAL FACTORS LTG was performed on 12 and 13 patients in the 3D and 2D groups, and LDG was done on 29 and 40 patients in the 3D and 2D groups, respectively, for one year before
and after introduction of the 3D system at our hospital. As shown in Table 1, there were no significant differences between the 3D vs. 2D groups for LTG and LDG with regard to age [LTG, 73
(66–80) years in 3D vs. 66 (64–69) years in 2D, _P_ = 0.26; LDG, 70 (63–76) vs. 68 (63–74), _P_ = 0.67], sex distribution (LTG, _P_ = 0.23; LDG, _P_ = 0.61) and body mass index (BMI;
<25/≥25 kg/m2) (LTG, _P_ = 1.00; LDG, _P_ = 0.58). These were also no differences between the 3D vs. 2D groups for LTG and LDG with regard to the following surgical factors: operator
with/without board certification (OwBC/OwoBC) (LTG, _P_ = 0.38; LDG, _P_ = 0.81), level of lymphadenectomy (D1 + /D2) (LTG, _P_ = 0.24; LDG, _P_ = 0.33) and anastomosis method (overlap/FEEA
in LTG, _P_ = 0.59; Roux-Y/Billroth-I/Billroth-II in LDG, _P_ = 0.99). All laparoscopic gastrectomies were completed without conversion to open surgery. Blood loss in LDG performed under 3D
was significantly less than under 2D [0 (0–0) mL in 3D vs. 3 (0–40) mL in 2D, _P_ = 0.010], although it was not different in LTG [12 (0–85) mL in 3D vs. 40 (0–55) mL, _P_ = 0.71] (Table 2).
The following pathological findings did not differ significantly between the groups; T factors (LTG, _P_ = 0.13; LDG, _P_ = 0.72), number of dissected lymph nodes [LTG, 49 (45–59) in 3D vs.
48 (41–56) in 2D, _P_ = 0.35; LDG, 41 (29–47) vs. 38 (30–49), _P_ = 0.77] and number of metastatic lymph nodes [LTG, 0 (0–1.5) in 3D vs. 0 (0–4.5) in 2D, _P_ = 0.85; LDG, 0 (0–2) vs. 0
(0–2), _P_ = 0.96] (Table 2). OPERATIVE TIME AND ASSOCIATED FACTORS Operative time was significantly shorter in both LTG and LDG when done under 3D stereoscopic visualization compared with
2D visualization [LTG, 351(335–380) min in 3D vs. 406 (367–465) min in 2D, _P_ = 0.026; LDG, 269 (243–326) min vs. 344 (288–402) min, _P_ < 0.01] (Table 2). It is conceivable that the
shorter operative time under 3D is the result of some shorter parts of the intracorporeal procedure compared to those under 2D. To address this possibility, we defined two laparoscopic
procedures, intracorporeal dissection and anastomosis, and determined the length of time required for each procedure. Advanced intracorporeal lymph node dissection is one of the most
difficult laparoscopic surgery procedures and the time required for intracorporeal dissection was significantly shorter under 3D in both LTG [183 (162–203) min in 3D vs. 232 (190–234) min in
2D, _P_ = 0.011] and LDG [161 (128–196) min vs. 213 (178–258) min, _P_ < 0.01]. The time required for intracorporeal anastomosis, which is also a difficult procedure during laparoscopic
gastrectomy, did not differ significantly between the groups [LTG, 21 (17–24) min in 3D vs. 24 (21–31) min in 2D, _P_ = 0.12; LDG, 18 (15–21) min vs. 19 (16–27) min, _P_ = 0.12].
