Spatiotemporal Characterizations of Spontaneously Beating Cardiomyocytes with Adaptive Reference Digital Image Correlation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

We developed an Adaptive Reference-Digital Image Correlation (AR-DIC) method that enables unbiased and accurate mechanics measurements of moving biological tissue samples. We applied the
AR-DIC analysis to a spontaneously beating cardiomyocyte (CM) tissue, and could provide correct quantifications of tissue displacement and strain for the beating CMs utilizing
physiologically-relevant, sarcomere displacement length-based contraction criteria. The data were further synthesized into novel spatiotemporal parameters of CM contraction to account for
the CM beating homogeneity, synchronicity, and propagation as holistic measures of functional myocardial tissue development. Our AR-DIC analyses may thus provide advanced non-invasive
characterization tools for assessing the development of spontaneously contracting CMs, suggesting an applicability in myocardial regenerative medicine.
Advanced tissue mechanical characterization and data visualization (Fig. S1) may lead to standardized measures of tissue mechanical functioning for in vitro tissues and for various in vivo
diagnosis (e.g., ultrasound speckle tracking, magnetic resonance elastography, etc.). The characterization of spatiotemporal mechanical properties of cardiac tissue is especially important
since the mechanical sensitivity of the heart is a recognized driver of cardiomyogenesis, homeostasis, and pathological hypertrophy1,2. For example, asynchrony of cardiac beating is
clinically used for the assessment of cardiac health. Current myocardial tissue assessment methods with patch clamp, electrode array, microforce transducer, voltage-sensitive dye, etc., are
invasive, require sophisticated instruments, or constrain the culture environment3,4. Since contraction quality is one of the most vital metrics to determine the functional health of
cardiomyocyte (CM) tissue, alternatively, digital image correlation (DIC) may be used to assess the tissue via non-invasive imaging and monitoring. However, DIC involving correlation
coefficient and median reference has only limited scope for biological samples5,6,7,8,9,10,11,12.
DIC is widely applicable to both hard and soft tissues at multiple length scales, and may be applied independently of mechanical tissue behavior (i.e., isotropicity, deformation range,
etc.)13. The resolution and sensitivity of DIC depends on many factors including the imaging resolution, frame rate, and choice of correlation parameters. These factors must be tailored to
capture the resolution at the tissue level. The non-contact nature of DIC excels for cell-based regenerative therapies since the tissues may be intended for transplantation9. However, the
tissue must be visually accessible for imaging and subsurface tissue characterizations may be difficult or impossible for a simple imaging setup. DIC is also dependent on trackable features
which may undergo non-affine deformations during contraction. Synthetic tracking molecules may be added to the tissue but, again, this is not ideal for an implantable tissue. Despite these
limitations, DIC has been utilized as a powerful characterization tool for measuring the beating characteristics of individual sarcomeres, individual cells, and cardiac tissues.
Quantifications using sarcomere banding, beating signal correlation with Ca2+, and dose-dependent drug responses are a sampling of the utility of DIC-based quantifications. See the review of
Laurila et al.14 for a more detailed comparison of DIC to traditional CM quantifications.
Here we developed Adaptive Reference-DIC (AR-DIC) to achieve robust, unbiased, and accurate kinematics and strain measurements of moving biological tissue samples, such as beating CMs, which
lack stationary reference frames thus producing noise build-up during the measurement. We further defined novel spatiotemporal analyses, including heat maps that reveal the beating
frequency and magnitude over time and position, and accumulative spatiotemporal maps that analyze interrelationships among moving tissue regions. In an application of AR-DIC (Figs. 1A and
S1) to spontaneously beating CMs, engineered myocardial tissue development with measures of tissue maturation and homogeneity regarding spontaneous contraction were evaluated. We further
demonstrate the utility for tracking tissue development using videos from Rajasingh et al.6 from three consecutive days. As a result, the “difficult-to-characterize” spontaneously beating CM
tissue model could be analyzed in the localization, synchronization, and development of CM beating motion. The AR-DIC may further be utilized to examine the functional aspects of cardiac
diseases via analyzing in vitro pathological hypertrophy model.