Furthermore, operators significantly preferred performing IKT when closing the anastomosis hole during intracorporeal anastomosis in both LTG and LDG (LTG, _P_ = 0.012; LDG, _P_ < 0.01)
under 3D visualization (Table 2), whereas they preferred EKT or LHS under 2D. IKT requires higher levels of spatial perception than does EKT or LHS. Therefore, these data suggest that when
using 3D visualization operators finished intracorporeal dissection within a shorter time frame and felt less stressed to perform IKT during intracorporeal anastomosis, which was usually the
final step in the operation. Given that surgeons repeatedly perform the same operation with the same team members, it is conceivable that operative times become shorter because of the
experience level of the surgeon and/or the surgical team. In order to assess whether the shorter operative time with 3D visualization was purely the result of surgical team experience, we
drew chronological trend lines for operative times before and after introduction of the 3D system (Fig. 3). These did not show clear chronological improvement, but rather clear gaps before
and after introduction of the 3D system. This suggests that shorter operative times resulted from the introduction of 3D laparoscopic system. Next, we compared operative times according to
several factors: type of visualization (3D vs. 2D), reconstruction method (overlap vs. FEEA in LTG, and Roux-Y vs. Billroth-I vs. Billroth-II in LDG), World Health Organization T factors (T1
vs. ≥T2), level of lymphadenectomy (D1 + vs. D2), operators (OwBC vs. OwoBC), BMI (<25 vs. ≥25 kg/m2) and sex (female vs. male) (Table 3). In the LTG group, univariate analyses revealed
that the type of visualization and BMI were factors significantly associated with operative time. In the LDG group, the type of visualization, the reconstruction method, and the level of
lymphadenectomy were significantly associated with operative time. We performed multivariate analyses with factors that showed associations with _P_ values < 0.2 in the univariate
analysis, with similar results. These analyses showed that the type of visualization (3D/2D) correlated with operative time for both LTG and LDG, and that 3D stereoscopic visualization
corresponded to significantly shorter operative time. SHORT-TERM OUTCOMES In our study, we also assessed whether laparoscopic gastrectomy with 3D stereoscopic visualization affected
postoperative short-term outcomes of the patients. There was no difference in the incidence of postoperative complications greater than Grade 2 of the Clavien-Dindo classification between
the groups (LTG, _P_ = 0.14; LDG, _P_ = 0.30) (Table 2 and Supplementary Table S1). However, the postoperative hospital stay of the 3D patients was significantly shorter in both LTG (_P_
< 0.01) and LDG (_P_ = 0.035). DISCUSSION This is the first study to show a direct benefit of 3D stereoscopic visualization over 2D for laparoscopic gastrectomy with advanced
lymphadenectomy and intracorporeal anastomosis. Namely, we found that the total operative time was significantly shorter with 3D than 2D visualization, and that the shorter operative time
was not the result of surgical team experience, based on drawing and comparing the chronological operative time trend lines. We tried to identify the exact reason for the shorter operative
times and found that intracorporeal dissection under 3D required shorter times than 2D in both LTG and LDG. Intracorporeal anastomosis is usually the final step after a long procedure of
lymph node and stomach dissection during laparoscopic gastrectomy. There was no significant difference in anastomosis time between the 3D and 2D groups in LTG and LDG. However, operators
significantly preferred IKT during anastomosis under 3D visualization, suggesting that they were less stressed during dissection using the 3D system and chose IKT, which required more
advanced skills than EKT or LHS, to close the anastomosis holes. Conversely, when using 2D visualization, they tended to use EKT in LTG or LHS in LDG. This may have been the reason for the
lack of difference in anastomosis times between the groups. For example, the use of a LHS can reduce the time required to close a linear stapler entry hole. Generally speaking, operators
tend to choose the easiest anastomosis procedure since they may be tired or feel stressed after the lengthy intracorporeal dissection even if it is more expensive such as using LHS.
Therefore, decreased use of LHS and shorter operative times with the 3D system may contribute to more cost-effective operations. The objective finding that operators performed more IKT when
using the 3D system compared with the 2D system suggests that they were less stressed throughout dissection under 3D. However, the limitation of this study was that we were not able to
assess subjective markers of the surgeons’ stress level, such as State-Trait Anxiety Inventory for Adults scores because of the retrospective nature of this study. During dry box training,
3D stereoscopic visualization increases the performance related to procedures requiring depth perception especially for novice25. In our study, surgeons tended to become LDG operators rather
than LTG operators before becoming board certified for laparoscopy (OwoBC ratio of 54% in LDG vs. 28% in LTG): therefore, it is conceivable that the improvement of operative time is greater
in LDGs (344 to 269 min, 21.8% reduction) than in LTGs (406 to 351 min, 13.5% reduction) although the operative time baseline under the 2D planar view was shorter in the LDG group. A
previous report by Kanaji _et al_. showed that 3D stereoscopic visualization shortened times for some scenes of lymphadenectomy and for E-J in LTG with D1 + lymphadenectomy (n = 15 in each
group), although there was no difference in total operative time12. In our study, we only defined the time required for the total dissection procedure, and did not analyse the time required
for each part of the lymphadenectomy since defining and identifying the start and end times can be difficult. We found a significant improvement in the time needed for 3D dissection. In
their study, they performed D1 + lymphadenectomy that required a shorter operative time than a D2 lymphadenectomy. This could be the reason for the lack of a significant difference in the
total operative time between the 3D and 2D groups. In contrast, our study included D2 lymphadenectomies that required more advanced lymph node dissection and longer intracorporeal dissection
times, which might have contributed to the significant difference in both total operative and intracorporeal dissection times for the 3D and 2D systems. A randomized controlled trial (RCT)
performed by Lu _et al_., showed that 3D stereoscopic visualization diminished blood loss in laparoscopic gastrectomies13. In our study, we found a significant reduction in blood loss with
the 3D system in the LDG group, but not in LTG. These results suggest that less bleeding under 3D visualization may also contribute to shorter operative times for LDGs. In their study,
however, they did not find a difference in total operative times between their 3D and 2D groups (n = 115 and 113, respectively). Generally speaking, LTG requires a longer operative time than
LDG (Table 2). Although this RCT was large, they combined LTG and LDG in their 3D and 2D groups, which could have been the reason that they found no difference in operative times between
the two groups. In conclusion, 3D stereoscopic visualization when compared with 2D, allowed for shorter total operative times in both LTGs and LDGs for gastric cancer. Intracorporeal
dissection required shorter times for both LTGs and LDGs under 3D, and the surgeons in this study preferred IKT for intracorporeal anastomosis under 3D. DATA AVAILABILITY The datasets in
this study are available from the corresponding author on reasonable request. REFERENCES * Haverkamp, L. _et al_. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a
systematic review and meta-analysis. _Surgical endoscopy_ 27, 1509–1520, https://doi.org/10.1007/s00464-012-2661-1 (2013). Article PubMed Google Scholar * Vinuela, E. F., Gonen, M.,
Brennan, M. F., Coit, D. G. & Strong, V. E. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized
studies. _Annals of surgery_ 255, 446–456, https://doi.org/10.1097/SLA.0b013e31824682f4 (2012). Article PubMed Google Scholar * Okabe, H. _et al_. Feasibility of Laparoscopic Radical
Gastrectomy for Gastric Cancer of Clinical Stage II or Higher: Early Outcomes in a Phase II Study (KUGC04). _Annals of surgical oncology_ 23, 516–523,
https://doi.org/10.1245/s10434-016-5383-0 (2016). Article PubMed Google Scholar * Tsunoda, S. _et al_. Short-term outcomes of totally laparoscopic total gastrectomy: experience with the
first consecutive 112 cases. _World journal of surgery_ 38, 2662–2667, https://doi.org/10.1007/s00268-014-2611-2 (2014). Article PubMed Google Scholar * Sakata, S., Grove, P. M., Hill,
A., Watson, M. O. & Stevenson, A. R. L. Impact of simulated three-dimensional perception on precision of depth judgements, technical performance and perceived workload in laparoscopy.