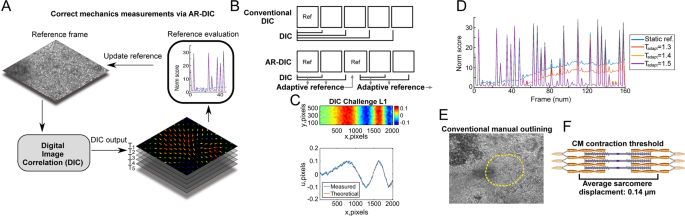
AR-DIC enables robust, unbiased, and accurate mechanics measurements of moving biological tissue samples such as spontaneously contacting CM tissue. (A) In AR-DIC, adaptive reference scheme
continually adjusts reference frames enabling correct quantification of displacements occurring during the spontaneous contraction of cardiomyocytes. (B) Unlike conventional DIC depending on
static reference frame, AR-DIC adaptively selects a new reference frame. (C) AR-DIC was validated using the 2D DIC Challenge 14-L1 of the Society for Experimental Mechanics
(sem.org/dicchallenge/). AR-DIC (Measured) describes well the given test set (Theoretical). (D) Iterative refinement of the adaptive reference threshold (Tadapt) reduces measurement error
build-up, enabling robust DIC application to beating CMs. Finding Tadapt to enable long-term monitoring of contracting CMs required three iterations (Tadapt from 1.3 to 1.5). (E)
Conventional manual outlining for beating area. (F) The average in vivo sarcomere displacement length of 0.14 µm was adopted in this study as a threshold over which displacement was
considered as CM contraction1.
Inspired by optimization methods, our AR-DIC Matlab toolbox automatically selects a reference frame after major CM contraction cycle (Fig. 1B) in order to resolve the measurement error
accrual in traditional DIC. The effectiveness of developed AR-DIC was tested by the DIC Challenge from the Society for Experimental Mechanics (sem.org/dicchallenge/) (Figs. 1C and S3).
Through iterative refinement of adaptive reference threshold, AR-DIC applied to CM tissues could almost completely remove the measurement error build-up (Fig. 1D, Tadapt = 1.5), enabling
robust application of DIC to assess the mechanics of CM contraction. Further, in contrast to manual outlining to define the contracting area (Fig. 1E), we adopted physiologically relevant
criteria such that regions with displacement greater than the average individual CM sarcomere contraction length (0.14 µm) (Fig. 1F) were considered as “spontaneously beating”1. We adopted
0.14 µm as a guide based on a published sarcomere average displacement however other researchers may wish to select contraction based on the properties of their expected tissue displacement.
This was achieved because AR-DIC was developed to have selection criteria adjustable and expandable. Of further utility, the adaptive reference scheme may be extended to other imaging
modalities such as photoacoustic imaging, magnetic resonance imaging, magnetic resonance elastography, ultrasound, etc., that commonly lack standard reference frames.
Measuring tissue displacement magnitude, beats per minute (BPM), and total contracting area may be a first step to assess contractile CM tissue health. The adaptive reference scheme enabled
the correct measurements of tissue displacement during the CM beating (Figs. 2A–D, S4 and S5). The maximum displacement of 3.62 µm for the contracting sample (Figs. 2A,C) implies concerted
effort of multiple (> two dozen) sarcomere units. Velocity field was then calculated from the displacement and time between frames (Fig. 2E). A horizontal trace through the maximum velocity
position reveals that velocity changes from the contraction center (Fig. 2F). The sarcomere displacement length criteria (0.14 µm) enabled physiology-based quantifications of contracting
area (Fig. 2G) and BPM (Fig. 2H) opposed to conventional manual drawing and counting. The beating area, assessed by the area showing displacement more than one sarcomere displacement (0.14
μm), had a maximum field of view coverage of 57.2% (or 6.99 × 105 µm2), indicating an ongoing engineered myocardial tissue development as the tissue was assessed on day 10 of CM
differentiation. Our method can also set other arbitrary contraction thresholds to define CM beating, e.g., four sarcomere displacement length (0.56 μm). This allows the method to be adapted
to each study based on the anticipated displacements and available imaging resolution. BPM, calculated by the fast Fourier transform (FFT) of the displacement signal and verified with an
automated peak counter, was 75.0 for the beating areas having displacement more than 0.14 μm; which is comparable to BPMs in previous studies including ours2,14,15.
In AR-DIC of beating CMs, accurate displacement measurement and combined sarcomere length-based physiological contraction criteria provide multiple quantitative biomechanics data critical
for assessing functional myocardial tissue development. Displacement at a time frame showing the maximum displacement for contracting (A) and non-contracting (B) samples, respectively. Scale
bar is 100 µm. Displacement change with respect to time at the maximum (C) and minimum (D) displacement locations, respectively (figures include both contracting and non-contracting cases).
(E) Velocity vector plot calculated from the displacement for the contracting sample. (F) A horizontal trace through the maximum velocity location shows contraction velocity change with
distance from the contraction center. (G) Surface coverage percentage of beating area calculated at the maximum contraction area frame. Area with displacement >0.14 μm (sarcomere
displacement length) was defined as contraction. Arbitrary threshold can also be set, e.g., displacement of four sarcomeres, 0.56 μm. (H) BPM calculated by FFT of displacement and verified
with an automated peak counter is also based on the sarcomere length criteria. (I–K) Horizontal (εxx), vertical (εyy), and shear (εxy) strains calculated from displacements for the
contracting sample at the maximum displacement frame. Positive strains values are tensile and negative strains are compressive. (L) Principal strains over time at the maximum compressive
strain location. (M) Maximum raw strains and maximum principal strains. Frame units in all relevant figures are in µm.
AR-DIC quantifications next enable the calculation of the tissue strain. Strain is known to play a critical role in the mechanical feedback loop assisting in cardiac development and
homeostasis16. Compressive strains were observed at the contraction center and tensile strains were present on either side of the compressive region (Figs. 2I,J and S6). The shear strain had
regions of opposing shear rotating away from the contraction center (Fig. 2K), which resembles the twisting of in vivo cardiac tissue during contraction16. Principal strains at the maximum
compression location (Fig. 2L) and values of maximum principal strains (Fig. 2M) may provide magnitude information on in vivo myocardial tissue strains during the heart beating. Plotting
transformed principal strains indicates a central region with compressive strains (Fig. S7). Having compressive strains implies a net decrease in the local tissue area, which could
potentially function as a contraction origin (related strain data in Figs. S8 and S9). For functional myocardial tissue engineering, mechanical loading at these identified strains (up to
about 10%) and frequencies (up to about 1.25 Hz corresponding to 75 BPM as noted above) may therefore be attempted for physiological relevancy consideration (as we adopted15).
Next, the homogeneity, propagation, and synchronicity of CM contraction which have significant clinical implications to evaluate the functional health of myocardial tissues17,18 could be
evaluated. AR-DIC processed images were arranged in spatial layers over time (Fig. 3A as schematic), from which spatial overlap of beating regions was analyzed (Fig. 3B). As a new spatial
assessment of CM beating, heat maps for beating frequency and displacement magnitude were constructed (Fig. 3C–F). Interestingly, centers of beating frequency and magnitude are not always
coincident (or, the most frequently beating region is not necessarily that having the largest displacement). The contraction volume (CV) (Fig. 3G) was quantified to provide a more holistic
measure of CM beating by combining beating area and displacement. When mechanics data are revisited with CV, regions with high CV (within 1σ below maximum CV) displayed significantly greater
velocity (Fig. 3H) and larger strains (Fig. 3I) relative to regions with low CV. These quantifications provide a metric of contraction development that may be tracked for an individual
tissue region over time or used for comparisons among tissues.
Holistic measures of functional myocardial tissue development assessing the homogeneity of CM tissue contraction can be achieved by AR-DIC with newly developed spatiotemporal parameters.
Contracting regions arranged in spatial layers over time (A) and overlaid as a single layer (B) (schematic illustrations). (C–F) Contraction frequency and magnitude heat maps are newly
defined to display spatial beating parameters accumulated over time. Contraction origins identified in ASC and ASTC maps (see Fig. 4) are marked as O′, O″, and O″′. (G) Contraction volume
(CV) for the entire viewing field, accounting for both beating area and displacement magnitude, is plotted over time. A dotted line denotes 1σ below the maximum CV. (H,I) Average maximum
velocity and strain plotted for high and low CV regions. High CV region was defined as to be within 1σ below the maximum CV, below which was considered as low CV. Mean ± standard error of
measurement. +++p