_The British journal of surgery_ 104, 1097–1106, https://doi.org/10.1002/bjs.10528 (2017). Article CAS PubMed PubMed Central Google Scholar * Harada, H. _et al_. The learning effect of
using stereoscopic vision in the early phase of laparoscopic surgical training for novices. _Surgical endoscopy_ 32, 582–588, https://doi.org/10.1007/s00464-017-5654-2 (2018). Article
PubMed Google Scholar * Patankar, S. B. & Padasalagi, G. R. Three-dimensional versus two-dimensional laparoscopy in urology: A randomized study. _Indian journal of urology: IJU:
journal of the Urological Society of India_ 33, 226–229, https://doi.org/10.4103/iju.IJU_418_16 (2017). Article Google Scholar * Yazawa, H., Takiguchi, K., Imaizumi, K., Wada, M. &
Ito, F. Surgical outcomes of total laparoscopic hysterectomy with 2-dimensional versus 3-dimensional laparoscopic surgical systems. _Fukushima journal of medical science_ 64, 38–45,
https://doi.org/10.5387/fms.2017-22 (2018). Article PubMed PubMed Central Google Scholar * Tang, F. J., Qi, L., Jiang, H. C., Tong, S. Y. & Li, Y. Comparison of the clinical
effectiveness of 3D and 2D imaging systems for laparoscopic radical cystectomy with pelvic lymph node dissection. _The Journal of international medical research_ 44, 613–619,
https://doi.org/10.1177/0300060515621445 (2016). Article PubMed PubMed Central Google Scholar * Dong, S. _et al_. Comparison of three-dimensional and two-dimensional visualization in
video-assisted thoracoscopic lobectomy. _Thoracic cancer_ 7, 530–534, https://doi.org/10.1111/1759-7714.12361 (2016). Article PubMed PubMed Central Google Scholar * Zeng, Q., Lei, F.,
Gao, Z., Wang, Y. & Gao, Q. K. Case-matched study of short-term effects of 3D vs 2D laparoscopic radical resection of rectal cancer. _World journal of surgical oncology_ 15, 178,
https://doi.org/10.1186/s12957-017-1247-8 (2017). Article PubMed PubMed Central Google Scholar * Kanaji, S. _et al_. Comparison of two- and three-dimensional display for performance of
laparoscopic total gastrectomy for gastric cancer. _Langenbeck’s archives of surgery_ 402, 493–500, https://doi.org/10.1007/s00423-017-1574-9 (2017). Article PubMed Google Scholar * Lu,
J. _et al_. Randomized, controlled trial comparing clinical outcomes of 3D and 2D laparoscopic surgery for gastric cancer: an interim report. _Surgical endoscopy_ 31, 2939–2945,
https://doi.org/10.1007/s00464-016-5310-2 (2017). Article PubMed Google Scholar * Tanigawa, N. _et al_. The Endoscopic Surgical Skill Qualification System for gastric surgery in Japan.
_Asian Journal of Endoscopic Surgery_ 4, 112–115, https://doi.org/10.1111/j.1758-5910.2011.00082.x (2011). Article CAS PubMed Google Scholar * Japanese gastric cancer treatment
guidelines 2014 (ver. 4). _Gastric Cancer_ 20, 1–19, https://doi.org/10.1007/s10120-016-0622-4 (2017). * Japanese gastric cancer treatment guidelines 2018 (ver. 5) in Japanese. Kanehara
Shuppan (2018). * Obama, K. _et al_. Feasibility of laparoscopic gastrectomy with radical lymph node dissection for gastric cancer: from a viewpoint of pancreas-related complications.
_Surgery_ 149, 15–21, https://doi.org/10.1016/j.surg.2010.04.014 (2011). Article PubMed Google Scholar * Okabe, H. _et al_. Medial approach for laparoscopic total gastrectomy with splenic
lymph node dissection. _Journal of the American College of Surgeons_ 211, e1–6, https://doi.org/10.1016/j.jamcollsurg.2010.04.006 (2010). Article PubMed Google Scholar * Okabe, H. _et
al_. Is laparoscopic total gastrectomy a safe operation? A review of various anastomotic techniques and their outcomes. _Surgery today_ 45, 549–558, https://doi.org/10.1007/s00595-014-0901-9
(2015). Article PubMed Google Scholar * Inaba, K. _et al_. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. _Journal of the American College
of Surgeons_ 211, e25–29, https://doi.org/10.1016/j.jamcollsurg.2010.09.005 (2010). Article PubMed Google Scholar * Kanaya, S. _et al_. The delta-shaped anastomosis in laparoscopic
distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. _Gastric Cancer_ 14, 365–371, https://doi.org/10.1007/s10120-011-0054-0 (2011).
Article PubMed Google Scholar * Okabe, H., Obama, K., Tsunoda, S., Tanaka, E. & Sakai, Y. Advantage of completely laparoscopic gastrectomy with linear stapled reconstruction: a
long-term follow-up study. _Annals of surgery_ 259, 109–116, https://doi.org/10.1097/SLA.0b013e31828dfa5d (2014). Article PubMed Google Scholar * Okabe, H. _et al_. Intracorporeal
esophagojejunal anastomosis after laparoscopic total gastrectomy for patients with gastric cancer. _Surgical endoscopy_ 23, 2167–2171, https://doi.org/10.1007/s00464-008-9987-8 (2009).
Article PubMed Google Scholar * Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and
results of a survey. _Annals of surgery_ 240, 205–213 (2004). Article Google Scholar * Harada, H. _et al_. The effect on surgical skills of expert surgeons using 3D/HD and 2D/4K resolution
monitors in laparoscopic phantom tasks. _Surgical endoscopy_, https://doi.org/10.1007/s00464-018-6169-1 (2018). Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department
of Surgery, Graduate School of Medicine, Kyoto University, Kyoto, 606-8507, Japan Yoshiro Itatani, Kazutaka Obama, Tatsuto Nishigori, Riki Ganeko, Shigeru Tsunoda, Shigeo Hisamori, Kyoichi
Hashimoto & Yoshiharu Sakai * Department of Surgery, Kyoto City Hospital, Kyoto, 604-8845, Japan Hisahiro Hosogi Authors * Yoshiro Itatani View author publications You can also search
for this author inPubMed Google Scholar * Kazutaka Obama View author publications You can also search for this author inPubMed Google Scholar * Tatsuto Nishigori View author publications You
can also search for this author inPubMed Google Scholar * Riki Ganeko View author publications You can also search for this author inPubMed Google Scholar * Shigeru Tsunoda View author
publications You can also search for this author inPubMed Google Scholar * Hisahiro Hosogi View author publications You can also search for this author inPubMed Google Scholar * Shigeo
Hisamori View author publications You can also search for this author inPubMed Google Scholar * Kyoichi Hashimoto View author publications You can also search for this author inPubMed Google
Scholar * Yoshiharu Sakai View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS K.O. designed the study concept. Y.I. and K.O. acquired the
data. Y.I. and T.N. analysed the results. Y.I. and K.O. drafted the manuscript. R.G., S.T., H.H., S.H., K.H. and Y.S. revised the manuscript. CORRESPONDING AUTHOR Correspondence to Yoshiro
Itatani. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE AND FIGURE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Itatani, Y., Obama, K., Nishigori, T. _et al._ Three-dimensional
Stereoscopic Visualization Shortens Operative Time in Laparoscopic Gastrectomy for Gastric Cancer. _Sci Rep_ 9, 4108 (2019). https://doi.org/10.1038/s41598-019-40269-3 Download citation *
Received: 05 November 2018 * Accepted: 11 February 2019 * Published: 11 March 2019 * DOI: https://doi.org/10.1038/s41598-019-40269-3 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative